Graphene-Based Films: Fabrication, Interfacial Modification, and Applications
Abstract
:1. Introduction
2. Preparation Techniques of Graphene-Based Film
2.1. Vacuum Filtration
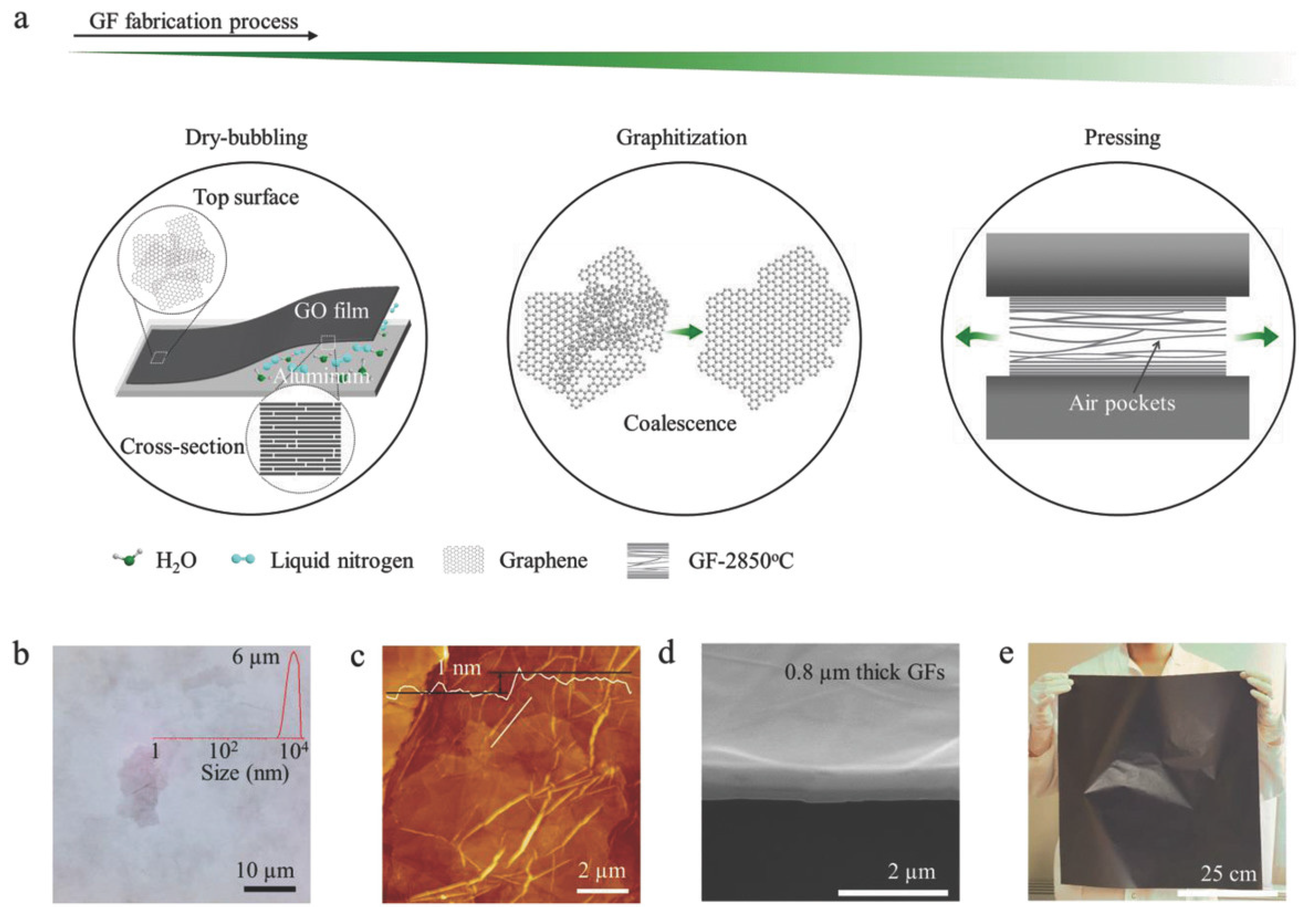
2.2. Evaporation-Induced Self-Assembly
2.3. Blade Coating
2.4. Spin Coating
2.5. Electro-Induced Preparation
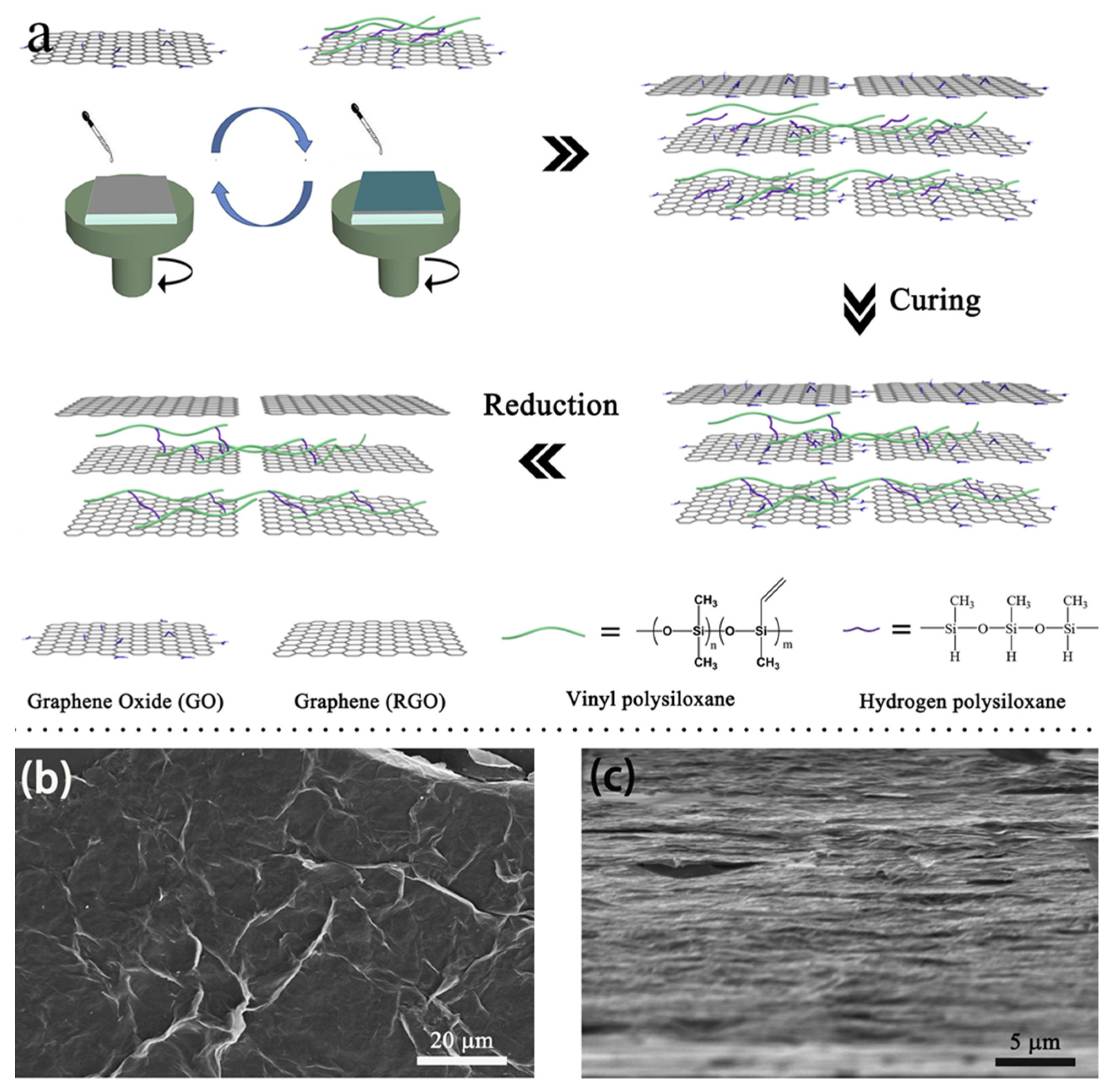
3. Interfacial Modification of Graphene-Based Film to Improve Its Mechanical Property
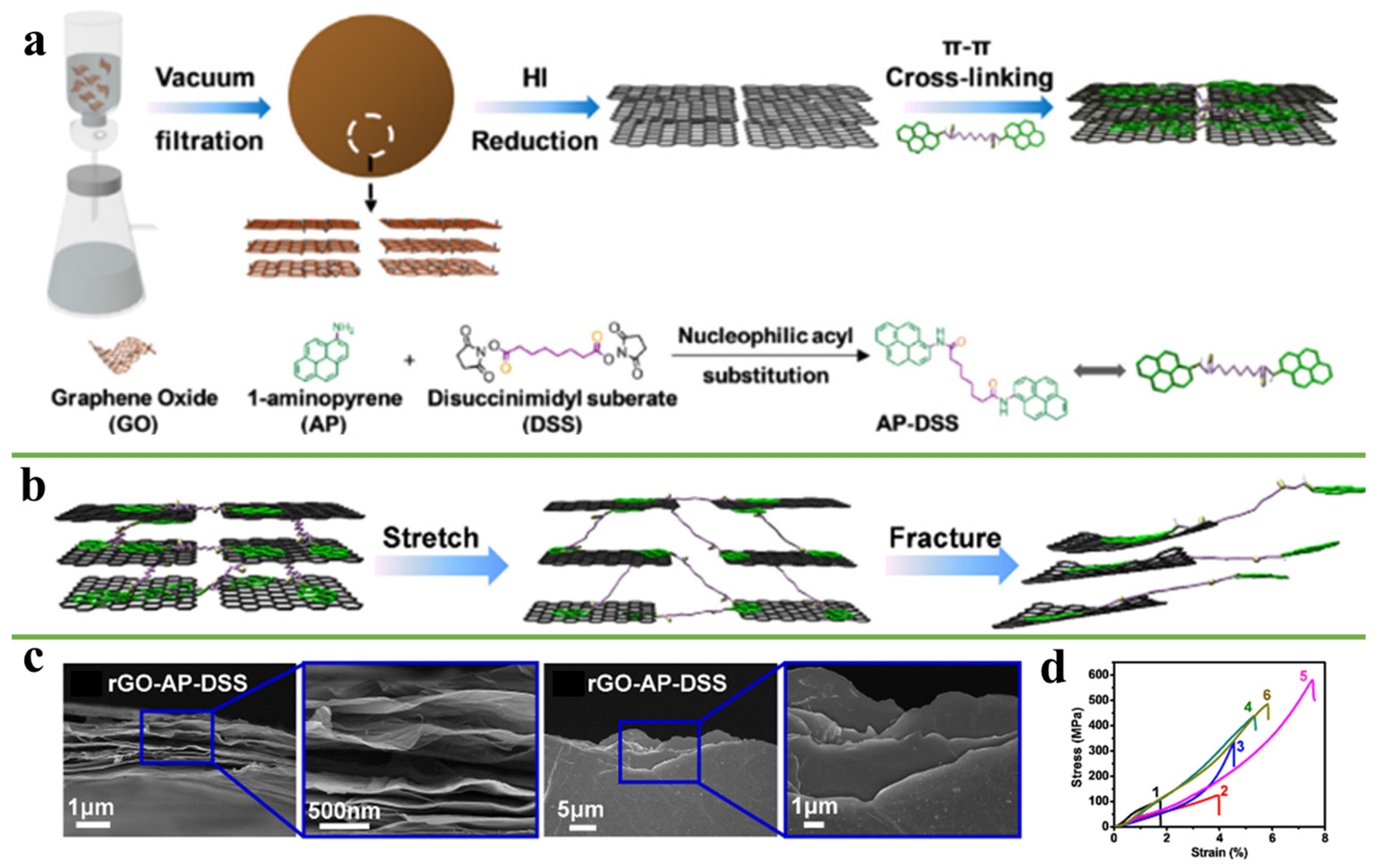
3.1. π-π Interactions
3.2. Hydrogen Bonding
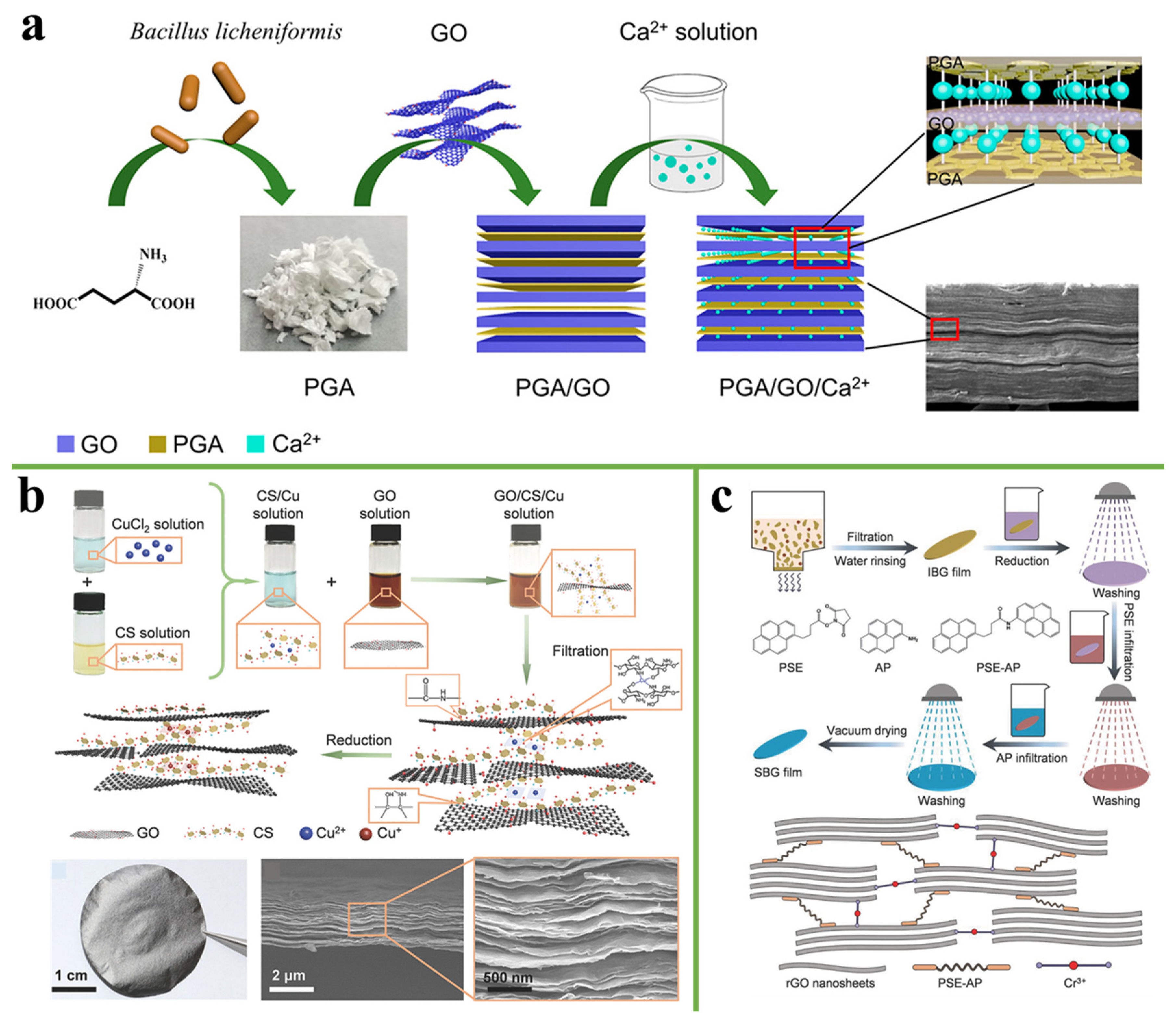
3.3. Ionic Bonding
3.4. Covalent Bonding
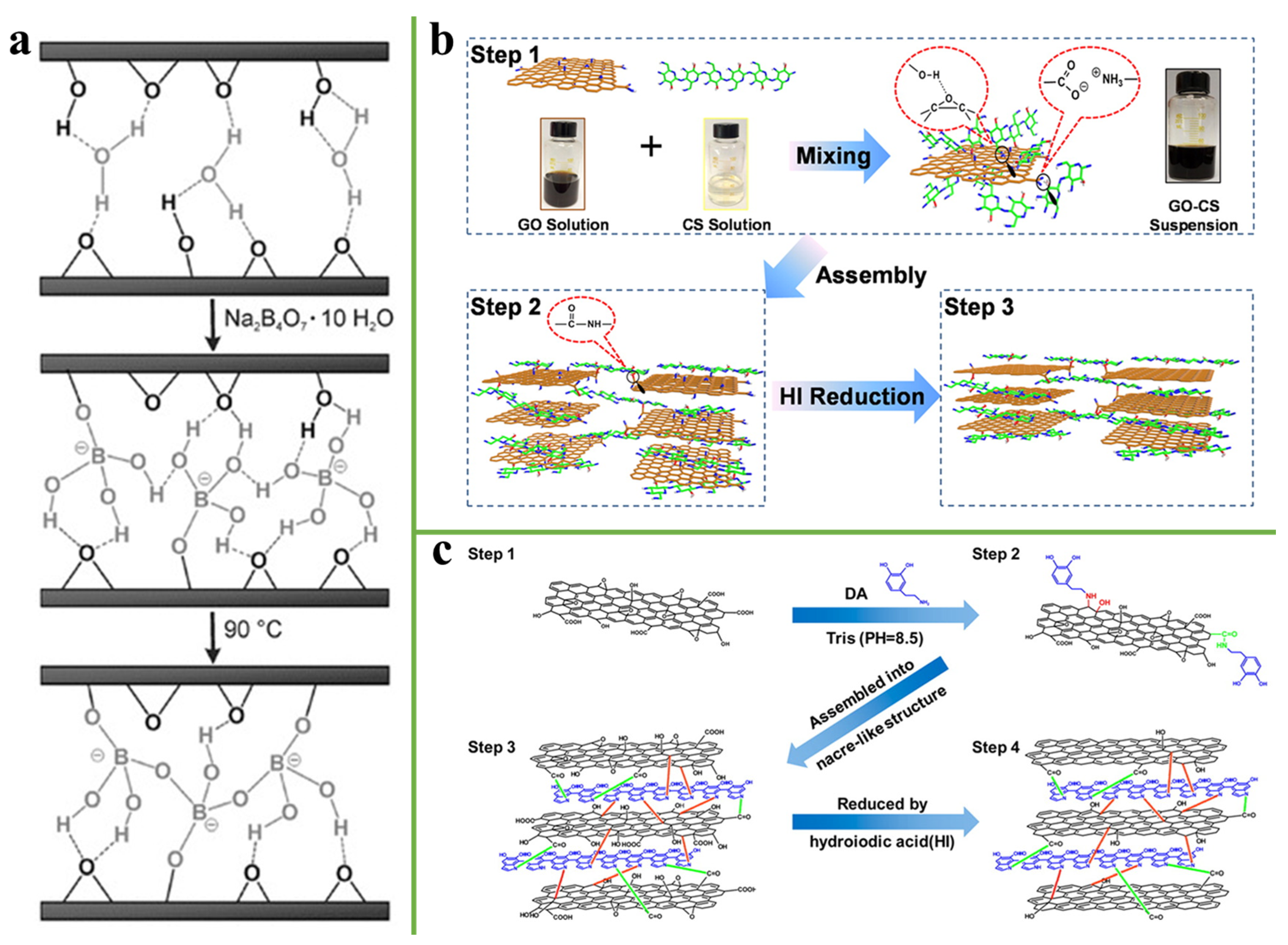
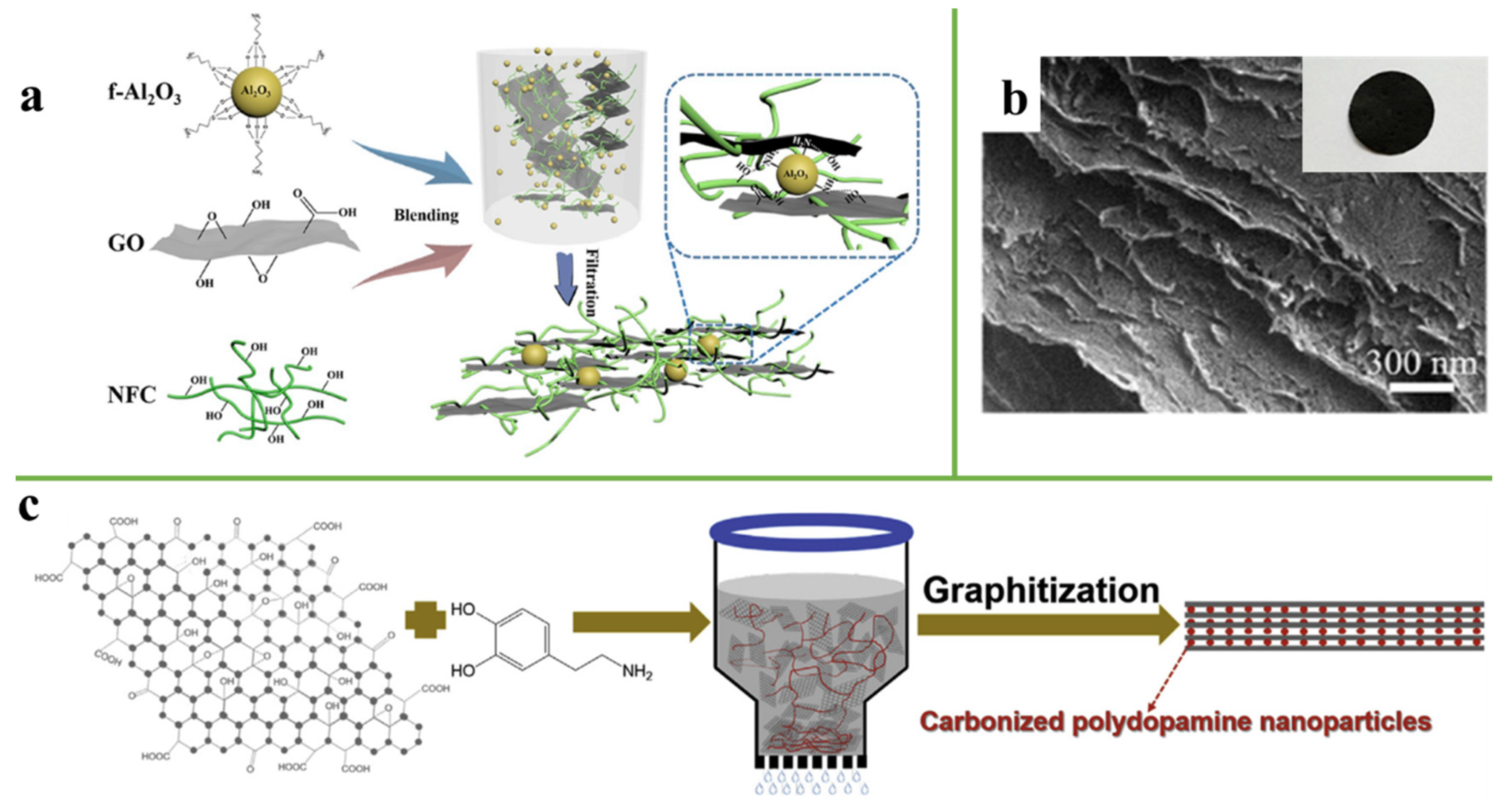
3.5. Synergistic Interaction
4. Applications
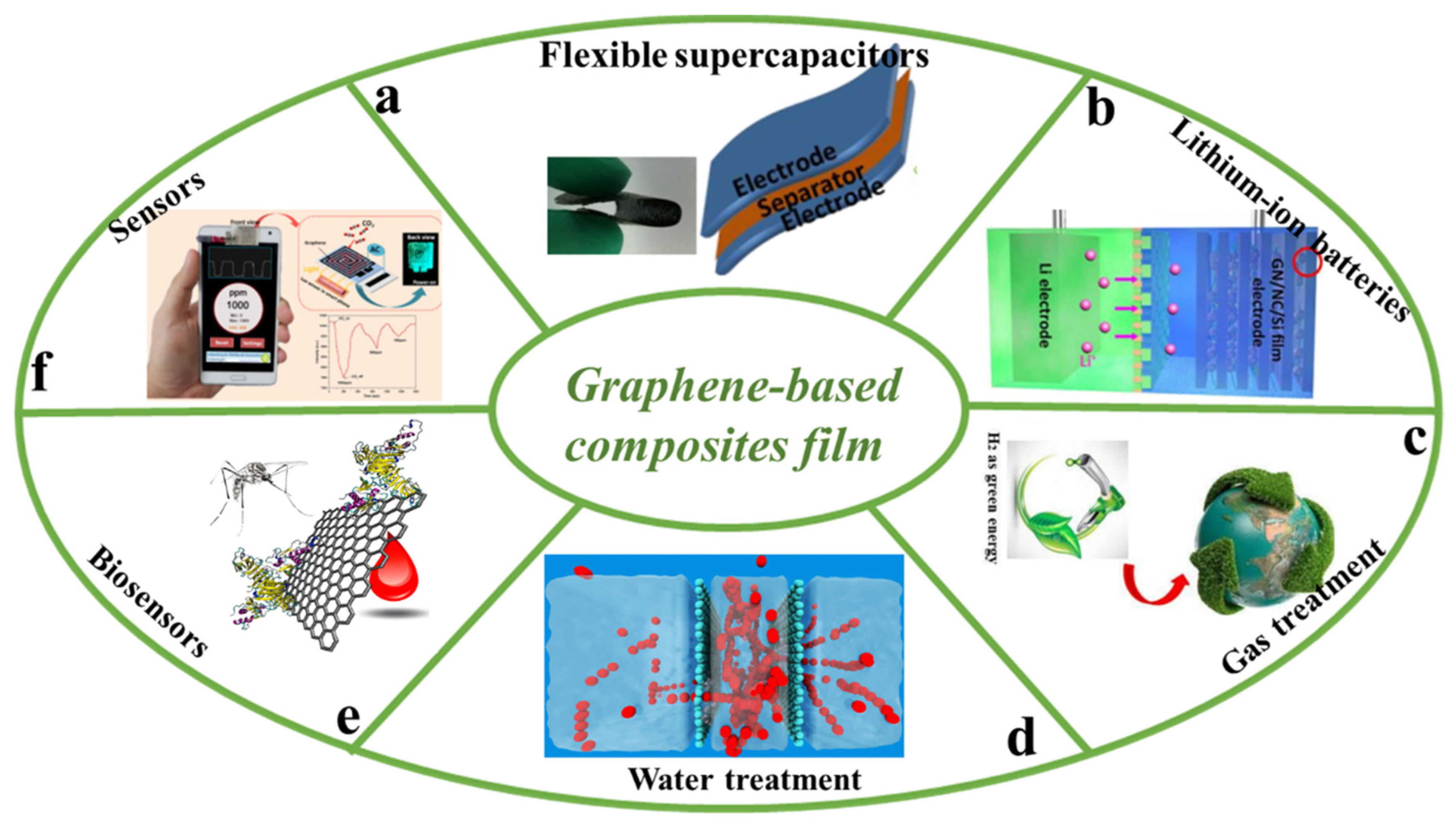
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B.; et al. Graphene related materials for thermal management. 2D Mater. 2019, 7, 012001. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, L.; Zehri, A.H.; Logothetis, N.; Liu, J. Fabrication and characterization of graphene based film. In Proceedings of the 2017 IMAPS Nordic Conference on Microelectronics Packaging, Gothenburg, Sweden, 18–20 June 2017; pp. 162–166. [Google Scholar]
- Zhang, M.; Chen, S.; Zhang, X.; Wang, N.; Ye, L.; Liu, J. Effect of pressure during graphitization on mechanical properties of graphene films. In Proceedings of the 2019 20th International Conference on Electronic Packaging Technology, Hong Kong, China, 11–15 August 2019. [Google Scholar]
- Liu, Y.; Chen, S.; Fu, Y.; Wang, N.; Liu, J. A lightweight and high thermal performance graphene heat pipe. Nano Sel. 2021, 2, 364–372. [Google Scholar] [CrossRef]
- Qin, S.; Liu, Y.; Jiang, H.; Xu, Y.; Shi, Y.; Zhang, R.; Wang, F. All-carbon hybrids for high-performance electronics, optoelectronics and energy storage. Sci. China Inform. Sci. 2019, 62, 220434. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Xu, J.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Chang, K.W.; Santos, I.A.; Nguyen, Y.; Hsu, C.C.; Hsieh, Y.P.; Hofmann, M. Electrostatic Control over the Electrochemical Reactivity of Graphene. Chem. Mater. 2018, 30, 7178–7182. [Google Scholar] [CrossRef]
- Wang, M.-H.; Li, Q.; Li, X.; Liu, Y.; Fan, L.-Z. Effect of oxygen-containing functional groups in epoxy/reduced graphene oxide composite coatings on corrosion protection and antimicrobial properties. Appl. Surf. Sci. 2018, 448, 351–361. [Google Scholar] [CrossRef]
- Okobiah, O.; Reidy, R.F. Surface Interactions: Functionalization of Graphene Oxide and Wetting of Graphene Oxide and Graphene. Prog. Mater. Sci. 2016, 20, 674–681. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Yang, H.; Luo, J. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar]
- Zhang, Y.; Gong, S.; Zhang, Q.; Ming, P.; Wan, S.; Cheng, Q. Graphene-based artificial nacre nanocomposites. Chem. Soc. Rev. 2016, 45, 2378–2395. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, G.; Huang, Y.; Chen, Z.; Yuan, H.; Du, F. Assembly and application advancement of organic-functionalized graphene-based materials: A review. J. Sep. Sci. 2020, 43, 1544–1557. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zheng, K.; Cao, X.; Fan, Q.; Ma, Y. Flexible Graphene Nanocomposites with Simultaneous Highly Anisotropic Thermal and Electrical Conductivities Prepared by Engineered Graphene with Flat Morphology. ACS Nano 2020, 14, 11733–11742. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Yang, S.; Xue, B.; Li, Y.; Li, X.; Zheng, Q. Controllable Ag-rGO heterostructure for highly thermal conductivity in layer-by-layer nanocellulose hybrid films. Chem. Eng. J. 2020, 383, 123072. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, H.; Kang, R.; Yun, L.; Nan, J.; Yu, J. Highly flexible biodegradable cellulose nanofiber/graphene heat-spreader films with improved mechanical properties and enhanced thermal conductivity. J. Mater. Chem. C 2018, 6, 12739–12745. [Google Scholar] [CrossRef]
- Li, L.; Ma, Z.; Xu, P.; Zhou, B.; Liu, C. Flexible and alternant-layered cellulose nanofiber/graphene film with superior thermal conductivity and efficient electromagnetic interference shielding. Compos. Part A Appl. Sci. 2020, 139, 106134. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Z.; Wu, K.; Huang, R.; Chen, F.; Fu, Q. Ultrathin flexible reduced graphene oxide/cellulose nanofiber composite films with strongly anisotropic thermal conductivity and efficient electromagnetic interference shielding. J. Mater. Chem. C 2017, 5, 3748–3756. [Google Scholar] [CrossRef]
- Song, N.; Jiao, D.; Cui, S.; Hou, X.; Ding, P.; Shi, L. Highly Anisotropic Thermal Conductivity of Layer-by-Layer Assembled Nanofibrillated Cellulose/Graphene Nanosheets Hybrid Films for Thermal Management. ACS Appl. Mater. Inter. 2017, 9, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Xu, L.; Qiao, L.; Chen, S.; Shi, Y.; Wang, X. Nanofibrillated Cellulose/MgO@rGO composite films with highly anisotropic thermal conductivity and electrical insulation. Chem. Eng. J. 2020, 392, 123714. [Google Scholar] [CrossRef]
- Guo, S.; Zheng, R.; Jiang, J.; Yu, J.; Dai, K.; Yan, C. Enhanced thermal conductivity and retained electrical insulation of heat spreader by incorporating alumina-deposited graphene filler in nano-fibrillated cellulose. Compos. Part B Eng. 2019, 178, 107489. [Google Scholar] [CrossRef]
- Yuan, G.; Xie, J.; Li, H.; Shan, B.; Liu, J.; Tian, Y. Thermally Reduced Graphene Oxide/Carbon Nanotube Composite Films for Thermal Packaging Applications. Materials 2020, 13, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, R.; Liu, F.; Hu, N.; Ning, H.; Jiang, X.; Zhou, X.; Yan, C. Carbonized polydopamine nanoparticle reinforced graphene films with superior thermal conductivity. Carbon 2019, 149, 173–180. [Google Scholar] [CrossRef]
- Li, H.; Dai, S.; Miao, J.; Wu, X.; Qiu, H.; Yang, J. Enhanced thermal conductivity of graphene/polyimide hybrid film via a novel “molecular welding” strategy. Carbon 2018, 126, 319–327. [Google Scholar] [CrossRef]
- Miao, J.; Li, H.; Qiu, H.; Wu, X.; Yang, J. Graphene/PANI hybrid film with enhanced thermal conductivity by in situ polymerization. J. Mater. Sci. 2018, 53, 8855–8865. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, X.; Liu, Y.; Gao, C. Tailorable graphene-based superconducting film via self-assembly and in-situ doping. Carbon 2019, 152, 527–531. [Google Scholar] [CrossRef]
- Hansson, J.; Samani, M.K.; Wang, N.; Nilsson, T.; Liu, J. Synthesis of a Graphene Carbon Nanotube Hybrid Film by Joule Self-Heating CVD for Thermal Applications. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2018; pp. 2450–2456. [Google Scholar]
- Wan, N.; Chen, S.; Nkansah, A.; Darmawan, C.C.; Liu, J. Highly Thermally Conductive and Light Weight Copper/Graphene Film Laminated Composites for Cooling Applications. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology, Shanghai, China, 8–11 August 2018; pp. 1588–1592. [Google Scholar]
- Tkacz, R.; Oldenbourg, R.; Fulcher, A.J.; Miansari, M.; Majumder, J. Capillary-Force-Assisted Self-Assembly (CAS) of Highly Ordered and Anisotropic Graphene-Based Thin Films. J. Phys. Chem. C 2013, 118, 259–267. [Google Scholar] [CrossRef]
- Sheath, P.; Majumder, M. Flux accentuation and improved rejection in graphene-based filtration membranes produced by capillary-force-assisted self-assembly. Philos. Trans. R. Soc. A 2016, 374, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Samani, M.K.; Li, H.; Chen, S.; Liu, J. Tailoring the Thermal and Mechanical Properties of Graphene Film by Structural Engineering. Small 2018, 14, 1801346. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh Thermal Conductive yet Superflexible Graphene Films. Adv. Mater. 2017, 29, 1700589. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Wang, F.; Fang, W.; Xu, Z.; Gao, W.; Gao, C. Rapid roll-to-roll production of graphene films using intensive Joule heating. Carbon 2019, 155, 462–468. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Liu, Y.; Li, Z.; Fang, W.; Peng, L.; Zhou, J.; Xu, Z.; Gao, C. Ultrathick and highly thermally conductive graphene films by self-fusion strategy. Carbon 2020, 167, 249–255. [Google Scholar] [CrossRef]
- Lin, S.; Su, J.; Zhang, J.; Gang, S.; He, Y.; Jiang, D. Ultrathin flexible graphene films with high thermal conductivity and excellent EMI shielding performance using large-sized graphene oxide flakes. RSC Adv. 2019, 9, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Wang, Y.; Wang, Y.; Wang, C.; Gao, X. Graphene induced carbonization of polyimide films to prepared flexible carbon films with improving-thermal conductivity. Ceram. Int. 2020, 46, 3332–3338. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Zhang, M.; Huang, R.; Liu, J. Scalable production of thick graphene film for next generation thermal management application. Carbon 2020, 167, 270–277. [Google Scholar] [CrossRef]
- Chi, C.; Wang, X.; Peng, Y.; Dong, J.; Zhao, D. Facile Preparation of Graphene Oxide Membranes for Gas Separation. Chem. Mater. 2016, 28, 2921–2927. [Google Scholar] [CrossRef]
- Fang, X.; Fan, Z.; Gu, Y.; Shi, J.; Chen, M.; Chen, X.; Qiu, X.; Zabihi, F.; Eslamian, M.; Chen, Q. A Solution Processable Flexible Transparent Conductive Graphene/PEDOT: PSS Film Fabricated by Spin and Blade Coating. J. Shanghai Jiao Tong Univ. Sci. 2018, 23, 106–111. [Google Scholar] [CrossRef]
- Ollik, K.; Lieder, M. Review of the Application of Graphene-Based Coatings as Anticorrosion Layers. Coatings 2020, 10, 883. [Google Scholar] [CrossRef]
- Kim, S.Y.; Gang, H.E.; Park, G.T.; Jeon, H.B.; Jeong, Y.G. Microstructure and electrothermal characterization of transparent reduced graphene oxide thin films manufactured by spin-coating and thermal reduction. Result Phys. 2021, 24, 104107. [Google Scholar] [CrossRef]
- Xiong, R.; Hu, K.; Grant, A.M.; Zhang, X.; Tsukruk, V.V. Ultrarobust Transparent Cellulose Nanocrystal-Graphene Membranes with High Electrical Conductivity. Adv. Mater. 2016, 28, 1501–1509. [Google Scholar] [CrossRef]
- Sreeja, V.G.; Hajara, P.; Reshmi, R.; Anila, E.I. Effects of reduced graphene oxide on nonlinear absorption and optical limiting properties of spin coated aluminium doped zinc oxide thin films. Thin Solid Films 2021, 722, 138580. [Google Scholar] [CrossRef]
- Oytun, F.; Basarir, F. Spin-assisted layer-by-layer assembled oppositely charged reduced graphene oxide films. Mater. Lett. 2019, 257, 126756. [Google Scholar] [CrossRef]
- Lee, T.; Min, S.H.; Gu, M.; Jung, Y.K.; Lee, W.; Kim, B.S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: Synthesis and Applications. Chem. Mater. 2015, 27, 3785–3796. [Google Scholar] [CrossRef]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.H.; Koo, C.M. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Cocemasov, A.; Nika, D.L.; Balandin, A.A. Strongly Anisotropic Thermal Conductivity of Free-Standing Reduced Graphene Oxide Films Annealed at High Temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Hong, S.H.; Yoo, S.S.; Yoo, P.J. Binder-free heat dissipation films assembled with reduced graphene oxide and alumina nanoparticles for simultaneous high in-plane and cross-plane thermal conductivities. J. Mater. Chem. C 2019, 7, 9380–9388. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Zhang, Y. High thermal conductivity and stretchability of layer-by-layer assembled silicone rubber/graphene nanosheets multilayered films. Compos. Part A Appl. Sci. 2018, 105, 1–8. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, R.; Zhan, K.; Bao, L.; Yang, J. Preparation of electro-reduced graphene oxide/copper composite foils with simultaneously enhanced thermal and mechanical properties by DC electro-deposition method. Mat. Sci. Eng. A 2021, 805, 140574. [Google Scholar] [CrossRef]
- Cui, R.; Han, Y.; Zhu, Z.; Chen, B.; Ding, Y.; Pei, F.; Ye, Z. Investigation of the structure and properties of electrodeposited Cu/graphene composite coatings for the electrical contact materials of an ultrahigh voltage circuit breaker. J. Alloy Compd. 2019, 777, 1159–1167. [Google Scholar] [CrossRef]
- Song, G.; Yang, Y.; Fu, Q.; Pan, C. Preparation of Cu-Graphene Composite Thin Foils via DC Electro-Deposition and Its Optimal Conditions for Highest Properties. J. Electrochem. Soc. 2017, 164, D652–D659. [Google Scholar] [CrossRef]
- Aghili, M.; Yazdi, M.K.; Ranjbar, Z.; Jafari, S.H. Anticorrosion performance of electro-deposited epoxy/amine functionalized graphene oxide nanocomposite coatings. Corros. Sci. 2021, 179, 109143. [Google Scholar] [CrossRef]
- An, Z.; Li, J.; Kikuchi, A.; Wang, Z.; Ono, T. Mechanically strengthened graphene-Cu composite with reduced thermal expansion towards interconnect applications. Microsyst. Nanoeng. 2019, 5, 2–11. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, J.; Yu, F.; Ye, N. Preparation of graphene nanoplatelets reinforcing copper matrix composites by electrochemical deposition. J. Alloy Compd. 2018, 766, 266–273. [Google Scholar] [CrossRef]
- Maharana, H.S.; Rai, P.K.; Basu, A. Surface-mechanical and electrical properties of pulse electrodeposited Cu–graphene oxide composite coating for electrical contacts. J. Mater. Sci 2016, 52, 1089–1105. [Google Scholar] [CrossRef]
- Pingala, A.D.; Belgamwar, S.U.; Rathore, J.S. The influence of graphene nanoplatelets (GNPs) addition on the microstructure and mechanical properties of Cu-GNPs composites fabricated by electro-co-deposition and powder metallurgy. Mater. Today 2020, 28, 2062–2067. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Finklea, H.; Liu, X. A review of electrophoretic deposition of metal oxides and its application in solid oxide fuel cells. Adv. Colloid Interfac. 2020, 276, 102102. [Google Scholar] [CrossRef]
- Ahmadizadeh, N.; Najafisayar, P. The effects of electrodeposition parameters on the wetting behavior of ceria coatings. Ceram. Int. 2020, 46, 19583–19592. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; He, H.; Shi, B. Enhanced the thermal conductivity of flexible copper foil by introducing graphene. Mater. Design 2020, 187, 108373. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.S.; Li, K.; Kang, T.J.; Kim, Y.H. High performance graphene foam emitter. Carbon 2016, 101, 345–351. [Google Scholar] [CrossRef]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Tang, M.; Zhu, S.; Liu, Z.; Jiang, C.; Wu, Y.; Wang, C. Tailoring π-Conjugated Systems: From π-π Stacking to High-Rate-Performance Organic Cathodes. Chem 2018, 4, 2600–2614. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Meng, F.; Huang, F.; Li, Y.; Zhou, Z. Ultrastrong Carbon Nanotubes/Graphene Papers via Multiple π-π Cross-Linking. ACS Appl. Mater. Inter. 2020, 12, 47811–47819. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ge, L.; Ni, K.; Fu, A.; Kan, X.; Chen, S.-M.; Gao, M.; Pan, F.; Yu, S.-H.; Zhu, Y.; et al. Strong and tough graphene papers constructed with pyrene-containing small molecules via π-π/H-bonding synergistic interactions. Sci. China Mater. 2021, 64, 1206–1218. [Google Scholar] [CrossRef]
- Ni, H.; Xu, F.; Tomsia, A.P.; Saiz, E.; Jiang, L.; Cheng, Q. Robust Bioinspired Graphene Film via π-π Cross-linking. ACS Appl. Mater. Inter. 2017, 9, 24987–24992. [Google Scholar] [CrossRef]
- Wan, S.; Chen, Y.; Wang, Y.; Li, G.; Cheng, Q. Ultrastrong Graphene Films via Long-Chain π-Bridging. Matter 2019, 1, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Meyers, M.A.; McKittrick, J.; Chen, P.Y. Structural biological materials: Critical mechanics-materials connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Bao, Q.; Liu, Y.; Wen, H.; Wang, Q. Hydrogen Bond Association to Prepare Flame Retardant Polyvinyl Alcohol Film with High Performance. ACS Appl. Mater. Inter. 2021, 13, 5508–5517. [Google Scholar] [CrossRef]
- Bhasha, S.; Shelkhar, S.; Gautam, S.; Sarkar, A.; Jain, P. Nanomechanical analysis of chemically reduced graphene oxide reinforced poly (vinyl alcohol) nanocomposite thin films. Polym. Test. 2018, 70, 458–466. [Google Scholar]
- Liu, S.; Yao, F.; Oderinde, O.; Li, K.; Zhang, Z.; Fu, G. Zinc ions enhanced nacre-like chitosan/graphene oxide composite film with superior mechanical and shape memory properties. Chem. Eng. J. 2017, 321, 502–509. [Google Scholar] [CrossRef]
- Jia, L.; Sun, W.; Zhou, C.; Yan, D.; Zhang, Q.; Li, Z. Integrated strength and toughness in graphene/calcium alginate films for highly efficient electromagnetic interference shielding. J. Mater. Chem. C 2018, 6, 9166–9174. [Google Scholar] [CrossRef]
- Duan, J.; Gong, S.; Gao, Y.; Xie, X.; Jiang, L.; Cheng, Q. Bioinspired Ternary Artificial Nacre Nanocomposites Based on Reduced Graphene Oxide and Nanofibrillar Cellulose. ACS Appl. Mater. Inter. 2016, 8, 10545–10550. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, L.; Guo, Y.; Yang, X. Theoretical insights into origin of graphene oxide acidity and relating behavior of oxygen-containing groups in water. Carbon 2021, 183, 355–361. [Google Scholar] [CrossRef]
- Xing, W.; Yuan, B.; Wang, X.; Hu, Y. Enhanced mechanical properties, water stability and repeatable shape recovery behavior of Ca2+ crosslinking graphene oxide-based nacre-mimicking hybrid film. Mater. Design 2017, 115, 46–51. [Google Scholar] [CrossRef]
- Lee, S.M.; Pippel, E.; Goesele, U.; Dresbach, C.; Qin, Y.; Braeuniger, T.; Hause, G.; Knez, M. Greatly Increased Toughness of Infiltrated Spider Silk. Science 2009, 324, 488–492. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R. Graphene Oxide Papers Modified by Divalent Lons-Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, Y.; Huang, F.; Yang, X.; Li, Y.; Li, J.; Meng, F.; Zhou, Z. Ultra-robust and high-toughness graphene oxide papers via synergistic strengthening by addition of carbon-nanotubes and copper ions. Carbon 2019, 147, 490–500. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- An, Z.; Compton, O.C.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Bio-inspired borate cross-linking in ultra-stiff graphene oxide thin films. Adv. Mater. 2011, 23, 3842–3846. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.; Liu, L.; Zu, S.; Peng, K.; Zhong, Z. The Effect of Interlayer Adhesion on the Mechanical Behaviors of Macroscopic Graphene Oxide Papers. ACS Nano 2011, 5, 2134–2141. [Google Scholar]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Wan, S.; Peng, J.; Li, Y.; Hu, H.; Jiang, L.; Cheng, Q. Use of Synergistic Interactions to Fabricate Strong, Tough, and Conductive Artificial Nacre Based on Graphene Oxide and Chitosan. ACS Nano 2015, 9, 9830–9836. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Tao, R.; Liu, X.; Cui, Y.; Liu, Y.; Zhang, Z. Enhanced thermal conductivity and mechanical properties of natural rubber-based composites co-incorporated with surface treated alumina and reduced graphene oxide. Diam. Relat. Mater. 2021, 116, 108438. [Google Scholar] [CrossRef]
- Cui, W.; Li, M.; Liu, J.; Wang, B.; Cheng, Q. A strong integrated strength and toughness artificial nacre based on dopamine cross-linked graphene oxide. ACS Nano 2014, 8, 9511–9517. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.; Boyce, M.C. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar]
- Li, M.; Miao, Y.; Zhai, X.; Yin, Y.; Liu, Z. Preparation of and research on bioinspired graphene oxide/nanocellulose/polydopamine ternary artificial nacre. Mater. Design. 2019, 181, 107961. [Google Scholar] [CrossRef]
- Song, P.; Xu, Z.; Wu, Y.; Cheng, Q.; Guo, Q.; Wang, H. Super-tough artificial nacre based on graphene oxide via synergistic interface interactions of π-π stacking and hydrogen bonding. Carbon 2017, 111, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wu, C.; Wang, Y.; Tomsia, A.; Cheng, Q. Super-tough MXene-functionalized graphene sheets. Nat. Commun. 2020, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Schmieden, D.T.; Meyer, A.S.; Aubin-Tam, M.E. Using bacteria to make improved, nacre-inspired materials. MRS Adv. 2016, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Spiesz, E.M.; Schmieden, D.; Aubin-Tam, M.E. Bioproduced Polymers Self-Assemble with Graphene Oxide into Nanocomposite Films with Enhanced Mechanical Performance. ACS Nano 2020, 14, 14731–14739. [Google Scholar] [CrossRef]
- Cheng, Y.; Peng, J.; Xu, H.; Cheng, Q. Glycera-Inspired Synergistic Interfacial Interactions for Constructing Ultrastrong Graphene-Based Nanocomposites. Adv. Funct. Mater. 2018, 28, 1800924. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Bryan, G.W. Copper-the Major Metal Component of Glycerid Polychaete Jaws. J. Mar. Biol. 1980, 60, 205–214. [Google Scholar] [CrossRef]
- Moses, D.N.; Pontin, M.G.; Waite, J.H.; Zok, F.W. Effects of hydration on mechanical properties of a highly sclerotized tissue. Biophys. J. 2008, 94, 3266–3272. [Google Scholar] [CrossRef] [Green Version]
- Hua, B.; Li, C.; Wang, X.; Shi, G. On the Gelation of Graphene Oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar]
- Wan, S.; Fang, S.; Jiang, L.; Cheng, Q.; Baughman, R.H. Strong, Conductive, Foldable Graphene Sheets by Sequential Ionic and π Bridging. Adv. Mater. 2018, 30, 1802733. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Wang, Q.; Zhang, X. Recent progress on transition metal oxides as advanced materials for energy conversion and storage. Energy Storage Mater. 2021, 42, 317–369. [Google Scholar] [CrossRef]
- Qi, B.; Ren, K.; Lin, Y.; Zhang, S.; Wei, T.; Fan, Z. Design of layered-stacking graphene assemblies as advanced electrodes for supercapacitors. Particuology 2021, 31, 13. [Google Scholar]
- Song, K.; Ni, H.F.; Fan, L.Z. Flexible Graphene-Based Composite Films for Supercapacitors with Tunable Areal Capacitance. Electrochim. Acta 2017, 235, 233–241. [Google Scholar] [CrossRef]
- Ning, L.; Tian, L.; Yao, Y.; Li, H.; Tao, C. Compact graphene/MoS2 composite films for highly flexible and stretchable all-solid-state supercapacitors. J. Mater. Chem. A 2017, 5, 3267–3273. [Google Scholar]
- Zhou, X.; Liu, Y.; Du, C.; Ren, Y.; Li, X.; Cheng, X.; Gao, Y. Free-Standing Sandwich-Type Graphene/Nanocellulose/Silicon Laminar Anode for Flexible Rechargeable Lithium Ion Batteries. ACS Appl. Mater. Inter. 2018, 10, 29638–29646. [Google Scholar] [CrossRef]
- Zhu, K.; Gao, H.; Hu, G. A flexible mesoporous Li4Ti5O12-rGO nanocomposite film as free-standing anode for high rate lithium ion batteries. J. Power Sources 2018, 375, 59–67. [Google Scholar] [CrossRef]
- Stergious, A.; Cantón-Vitoria, R.; Psarrou, M.N.; Economopoulos, S.P.; Tagmatarchis, N. Functionalized graphene and targeted applications-Highlighting the road from chemistry to applications. Prog. Mater. Sci. 2020, 114, 100683. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Zhu, Y.; Caro, J. Bicontinuous zeolitic imidazolate framework ZIF-8@GO membrane with enhanced hydrogen selectivity. J. Am. Chem. Soc. 2014, 136, 14686–14689. [Google Scholar] [CrossRef] [PubMed]
- Zeynali, R.; Ghasemzadeh, K.; Sarand, A.B.; Kheiri, F.; Basile, A. Performance evaluation of graphene oxide (GO) nanocomposite membrane for hydrogen separation: Effect of dip coating sol concentration. Sep. Purif. Technol. 2018, 200, 169–176. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Lin, L.C.; Grossman, J.C. Multilayer Nanoporous Graphene Membranes for Water Desalination. Nano Lett. 2016, 16, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernaez, M. Applications of Graphene-Based Materials in Sensors. Sensors 2020, 20, 3196. [Google Scholar] [CrossRef]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Barron, F.; Goldsmith, B.R. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88. [Google Scholar] [CrossRef]
- Zhang, C.; Ping, J.; Ying, Y. Evaluation of trans-resveratrol level in grape wine using laser-induced porous graphene-based electrochemical sensor. Sci. Total Environ. 2020, 714, 136687. [Google Scholar] [CrossRef] [PubMed]
- Seekaew, Y.; Wongchoosuk, C. A novel graphene-based electroluminescent gas sensor for carbon dioxide detection. Appl. Surf. Sci. 2019, 479, 525–531. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Chen, J.; Zhang, Y.; Liu, J. Graphene-Based Films: Fabrication, Interfacial Modification, and Applications. Nanomaterials 2021, 11, 2539. https://doi.org/10.3390/nano11102539
Guo S, Chen J, Zhang Y, Liu J. Graphene-Based Films: Fabrication, Interfacial Modification, and Applications. Nanomaterials. 2021; 11(10):2539. https://doi.org/10.3390/nano11102539
Chicago/Turabian StyleGuo, Sihua, Jin Chen, Yong Zhang, and Johan Liu. 2021. "Graphene-Based Films: Fabrication, Interfacial Modification, and Applications" Nanomaterials 11, no. 10: 2539. https://doi.org/10.3390/nano11102539
APA StyleGuo, S., Chen, J., Zhang, Y., & Liu, J. (2021). Graphene-Based Films: Fabrication, Interfacial Modification, and Applications. Nanomaterials, 11(10), 2539. https://doi.org/10.3390/nano11102539





