CVD Synthesis of Intermediate State-Free, Large-Area and Continuous MoS2 via Single-Step Vapor-Phase Sulfurization of MoO2 Precursor
Abstract
:1. Introduction
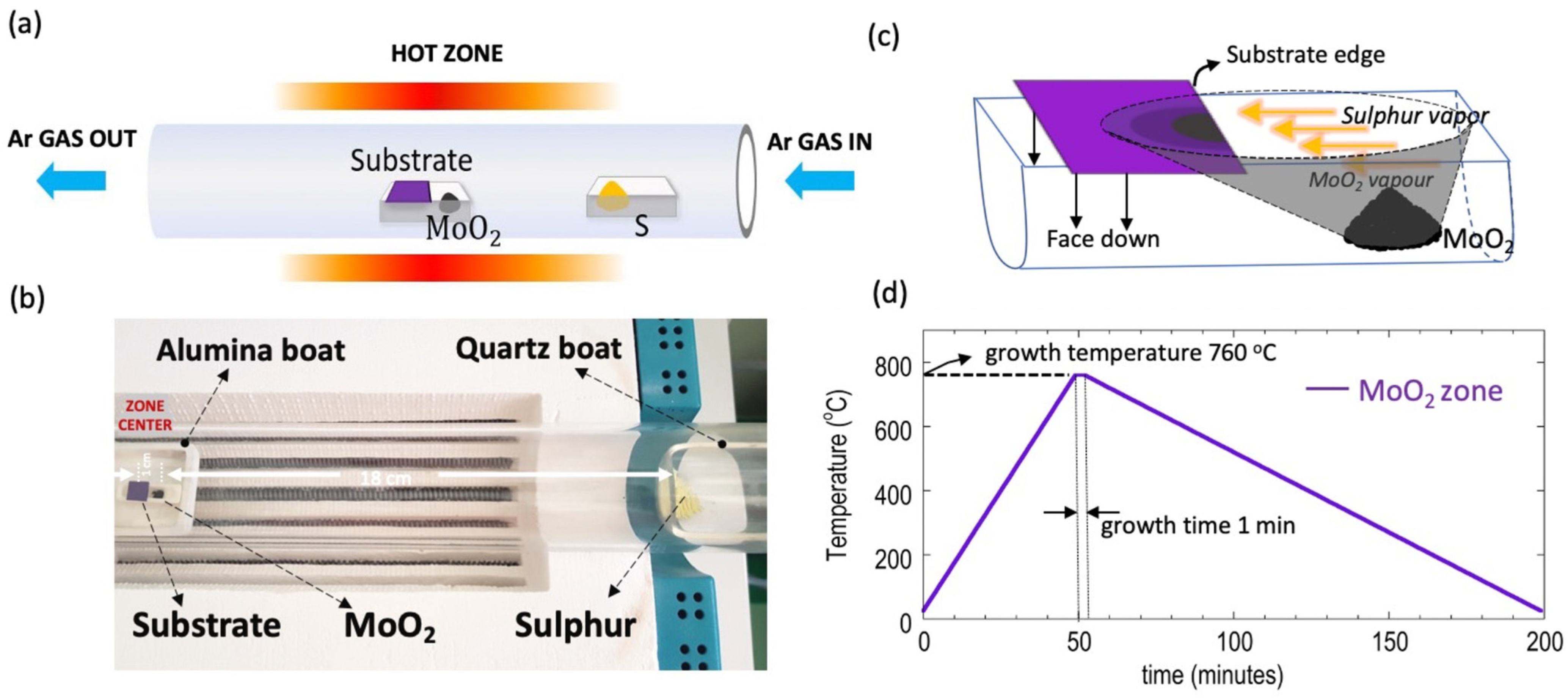
2. Materials and Methods
2.1. CVD Synthesis of MoS2 Using Direct MoO2 Precursor
2.2. MoS2 Characterization
3. Results and Discussions
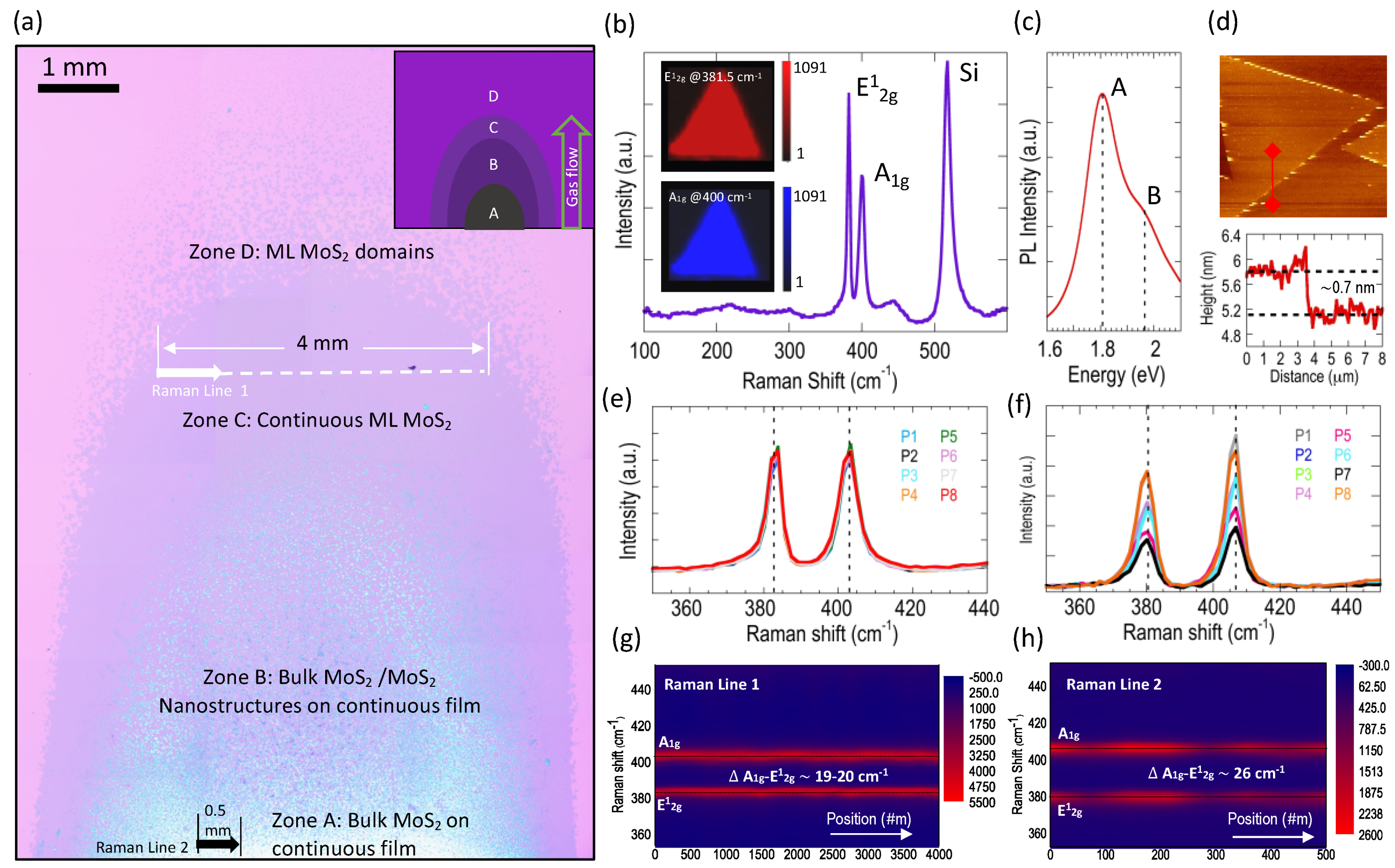
3.1. Zonal Deposition Pattern of MoS2
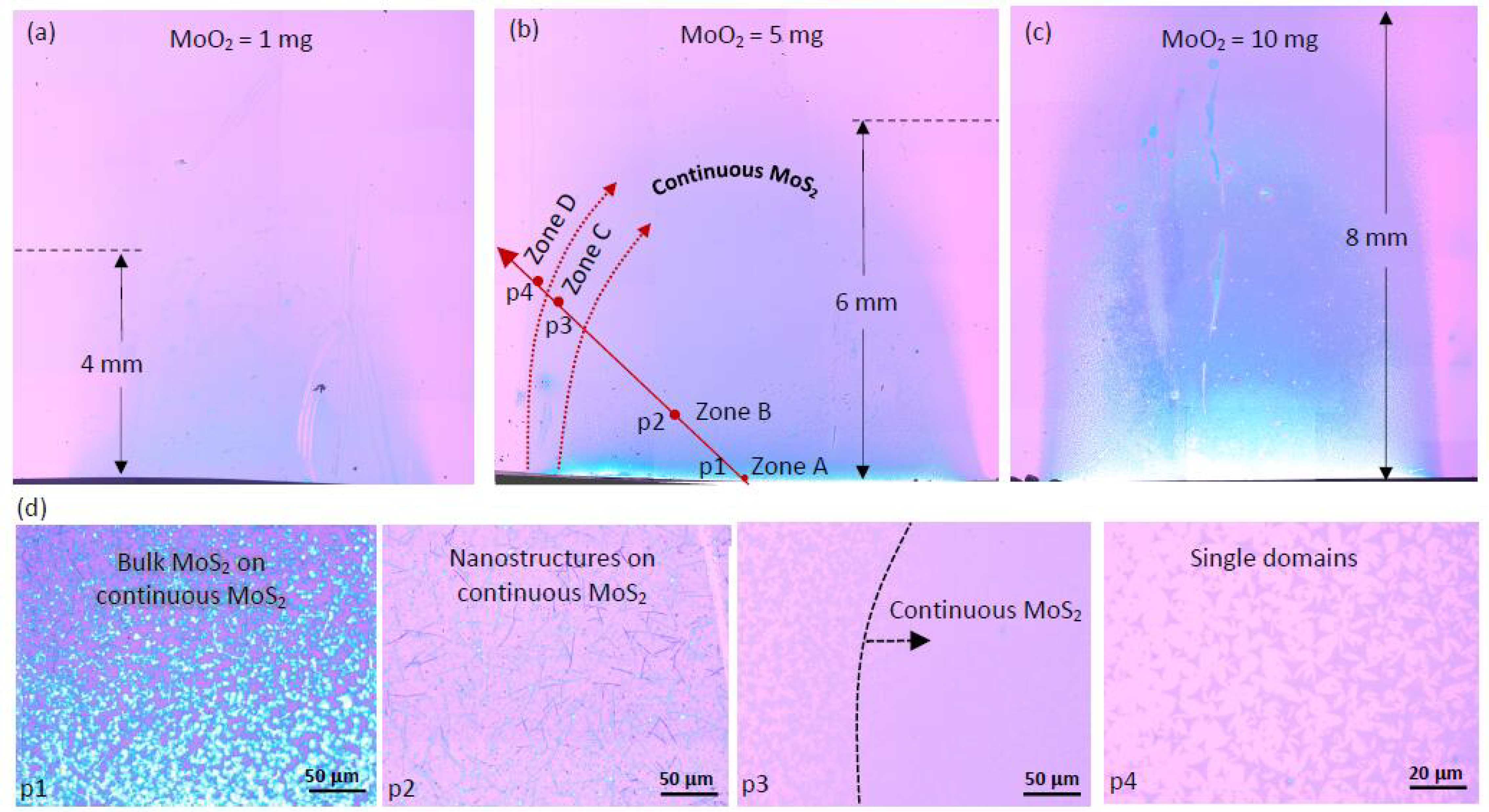
3.2. Effect of MoO2 Precursor Quantity
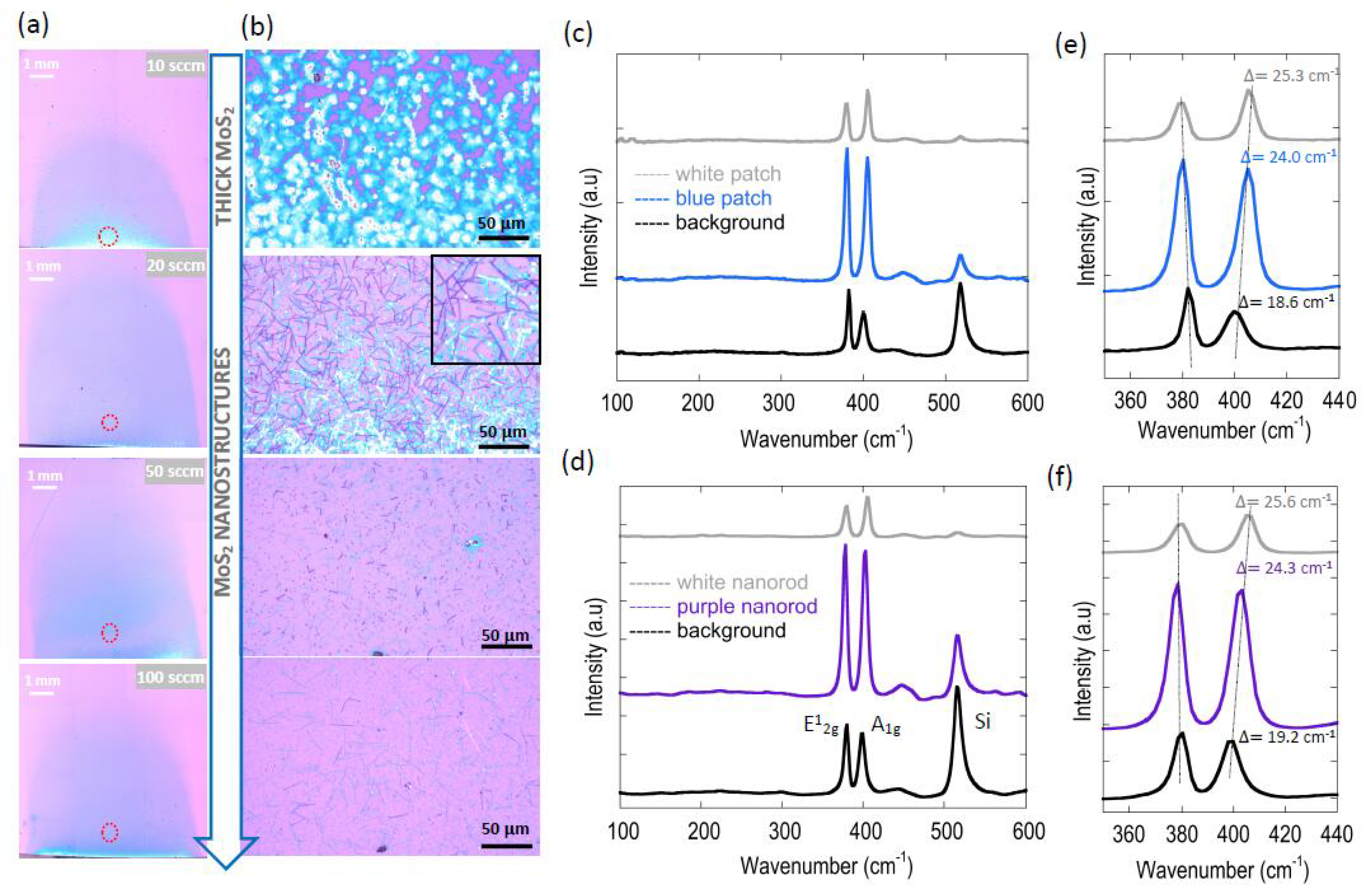
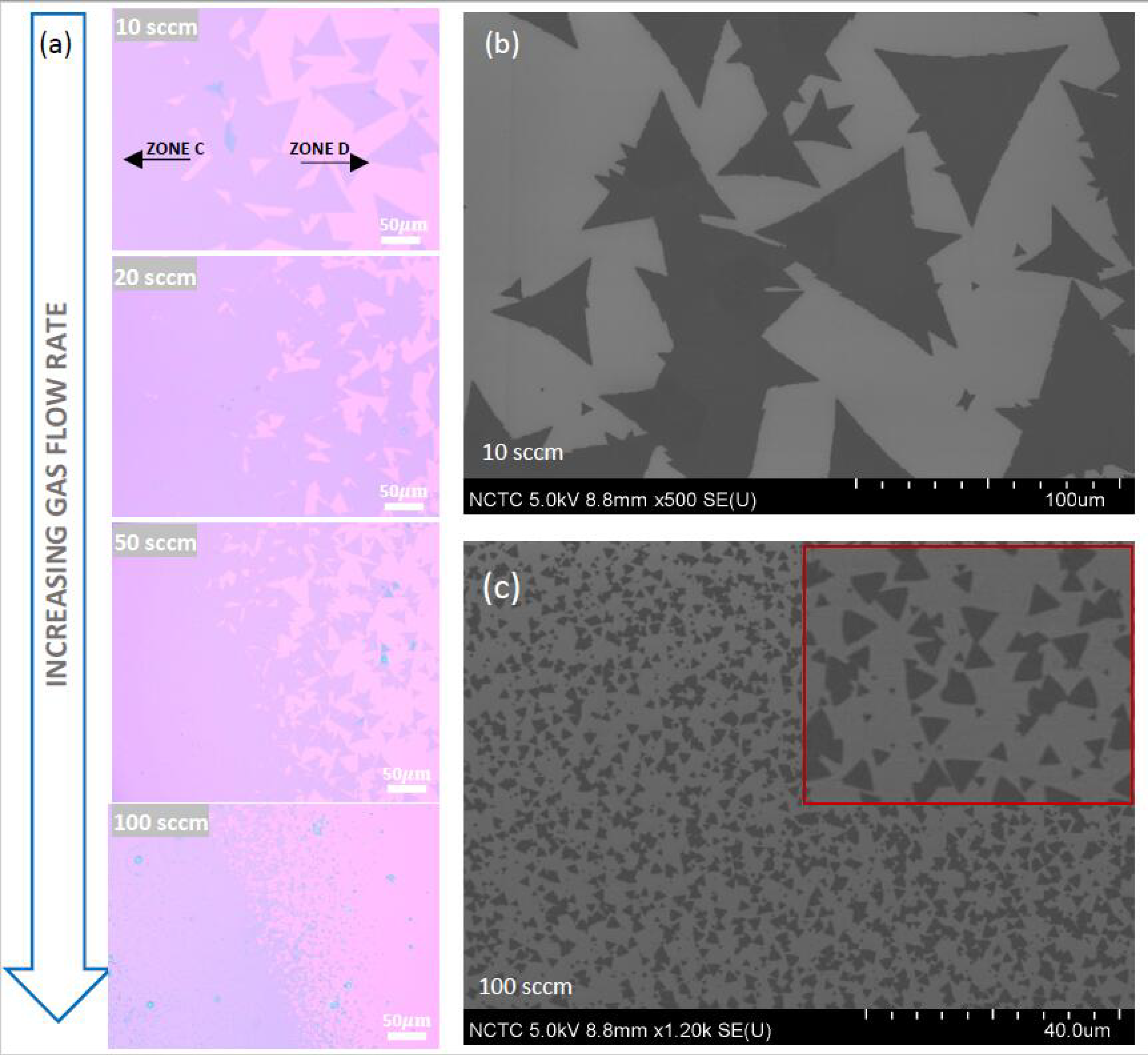
3.3. Effect of Gas-Flow Rate on MoS2 Morphology
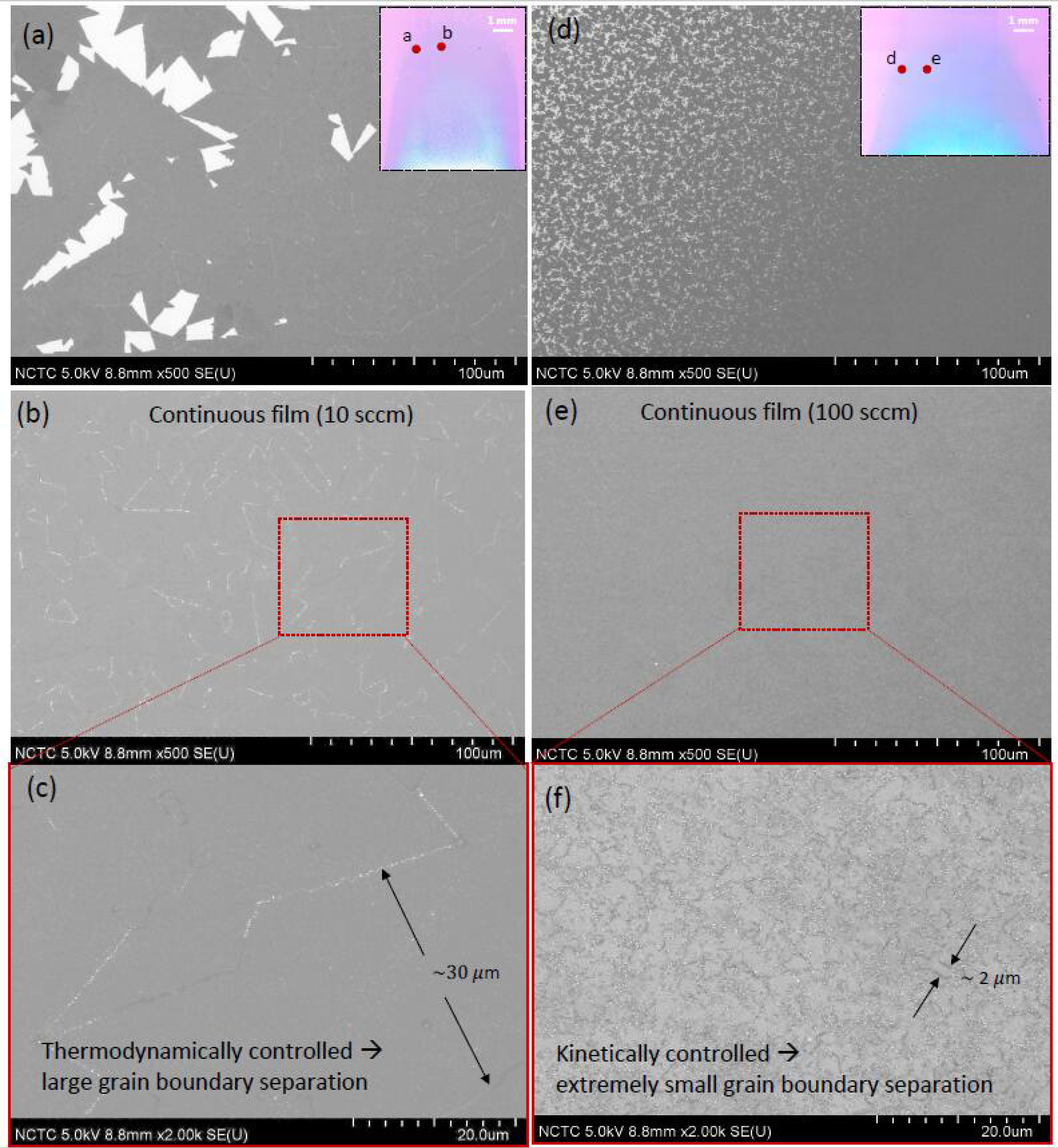
3.4. Effect of Gas-Flow Rate on Domain Size and Grain-Boundary Separation of Continuous MoS2
3.5. Influence of Critical Growth Parameters on MoS2 Domain Shape and Size
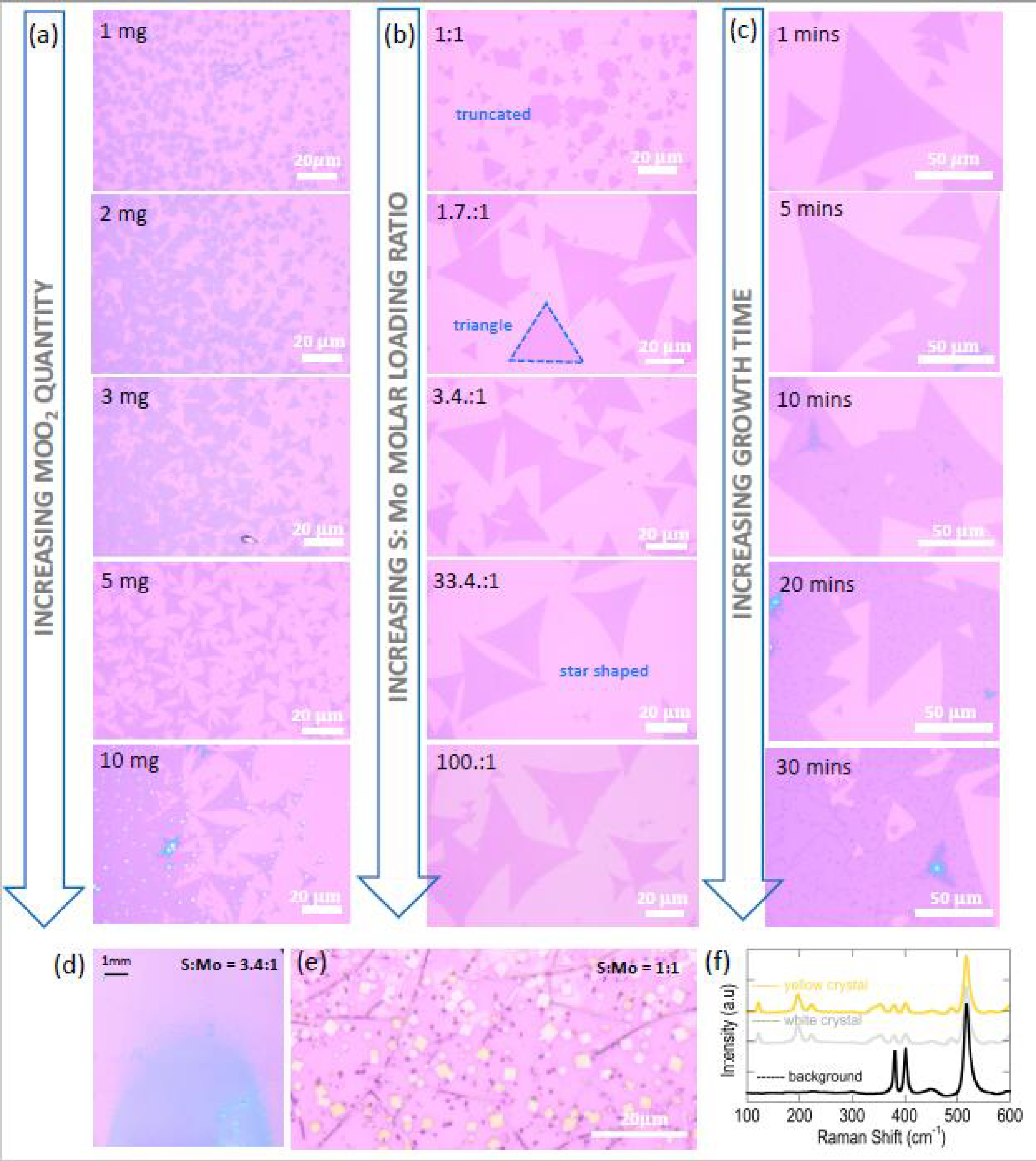
3.5.1. Domain-Size Dependency on MoO2 Precursor Amount
3.5.2. Domain Shape Dependency on S:Mo Molar Loading Ratio and Identifying the Regime of Intermediate-State-Free Growth of MoS2
3.5.3. Influence of CVD Growth Time on Nanoparticle Formations
3.6. Effect of Precursor Spatial Distribution on Wafer Coverage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Li, H.; Li, H.; Jiang, L.; Shi, Y.; Sun, Y.; Lu, G.; Zhang, Q.; Chen, X.; Zhang, H. Single-layer MoS2 phototransistors. ACS Nano 2012, 6, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Late, D.J.; Huang, Y.K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing behavior of atomically thin-layered MoS2 transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef]
- Mak, K.F.; He, K.; Lee, C.; Lee, G.H.; Hone, J.; Heinz, T.F.; Shan, J. Tightly bound trions in monolayer MoS2. Nat. Mater. 2013, 12, 207–211. [Google Scholar] [CrossRef]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.M.; Jernigan, G.G.; Jonker, B.T. Chemical vapor sensing with monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef]
- Salehzadeh, O.; Tran, N.H.; Liu, X.; Shih, I.; Mi, Z. Exciton kinetics, quantum efficiency, and efficiency droop of monolayer MoS2 light-emitting devices. Nano Lett. 2014, 14, 4125. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef]
- Xue, F.; Chen, L.; Wang, L.; Pang, Y.; Chen, J.; Zhang, C.; Wang, Z.L. MoS2 tribotronic transistor for smart tactile switch. Adv. Funct. Mater. 2016, 26, 2104–2109. [Google Scholar] [CrossRef]
- He, G.; Ghosh, K.; Singisetti, U.; Ramamoorthy, H.; Somphonsane, R.; Bohra, G.; Matsunaga, M.; Higuchi, A.; Aoki, N.; Najmaei, S.; et al. Conduction mechanisms in CVD-Grown monolayer MoS2 transistors: From variable-range hopping to velocity saturation. Nano Lett. 2015, 15, 5052–5058. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Park, Y.J.; Chen, X.; Park, Y.K.; Kim, M.S.; Ahn, J.H. MoS2-Based tactile sensor for electronic skin applications. Adv. Mater. 2016, 28, 2556–2562. [Google Scholar] [CrossRef]
- Nikam, R.D.; Sonawane, P.A.; Sankar, R.; Chen, Y.T. Epitaxial growth of vertically stacked p-MoS2/n-MoS2 heterostructures by chemical vapor deposition for light emitting devices. Nano Energy 2017, 32, 454–462. [Google Scholar] [CrossRef]
- Singh, E.; Singh, P.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Flexible Molybdenum Disulfide (MoS2) Atomic Layers for Wearable Electronics and Optoelectronics. ACS Appl. Mater. Interfaces 2019, 11, 11061–11105. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 2012, 8, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Bilgin, I.; Liu, F.; Vargas, A.; Winchester, A.; Man, M.K.L.; Upmanyu, M.; Dani, K.M.; Gupta, G.; Talapatra, S.; Mohite, A.D.; et al. Chemical vapor deposition synthesized atomically thin molybdenum disulfide with optoelectronic-grade crystalline quality. ACS Nano 2015, 9, 8822–8832. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Wang, Z.; Zhan, Y.; Zhang, P.; Wu, R.; Jiang, T.; Wu, S.; Wang, H.; Zhao, Y.; Nan, T.; et al. Controllable growth of monolayer MoS2 by chemical vapor deposition via close MoO2 precursor for electrical and optical applications. Nanotechnology 2017, 28, 2–3. [Google Scholar] [CrossRef]
- Zhu, D.; Shu, H.; Jiang, F.; Lv, D.; Asokan, V.; Omar, O.; Yuan, J.; Zhang, Z.; Jin, C. Capture the growth kinetics of CVD growth of two-dimensional MoS2. npj 2D Mater. Appl. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Verhagen, T.; Rodriguez, A.; Vondráček, M.; Honolka, J.; Funke, S.; Zlámalová, M.; Kavan, L.; Kalbac, M.; Vejpravova, J.; Frank, O. Chemical vapor deposition of MoS2 for energy harvesting: Evolution of the interfacial oxide layer. ACS Appl. Nano Mater. 2020, 3, 6563–6573. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Zhang, X.-Q.; Zhang, W.; Chang, M.-T.; Lin, C.-T.; Chang, K.-D.; Yu, Y.-C.; Wang, J.T.-W.; Chang, C.-S.; Li, L.-J.; et al. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [Green Version]
- Van Der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najmaei, S.; Liu, Z.; Zhou, W.; Zou, X.; Shi, G.; Lei, S.; Yakobson, B.I.; Idrobo, J.C.; Ajayan, P.M.; Lou, J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rong, Y.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape evolution of monolayer MoS2 crystals grown by chemical vapor deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Tam, L.C.; Kim, Y.S.; Sim, Y.; Seong, M.J.; Jang, J.I. Direct vapor phase growth process and robust photoluminescence properties of large area MoS2 layers. Nano Res. 2014, 7, 1759–1768. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Zhou, C.; Zhong, R.; Wang, X.; Tsang, Y.H.; Chai, Y. Controllable growth of large-size crystalline MoS2 and resist-free transfer assisted with a cu thin film. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ganorkar, S.; Kim, J.; Kim, Y.H.; Kim, S.-I. Effect of precursor on growth and morphology of MoS2 monolayer and multilayer. J. Phys. Chem. Solids 2015, 87, 32–37. [Google Scholar] [CrossRef]
- Wang, S.; Pacios, M.; Bhaskaran, H.; Warner, J.H. Substrate control for large area continuous films of monolayer MoS2 by atmospheric pressure chemical vapor deposition. Nanotechnology 2016, 27, 085604. [Google Scholar] [CrossRef]
- Kataria, S.; Wagner, S.; Cusati, T.; Fortunelli, A.; Iannaccone, G.; Pandey, H.; Fiori, G.; Lemme, M.C. Growth-induced strain in chemical vapor deposited monolayer MoS2: Experimental and theoretical investigation. Adv. Mater. Interfaces 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Xie, S.; Huang, Z.; Fu, W.; Cai, Y.; Yang, X.; Chen, H.; Ma, X.; Iwai, H.; Fujita, D.; et al. Elucidation of zero-dimensional to two-dimensional growth transition in MoS2 chemical vapor deposition synthesis. Adv. Mater. Interfaces 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Yang, S.Y.; Shim, G.W.; Seo, S.B.; Choi, S.Y. Effective shape-controlled growth of monolayer MoS2 flakes by powder-based chemical vapor deposition. Nano Res. 2017, 10, 255–262. [Google Scholar] [CrossRef]
- Ozden, A.; Ay, F.; Sevik, C.; Perkgöz, N.K. CVD growth of monolayer MoS2: Role of growth zone configuration and precursors ratio. Jpn. J. Appl. Phys. 2017, 56, 06GG05. [Google Scholar] [CrossRef]
- Kumar, N.; Tomar, R.; Wadehra, N.; Devi, M.M.; Prakash, B.; Chakraverty, S. Growth of highly crystalline and large scale monolayer MoS2 by CVD: The role of substrate position. Cryst. Res. Technol. 2018, 53, 1–7. [Google Scholar] [CrossRef]
- Han, T.; Liu, H.; Wang, S.; Li, W.; Chen, S.; Yang, X.; Cai, M. Research on the factors affecting the growth of large-size monolayer MoS2 by APCVD. Materials 2018, 11, 2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, C.M.; Choi, J.H.; Lee, S.W.; Park, J.H.; Lee, K.T.; Ahn, J.H. Synthesis mechanism of MoS2 layered crystals by chemical vapor deposition using MoO3 and sulfur powders. J. Alloys Compd. 2018, 765, 380–384. [Google Scholar] [CrossRef]
- Pondick, J.V.; Woods, J.M.; Xing, J.; Zhou, Y.; Cha, J.J. Stepwise sulfurization from MoO3 to MoS2 via chemical vapor deposition. ACS Appl. Nano Mater. 2018, 1, 5655–5661. [Google Scholar] [CrossRef]
- Zhou, D.; Shu, H.; Hu, C.; Jiang, L.; Liang, P.; Chen, X. Unveiling the growth mechanism of MoS2 with chemical vapor deposition: From two-dimensional planar nucleation to self-seeding nucleation. Cryst. Growth Des. 2018, 18, 1012–1019. [Google Scholar] [CrossRef]
- Şar, H.; Özden, A.; Demiroğlu, İ.; Sevik, C.; Perkgoz, N.K.; Ay, F. Long-term stability control of CVD-grown monolayer MoS2. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Chen, J.Y.; Liu, L.; Li, C.X.; Xu, J.P. Chemical Vapor Deposition Growth of Large-Area Monolayer MoS2 and Fabrication of Relevant Back-Gated Transistor. Chin. Phys. Lett. 2019, 36, 037301. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Lu, J.; Geng, X.; Wan, Y.; Wu, M.; Yang, P. Substrate effects on the CVD growth of MoS2 and WS2. J. Mater. Sci. 2020, 55, 990–996. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Zhang, W.; Huang, J.-K.; Liu, K.-K.; Lee, Y.-H.; Liang, C.-T.; Chu, C.-W.; Li, L.-J. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 2012, 4, 6637–6641. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Wang, S.; Chu, L.; Toh, M.; Kumar, R.; Zhao, W.; Castro Neto, A.H.; Martin, J.; Adam, S.; Özyilmaz, B.; et al. Transport properties of monolayer MoS2 grown by chemical vapor deposition. Nano Lett. 2014, 14, 1909–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzari, N.; Sanchez, O.L.; Kung, Y.; Krasnozhon, D.; Chen, M.; Bertolazzi, S.; Gillet, P.; Fontcuberta, A.; Radenovic, A.; Kis, A. large-area epitaxial monolayer MoS2. ACS Nano 2015, 4, 4611–4620. [Google Scholar]
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.Y.; Mak, K.F.; Kim, C.J.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Wu, K.L.; Yip, P.S.; Wang, X.; Chai, Y.; Lau, K.M. Selectable synthesis of 2-D MoS2 and its electronic devices: From isolated triangular islands to large-area continuous thin film. IEEE Trans. Nanotechnol. 2016, 15, 310–317. [Google Scholar] [CrossRef]
- Qian, S.; Yang, R.; Lan, F.; Xu, Y.; Sun, K.; Zhang, S.; Zhang, Y.; Dong, Z. Growth of continuous MoS2 film with large grain size by chemical vapor deposition. Mater. Sci. Semicond. Process. 2019, 93, 317–323. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Tang, J.; Zhu, J.; Zhang, Q.; Jia, Q.; Lu, Y.; Wei, Z.; Yu, H.; Zhao, Y.; et al. Wafer-Scale Highly Oriented Monolayer MoS2 with Large Domain Sizes. Nano Lett. 2020, 20, 7193–7199. [Google Scholar] [CrossRef]
- Durairaj, S.; Krishnamoorthy, P.; Raveendran, N.; Ryu, B.D.; Hong, C.H.; Seo, T.H.; Chandramohan, S. Barrier-assisted vapor phase CVD of large-area MoS2 monolayers with high spatial homogeneity. Nanoscale Adv. 2020, 2, 4106–4116. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, C.; He, Y.; Wei, H.; Liu, S.; Qiu, P.; Song, Y.; Wei, S.; Wang, Z.; Zheng, X.; et al. Reaction mechanism transformation of LPCVD-grown MoS2 from isolated triangular grains to continuous films. J. Alloys Compd. 2021, 853, 157374. [Google Scholar] [CrossRef]
- Lince, J.R.; Hilton, M.R.; Bommannavar, A.S. Oxygen substitution in sputter-deposited MoS2 films studied by extended X-ray absorption fine structure, X-ray photoelectron spectroscopy and X-ray diffraction. Surf. Coat. Technol. 1990, 43–44, 640–651. [Google Scholar] [CrossRef]
- Li, X.L.; Li, Y.D. Formation of MoS2 inorganic fullerenes (IFs) by the reaction of MoO3 nanobelts and S. Chem. A Eur. J. 2003, 9, 2726–2731. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Muijsers, J.C.; Van Wolput, J.H.M.C.; Verhagen, C.P.J.; Niemantsverdriet, J.W. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2, as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 1996, 100, 14144–14150. [Google Scholar] [CrossRef] [Green Version]
- Vilá, R.A.; Momeni, K.; Wang, Q.; Bersch, B.M.; Lu, N.; Kim, M.J.; Chen, L.Q.; Robinson, J.A. Bottom-up synthesis of vertically oriented two-dimensional materials. 2D Mater. 2016, 3, 041003. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [Green Version]
- Dobkin, D.M.; Zuraw, M.K. Principles of Chemical Vapor Deposition; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-94-017-0369-7. [Google Scholar]
- Shi, J.; Wu, D.; Zheng, X.; Xie, D.; Song, F.; Zhang, X.; Jiang, J.; Yuan, X.; Gao, Y.; Huang, H. From MoO2@MoS2 Core–Shell Nanorods to MoS2 Nanobelts. Phys. Status Solidi Basic Res. 2018, 255, 1–7. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Zhan, X.; Wang, S.; Lei, L.; Liang, Y.; Kang, H.; Zhang, Y.; Chen, Z.; Sui, Y.; et al. Aligned monolayer MoS2 ribbons growth on sapphire substrate via NaOH-assisted chemical vapor deposition. Sci. China Mater. 2020, 63, 1065–1075. [Google Scholar] [CrossRef] [Green Version]
- Stranski INand Krastanov LV1938 Akad. Wiss. Wien, Math-Naturwiss. K1IIb 146 797.
- Gao, L.; Ren, W.; Xu, H.; Jin, L.; Wang, Z.; Ma, T.; Ma, L.P.; Zhang, Z.; Fu, Q.; Peng, L.M.; et al. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 2012, 3, 699. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Louie, S.G. Electronic transport in polycrystalline graphene. Nat. Mater. 2010, 9, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.K.; Lee, H.S. Study on growth parameters for monolayer MoS2 synthesized by CVD using solution-based metal precursors. Appl. Sci. Converg. Technol. 2019, 28, 159–163. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiawchan, T.; Ramamoorthy, H.; Buapan, K.; Somphonsane, R. CVD Synthesis of Intermediate State-Free, Large-Area and Continuous MoS2 via Single-Step Vapor-Phase Sulfurization of MoO2 Precursor. Nanomaterials 2021, 11, 2642. https://doi.org/10.3390/nano11102642
Chiawchan T, Ramamoorthy H, Buapan K, Somphonsane R. CVD Synthesis of Intermediate State-Free, Large-Area and Continuous MoS2 via Single-Step Vapor-Phase Sulfurization of MoO2 Precursor. Nanomaterials. 2021; 11(10):2642. https://doi.org/10.3390/nano11102642
Chicago/Turabian StyleChiawchan, Tinna, Harihara Ramamoorthy, Kanokwan Buapan, and Ratchanok Somphonsane. 2021. "CVD Synthesis of Intermediate State-Free, Large-Area and Continuous MoS2 via Single-Step Vapor-Phase Sulfurization of MoO2 Precursor" Nanomaterials 11, no. 10: 2642. https://doi.org/10.3390/nano11102642
APA StyleChiawchan, T., Ramamoorthy, H., Buapan, K., & Somphonsane, R. (2021). CVD Synthesis of Intermediate State-Free, Large-Area and Continuous MoS2 via Single-Step Vapor-Phase Sulfurization of MoO2 Precursor. Nanomaterials, 11(10), 2642. https://doi.org/10.3390/nano11102642





