Fluorescence Based Comparative Sensing Behavior of the Nano-Composites of SiO2 and TiO2 towards Toxic Hg2+ Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 4-((4-Oxo-4H-chromen-3-yl)methyleneamino)benzenesulfonamide (3)
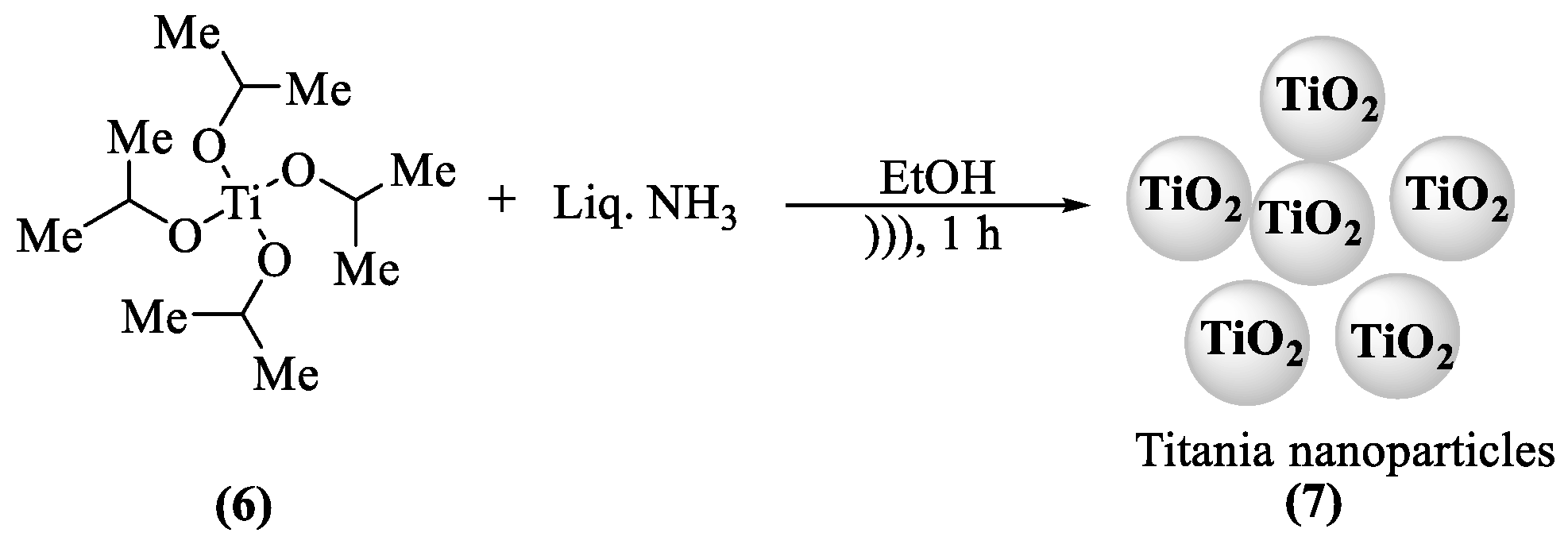
2.2. Synthesis of Nano-Composites of SiO2 (7) and TiO2 (9)
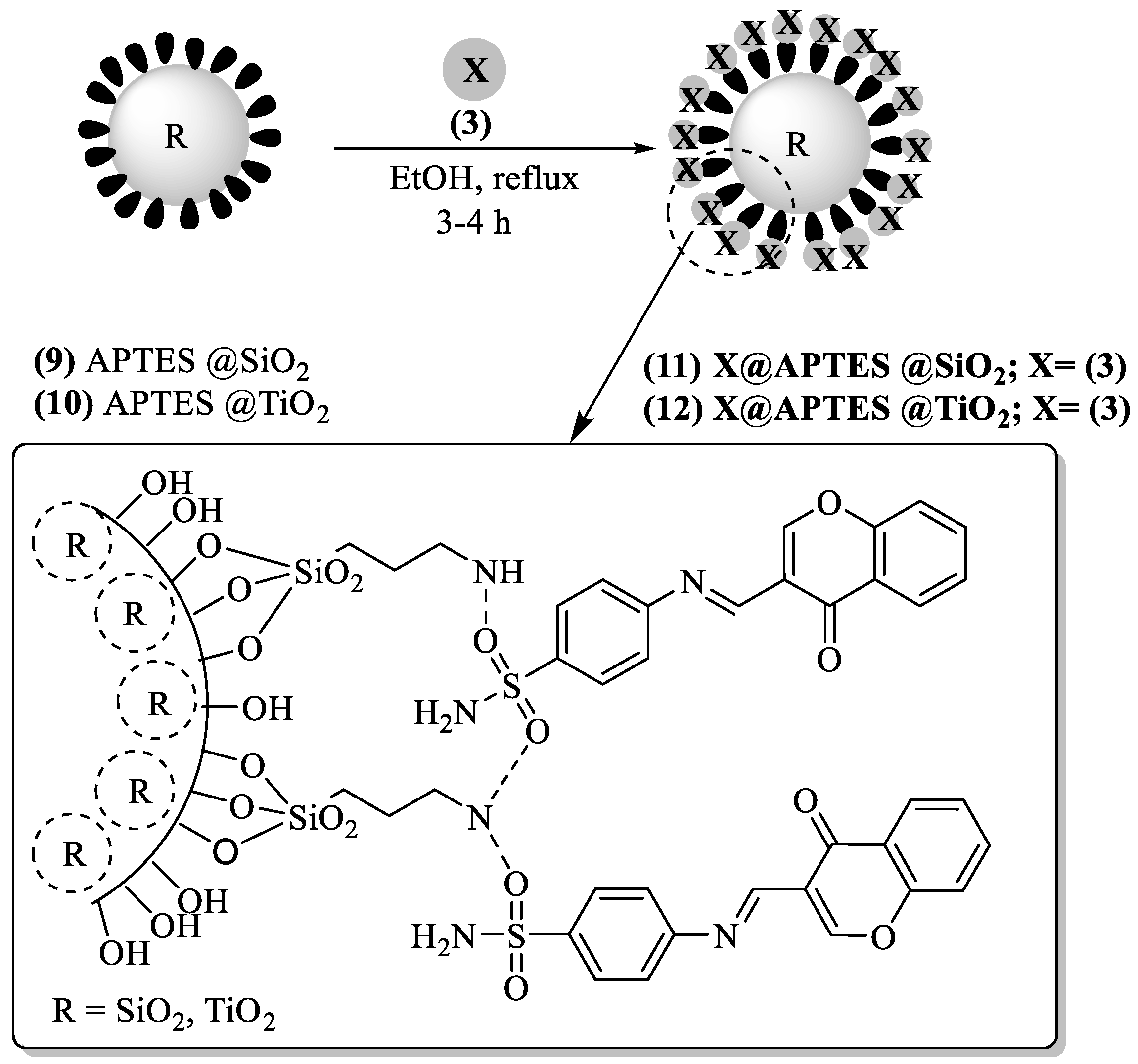
2.3. Functionalization of SiO2 and TiO2 with 3-Aminopropyl Triethoxysilane (APTES)
2.4. Synthesis of the Organic-Inorganic Nano-Composites (11, 12) of Chemosensor (3)
3. Results and Discussion
3.1. Chemoreceptor Spectral Studies
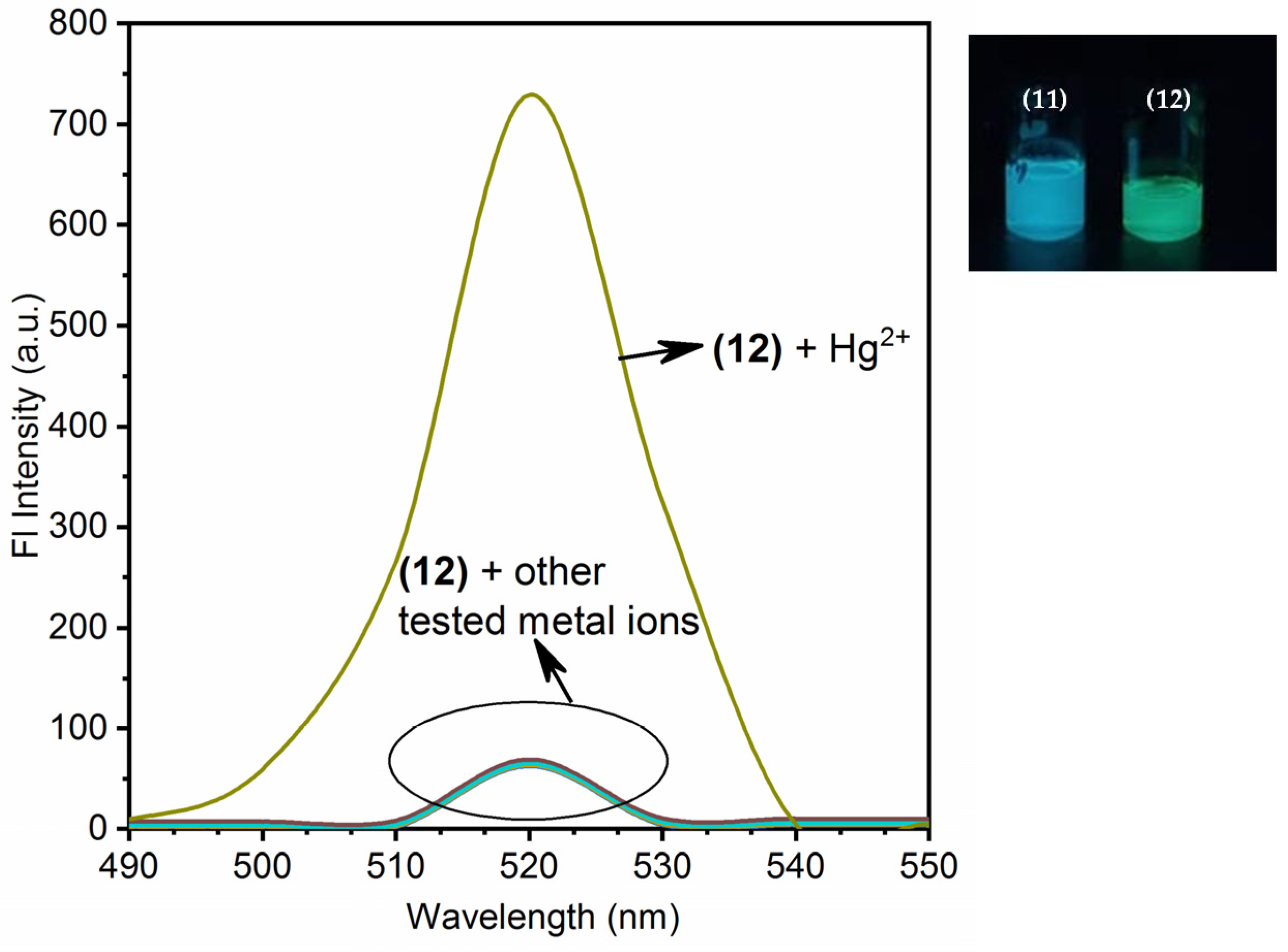
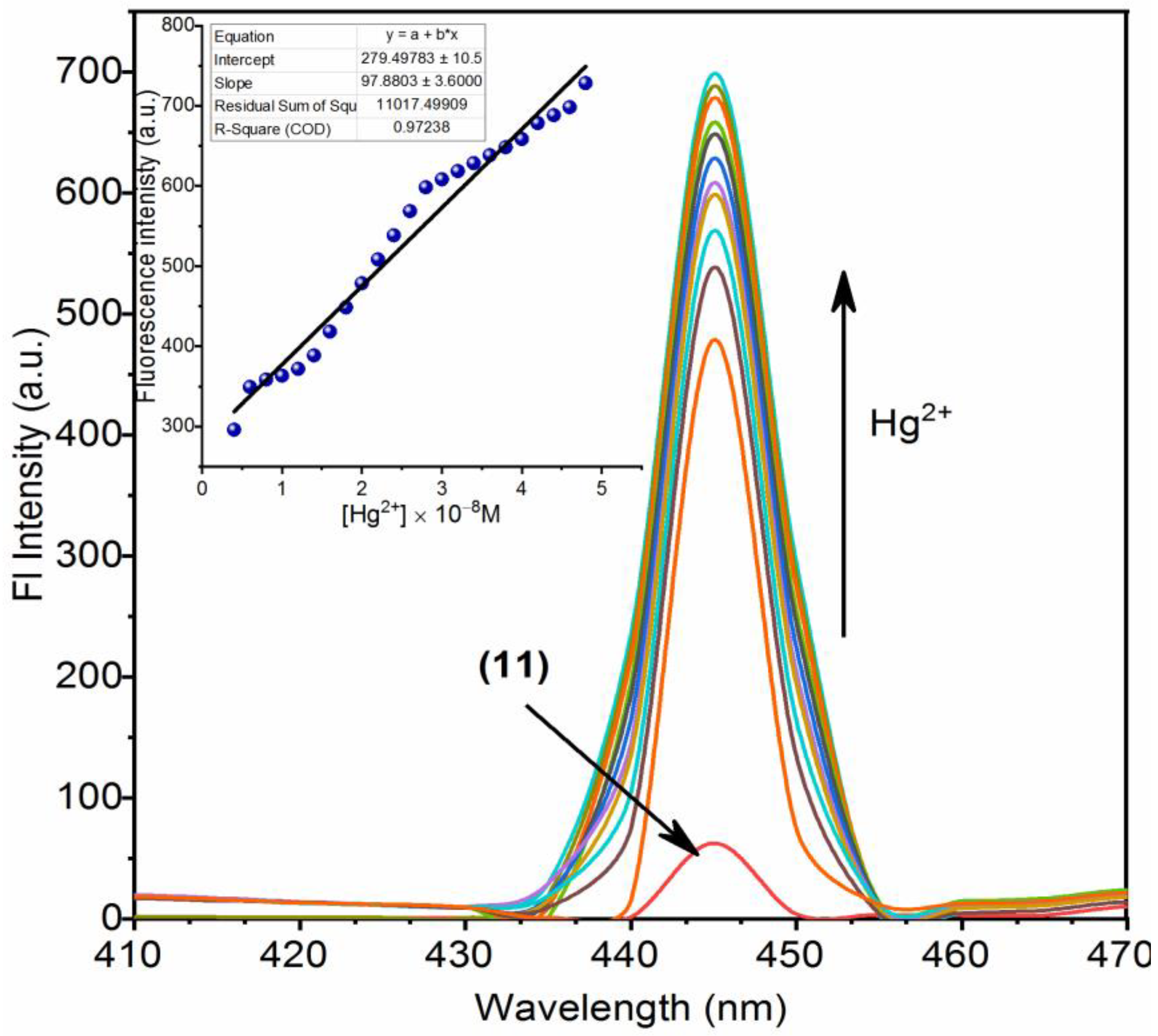
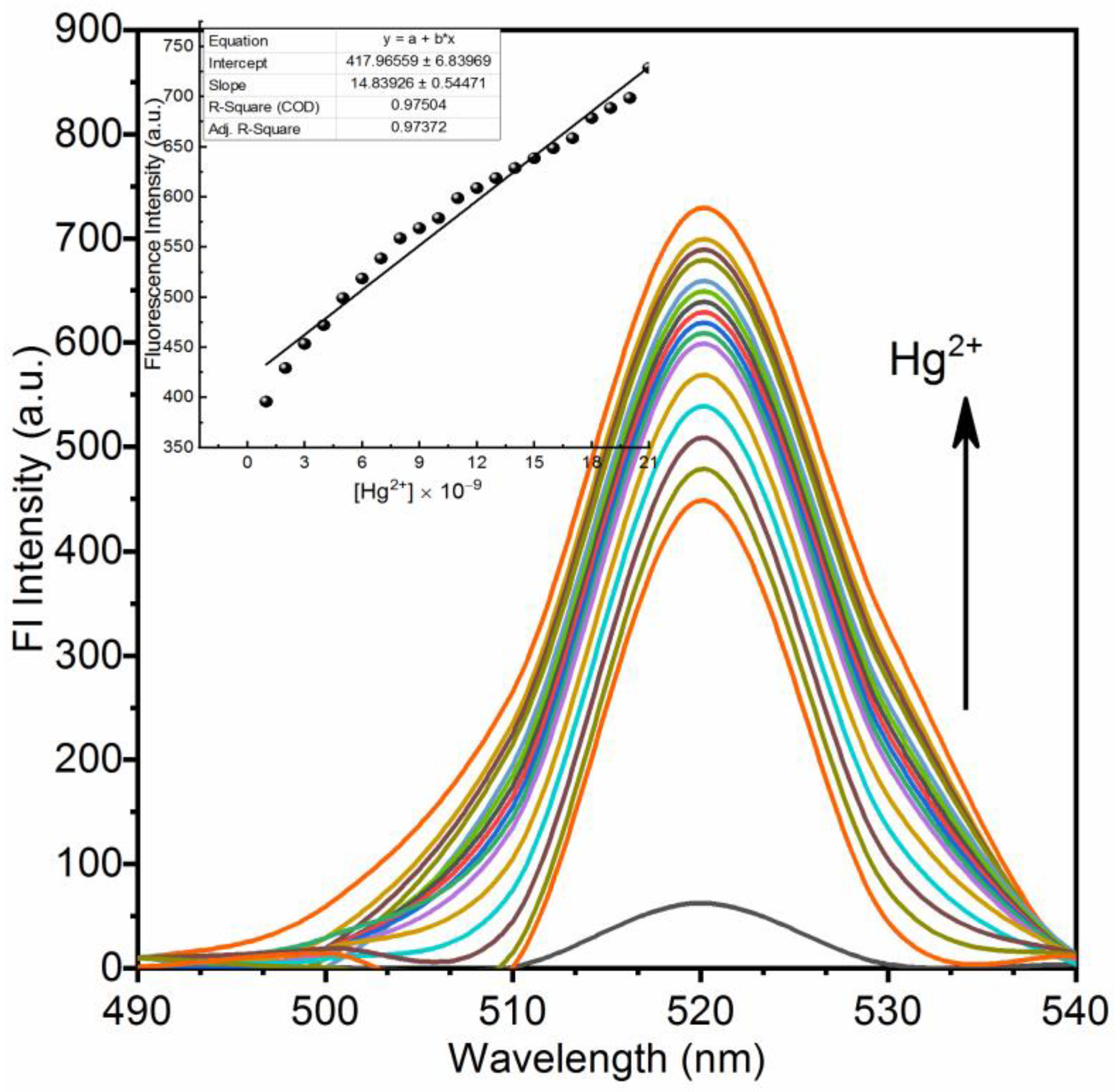
3.2. Chemistry of Nano-Composites (11) and (12) and Their Turn-On Emission Due to Hg2+ Ions
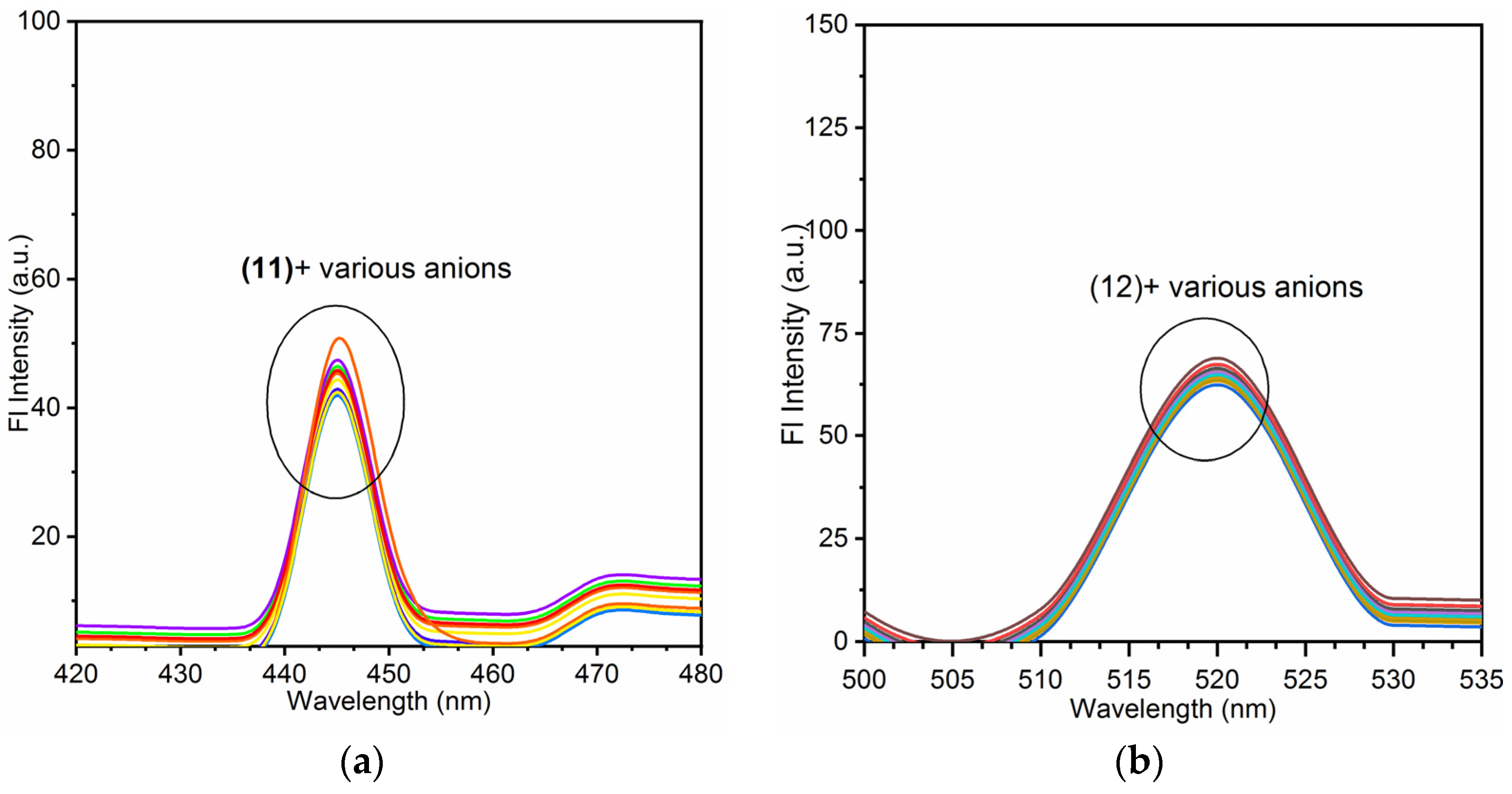
3.3. Anion Sensing Analysis of Nano-Composites (11) and (12)
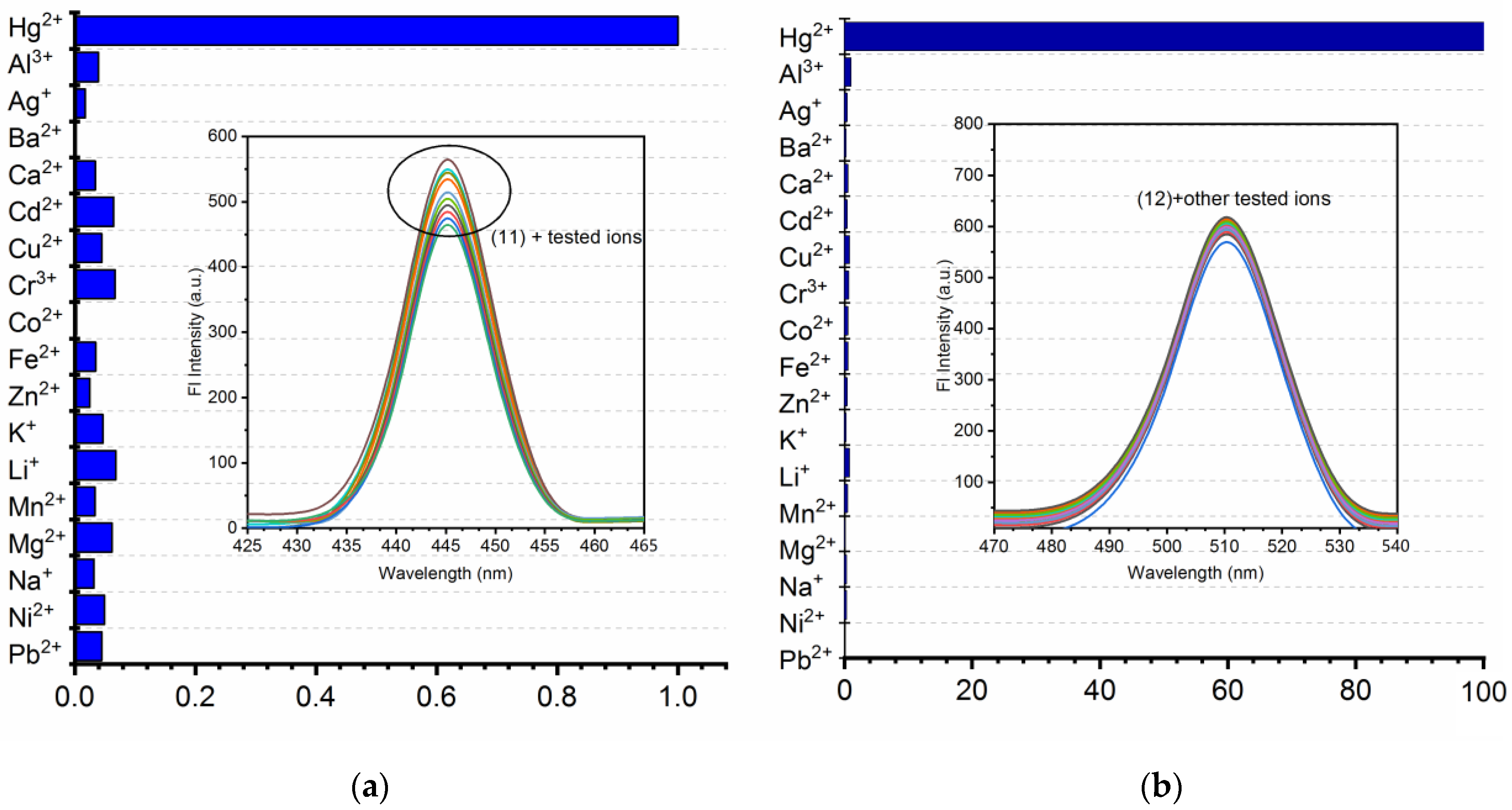
3.4. Competitive Binding Analysis (Interference Analysis)
3.5. Plausible Mechanism of Sensing of (11) and (12)
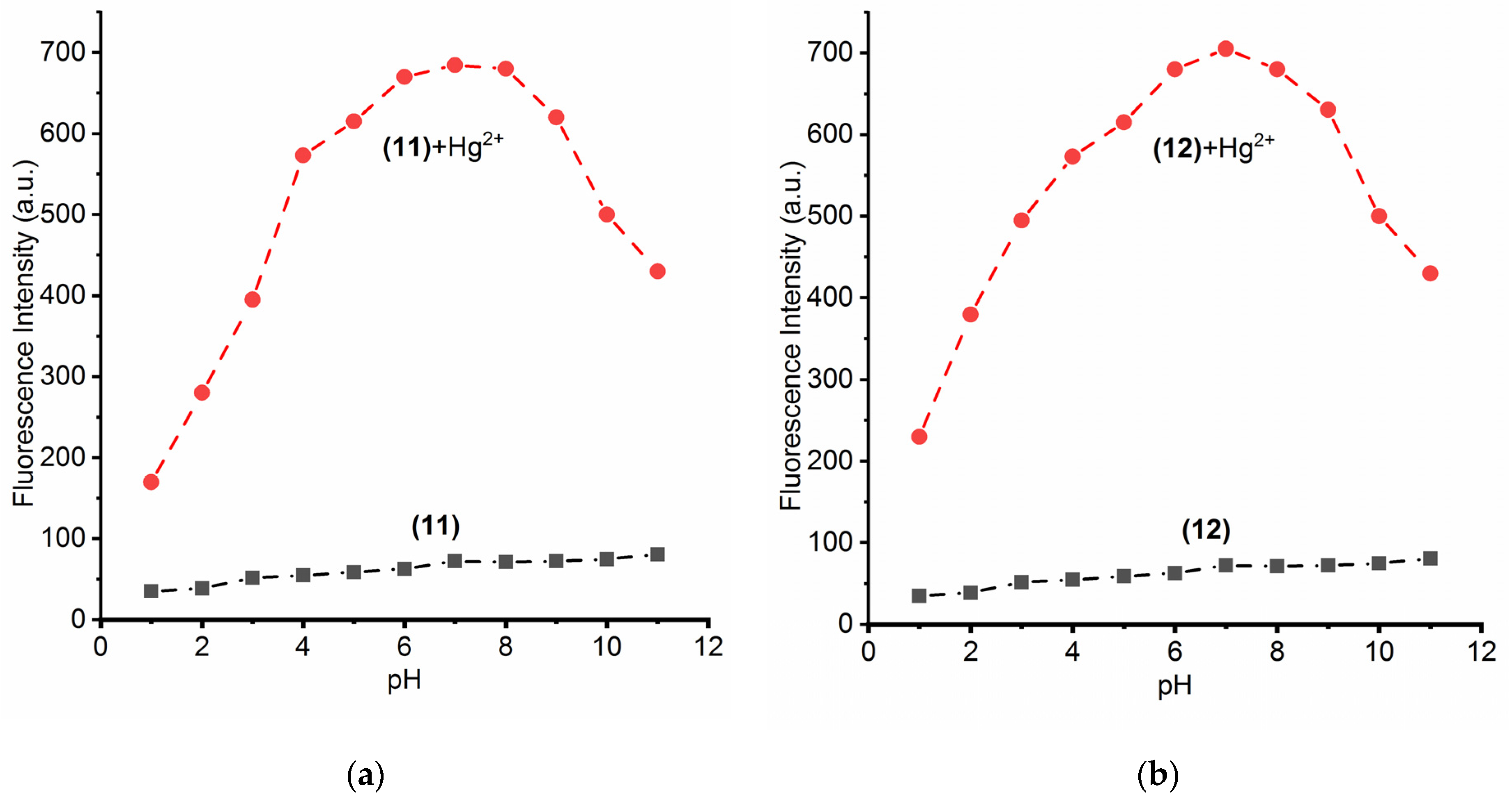
3.6. Effect of pH on Nano-Composites (11) and (12) with Hg2+ Ions
3.7. Characterization of Nano-Composites (11) and (12)
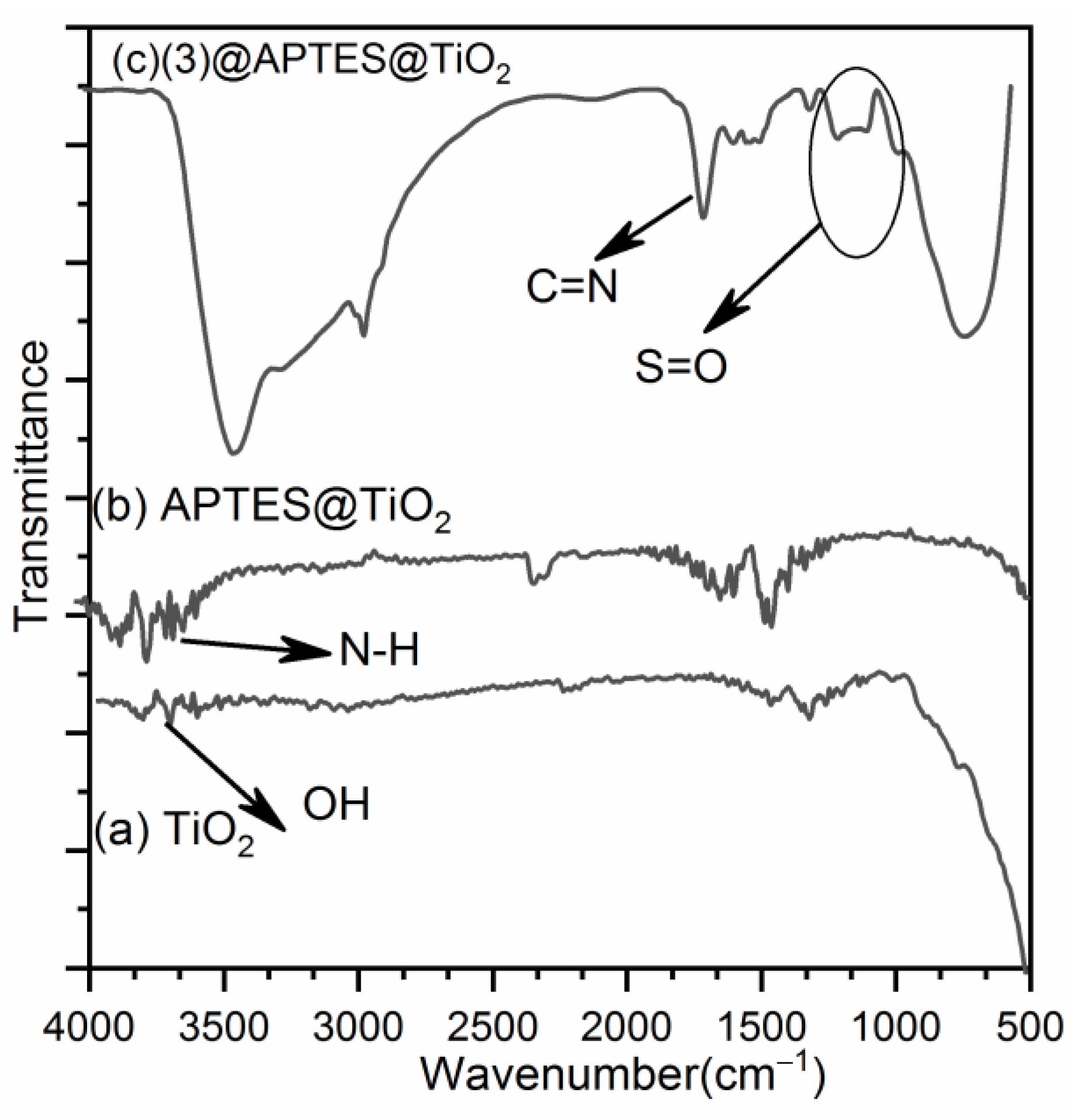
3.7.1. FT-IR Studies
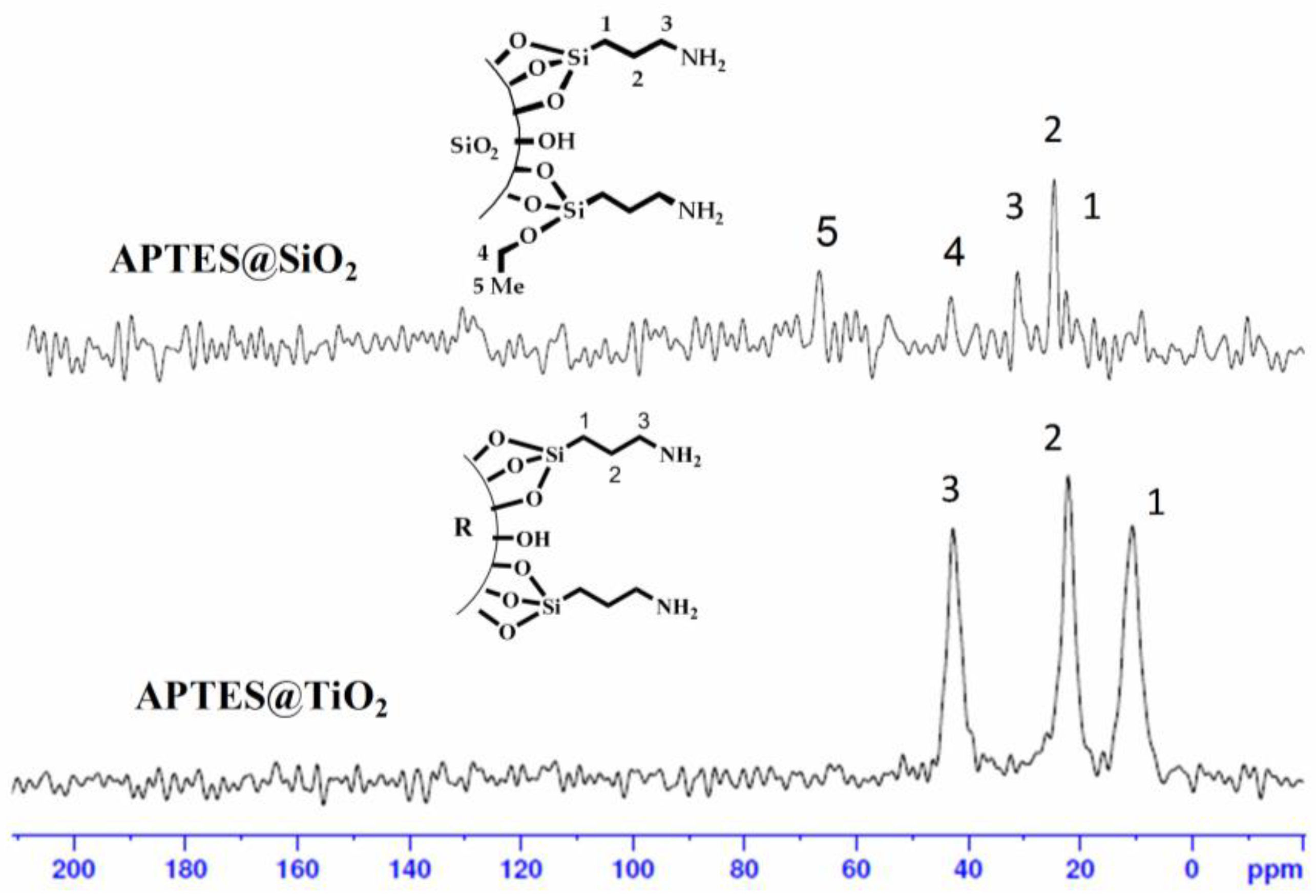
3.7.2. Solid-State 13C CPMAS and 29Si CPMAS NMR Spectroscopy
3.7.3. Elemental (C, H, N) and Surface Area Analysis (BET Studies)
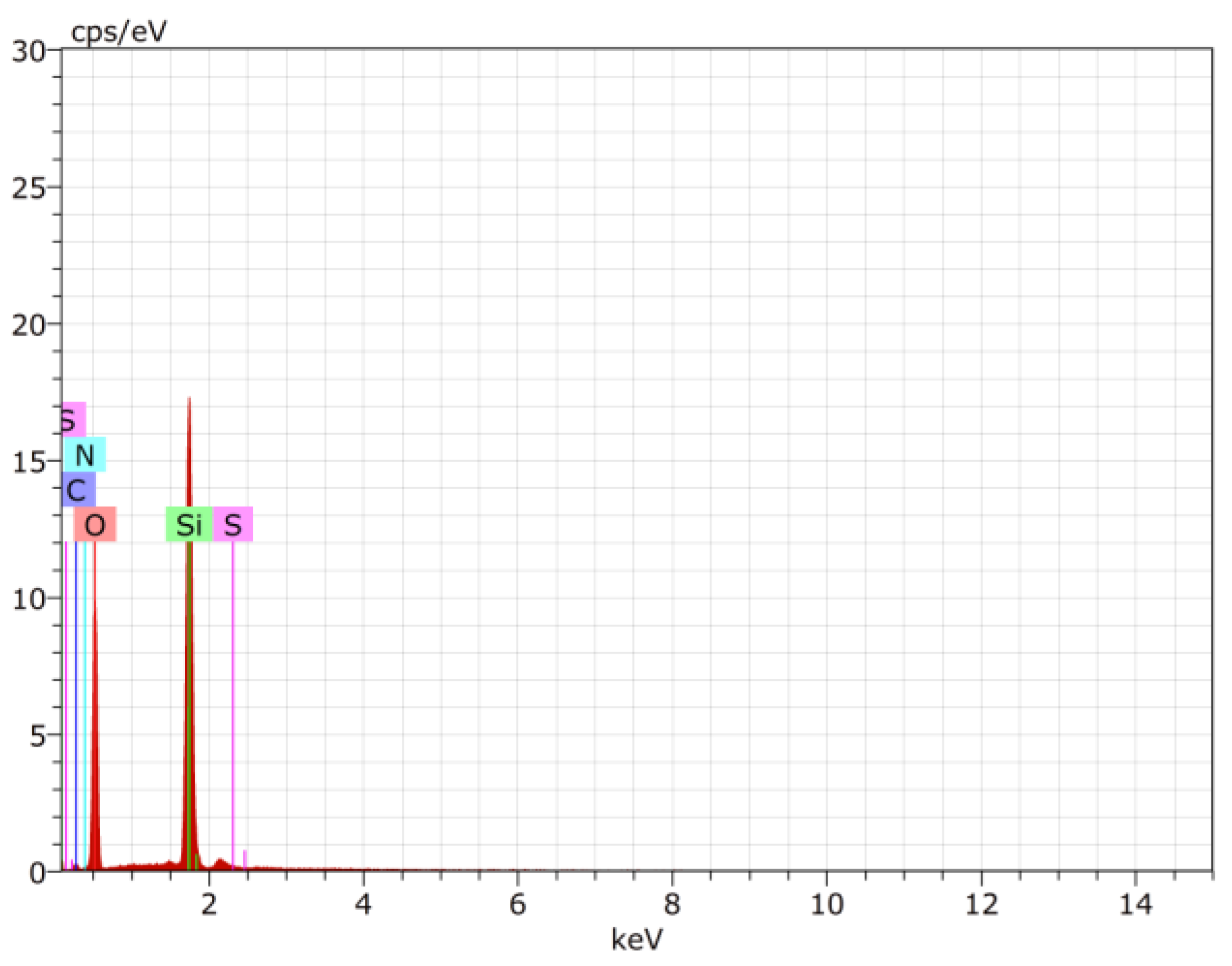
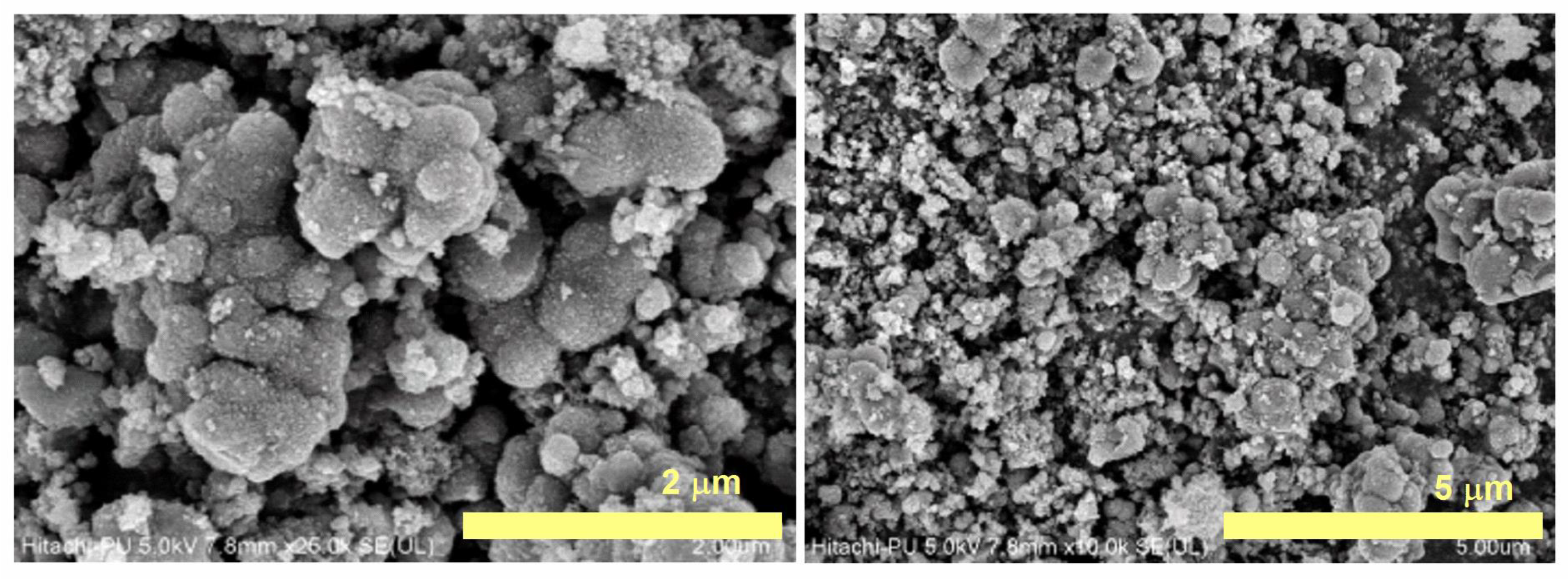
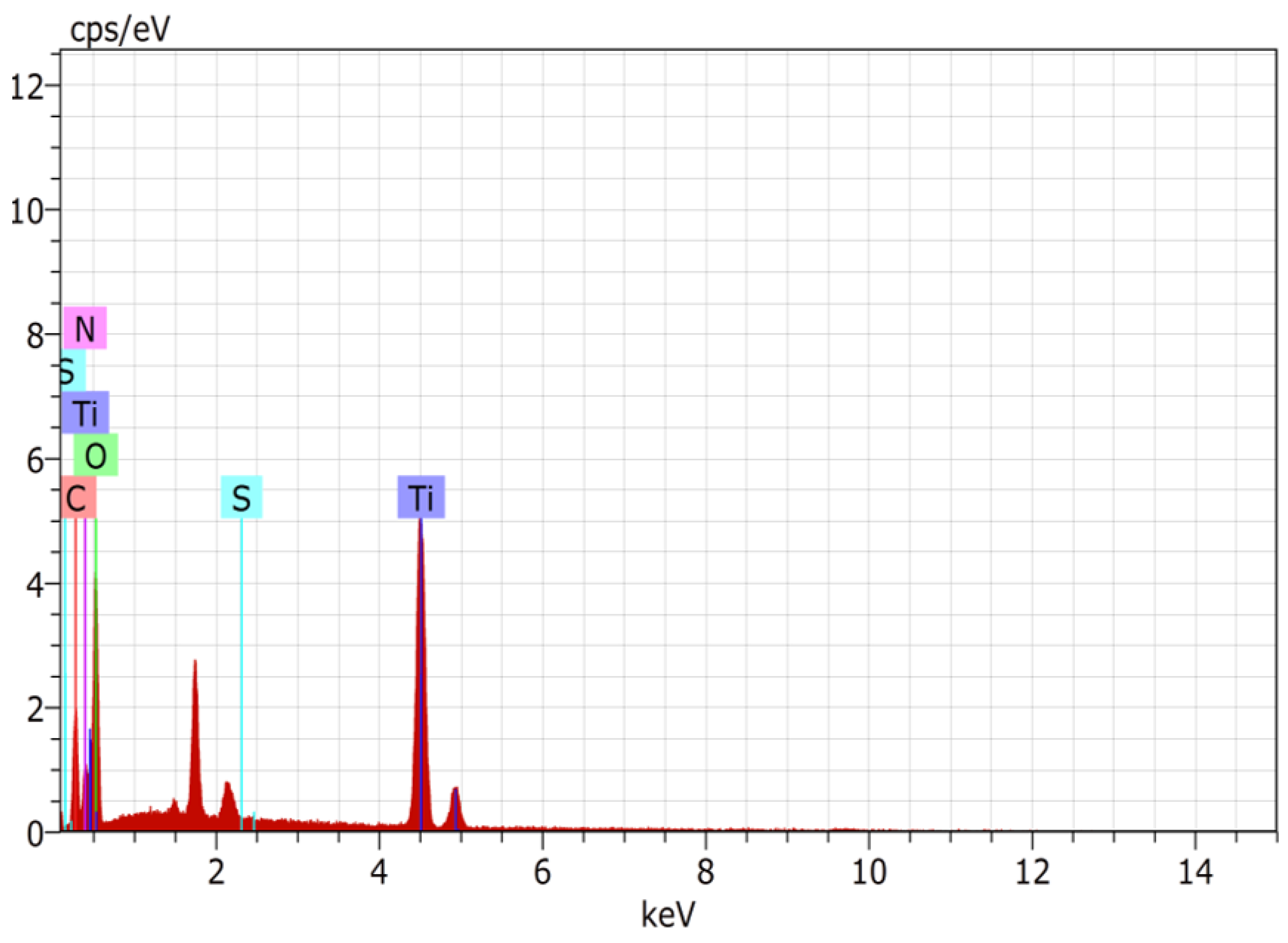
3.7.4. Field Emission Stimulated Electron Emission (FE SEM) and Energy Dispersive X-ray Analysis (EDX)
3.7.5. Transmission Electron Microscopy (TEM) Analysis
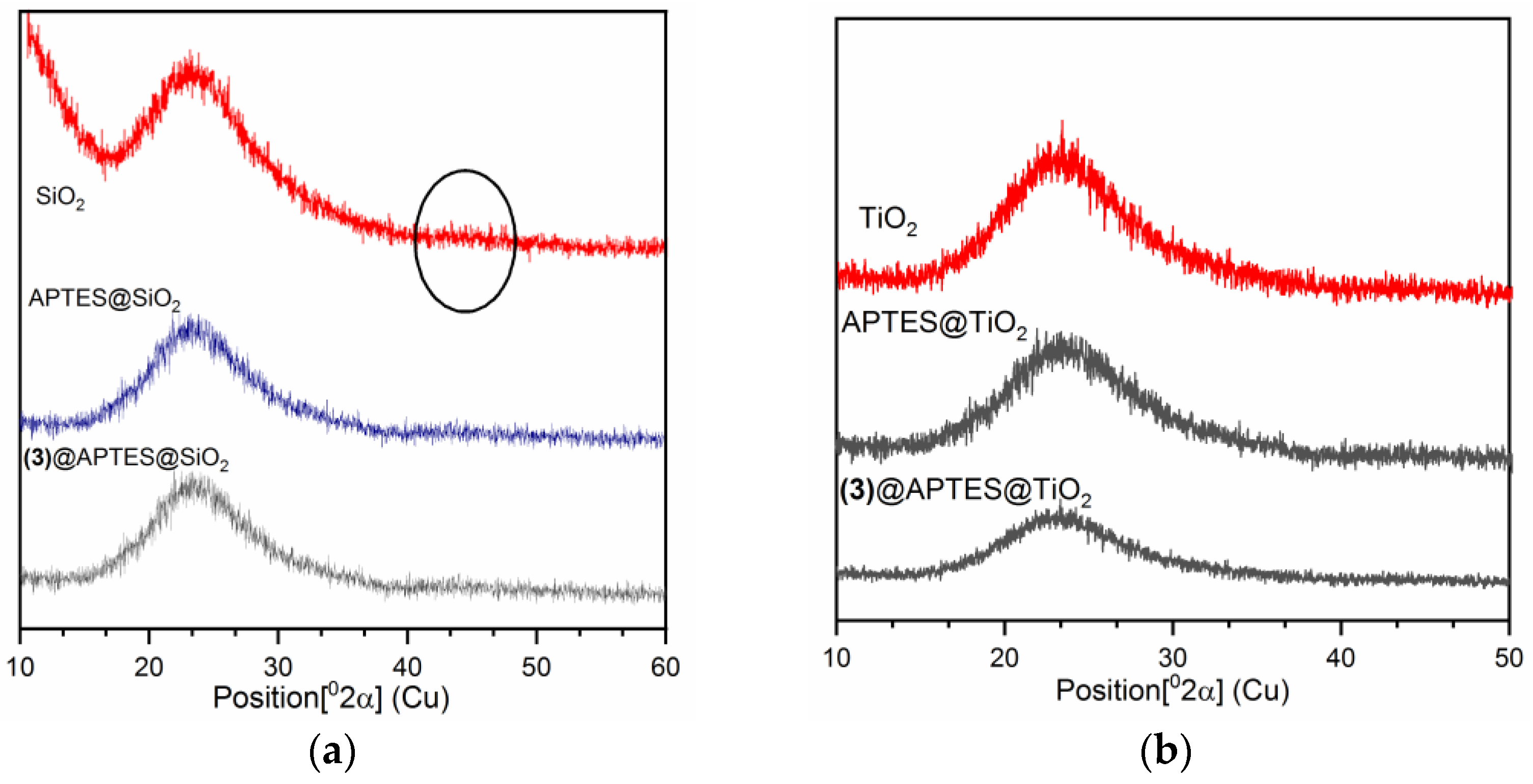
3.7.6. X-ray Diffraction (XRD) Studies
3.8. Application on Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Feng, X.; Anderson, C.W.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metals toxicity and the environment. NIH Public Access 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Risher, J.F.; Nickle, R.A.; Amler, S.N. Elemental mercury poisoning in occupational and residential settings. Int. J. Hyg. Environ. Health 2003, 206, 371–379. [Google Scholar] [CrossRef]
- Hamdy, M.K.; Noyes, O.R. Formation of methyl mercury by bacteria. Appl. Microbiol. 1975, 30, 424–432. [Google Scholar] [CrossRef]
- Lee, C.S.; Fisher, N.S. Microbial generation of elemental mercury from dissolved methylmercury in seawater. Limnol. Oceanogr. 2019, 64, 679–693. [Google Scholar] [CrossRef]
- Regnell, O.; Watras, C.J. Microbial mercury methylation in aquatic environments: A critical review of published field and laboratory studies. Environ. Sci. Technol. 2019, 53, 4–19. [Google Scholar] [CrossRef]
- Date, S.S.; Parks, J.M.; Rush, K.W.; Wall, J.D.; Ragsdale, S.W.; Johs, A. Kinetics of enzymatic mercury methylation at nanomolar concentrations catalyzed by HgcAB. Appl. Environ. Microbiol. 2019, 85, e00438-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Lin, X.; Zhao, J.; Cui, L.; Wang, L.; Gao, Y.; Li, B.; Chen, C.; Li, Y.F. Intestinal methylation and demethylation of mercury. Bull. Environ. Contam. Toxicol. 2019, 102, 597–604. [Google Scholar] [CrossRef]
- Gardner, R.M.; Nyland, J.F.; Silbergeld, E.K. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol. Lett. 2010, 198, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Mahaffey, K.R. Methylmercury: A new look at the risks. Public Health Rep. 1999, 114, 396–399. [Google Scholar]
- Johnson, F.O.; Atchison, W.D. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 2009, 30, 761–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, B.F.; Furieri, L.B.; Pec¸anha, F.M.; Wiggers, G.A.; Vassallo, P.F.; Simoes, M.R.; Fiorim, J.; de Batista, P.R.; Fioresi, M.; Rossoni, L.; et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef]
- Houstan, C.M. Role of mercury toxicity in hypertension, cardiovascular diseases and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern. Ther. Health Med. 2007, 13, 128–133. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Mercury; US Department of Health and Human Services: Washington, DC, USA, 1999. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp46.pdf (accessed on 15 August 2021).
- García, J.D.D.; Arceo, E. Renal damage associated with heavy metals: Review work. Rev. Colomb. Nefrol. 2018, 5, 45–53. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; International Program on Chemical Safety. Inorganic Mercury: Environmental Health Criteria 118; World Health Organization: Geneva, Switzerland, 1991; Available online: https://inchem.org/documents/ehc/ehc/ehc118.htm (accessed on 15 August 2021).
- Abrham, F.; Gholap, A.V. Analysis of heavy metal concentration in some vegetables using atomic absorption spectroscopy. Pollution 2021, 7, 205–216. [Google Scholar] [CrossRef]
- Jinadasa, K.K.; Herbello-Hermelo, P.; Pena-Vấzquez, E.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Mercury speciation in edible seaweed by liquid chromatography-Inductively coupled plasma mass spectrometry after ionic imprinted polymer-solid phase extraction. Talanta 2021, 224, 121841. [Google Scholar] [CrossRef]
- Ali, R.; Ali, I.A.I.; Messaoud, S.; Alminderej, F.M.; Saleh, S.M. An effective optical chemosensor film for selective detection of mercury ions. J. Mol. Liq. 2021, 336, 116122. [Google Scholar] [CrossRef]
- Rajadurai, M.; Reddy, E.R. Tuning the sensitivity towards mercury viacooperative binding to D-fructose dual chemosensor based on 1,8-naphthyridine-boronic acid derivative. RSC Adv. 2021, 11, 14862. [Google Scholar] [CrossRef]
- Chan, C.; Liu, H.; Xue, Z. Chromogenic and fluorescent probe for detection of mercury (II) ion based on mono-pyrrolyl substituted BODIPY. Microchem. J. 2021, 166, 106247. [Google Scholar] [CrossRef]
- Hu, J.H.; Long, C.; Fu, Q.Q.; Ni, P.W.; Yin, Z.Y. A novel highly selective colorimetric and “turn-on” fluorimetricchemosensor for detecting Hg2+ based on Rhodamine B hydrazide derivatives in aqueous media. J. Photochem. Photobiol. A Chem. 2019, 379, 105–111. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallared, M.R.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementary upon studying nanoparticles. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Noah, N.M. Design and Synthesis of nanostructured materials for sensor applications. J. Nanomater. 2020, 2020, 8855321. [Google Scholar] [CrossRef]
- Zhang, B. Physical fundamentals of nanomaterials micro and nano technologies. In Optical Properties of Nanomaterials; William Andrew: Norwich, NY, USA, 2018; pp. 291–335. [Google Scholar]
- Siddique, S.; Chow, J.C.L. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.P.; Cawthorne, C.; Archibald, S.J. Multimodal nanoparticle imaging agents: Design and applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170261. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Aplications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photo thermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Prodi, L. Luminescent Chemosensors: From molecule to nanparticles. New J. Chem. 2005, 29, 20–31. [Google Scholar] [CrossRef]
- Keane, D.A.; Hanrahanb, J.P.; Copley, M.P.; Holmes, J.D.; Morris, M.A. A modified Stöber process for the production of mesoporous Sub 2 micron silica microspheres; applications in HPLC D. J. Porous Mater. 2010, 17, 145–152. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Bazarganipour, M.; Noori, E. Synthesis and characterization of spherical silica nanoparticles by modified Stöber process assisted by organic ligand. Superlattices Microstruct. 2013, 61, 33–41. [Google Scholar] [CrossRef]
- Karade, V.C.; Sharma, A.; Dhavale, R.P.; Shingte, S.R.; Patil, P.S.; Kim, J.H.; Zahn, D.R.T.; Chougale, A.D.; Salvan, G.; Patil, P.B. APTES monolayer coverage on self-assembled magnetic nanospheres for controlled release of anticancer drug Nintedanib. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Loginova, N.V.; Harbatsevich, H.I.; Osipovich, N.P.; Ksendzova, G.A.; Koval’chuk, T.V.; Polozov, G.I. Metal Complexes as Promising Agents for Biomedical Applications. Curr. Med. Chem. 2020, 27, 5213–5249. [Google Scholar] [CrossRef]
- Xue, S.; Wang, P.; Chen, K. A novel fluorescent chemosensor for detection of mercury (II) ions based on dansyl-peptide and its application in real water samples and living LNcap cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117616. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Z.; Hou, L.; Qu, Y.; Deng, Y.; Zhang, C.; Xie, C.; Wu, Z. Selective mercury (II) detection in aqueous solutions upon the absorption changes corresponding to the transition moments polarized along the short axis of an azobenzene chemosensor. Analyst 2020, 145, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.K.K.; Chauhan, P.; Chaudhary, S. A novel coumarin-tagged ditopic scaffold as a selectively sensitive fluorogenic receptor of zinc (II) ion. Sens. Actuators B Chem. 2020, 330, 129328. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sahoo, P.R.; Kumar, S. A light controlled, reversible, sensitive and highly selective colorimetric sensor for mercuric ions in water. J. Mol. Struct. 2020, 1206, 127702. [Google Scholar] [CrossRef]
- Karuppusamy, P.; Senthilvelan, J.; Vijayakumar, V.; Sarveswari, S. A Pyrazole-based highly selective colorimetric chemosensor for Hg2+ ion in semi-aqueous medium. ChemistrySelect 2020, 5, 49–53. [Google Scholar] [CrossRef]
- Tripathy, M.; Subuddhi, U.; Patel, S. An azo dye based D-π-A chromogenic probe for selective naked-eye detection of Hg2+ ion: Application in logic gate operation. ChemistrySelect 2020, 5, 4803–4815. [Google Scholar] [CrossRef]
- Kim, A.; Kim, S.; Kim, C. A Conjugated Schiff Base-Based Chemosensor for Selectively Detecting Mercury Ion. J. Chem. Sci. 2020, 132, 1–7. [Google Scholar] [CrossRef]
- Madhesan, T.; Mohan, A.M. Porous silica and polymer monolith architectures as solid-state optical chemosensors for Hg2+ ions. Anal. Bioanal. Chem. 2020, 412, 7357–7370. [Google Scholar] [CrossRef]
- Dhaka, G.; Jindal, G.; Kaur, R.; Rana, S.; Gupta, A.; Kaur, N. Multianalyte azo dye as an on-site assay kit for colorimetric detection of Hg2+ ions and electrochemical sensing of Zn2+ ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117869. [Google Scholar] [CrossRef]
- Raju, V.; Kumar, R.S.; Kumar, S.A.; Madhu, G.; Bothra, S.; Sahoo, S.K. A ninhydrin-thiosemicarbazone based highly selective and sensitive chromogenic sensor for Hg2+ and F− ions. J. Chem. Sci. 2020, 132, 1–11. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, A.; Kaur, N. A novel anthracene-based naked eye probe for detecting Hg2+ ions in aqueous as well as solid state media. Microchem. J. 2020, 153, 104508. [Google Scholar] [CrossRef]
- Singh, G.; Satija, P.; Singh, A.; Sharma, G. First report on the synthesis of antipyrine crowned siloxy framework: Optical recognition of Fe2+ and Hg2+ ions. ChemistrySelect 2020, 5, 8823–8830. [Google Scholar] [CrossRef]
- Venkatesan, V.; Kumar, R.S.; Kumar, S.K.A.; Sahoo, S.K. Dual optical properties of new Schiff base based on bisthiophene forsensing of Cu2+ in protic media. J. Mol. Strut. 2019, 1198, 126906. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L. Infrared Spectrum of Hg(OH)2 in Solid Neon and Argon. Inorg. Chem. 2005, 44, 108–113. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, S.; Gulati, S.; Pandey, A. Fabrication of a novel nano-composite carbon paste sensor based on silica-nanospheres functionalized with isatin thiosemicarbazone for potentiometric monitoring of Cu2+ ions in real samples. Anal. Methods 2013, 5, 1414. [Google Scholar] [CrossRef]
- Young, J.F.; Sun, G. 15-Silicate Polymerization Analysis. In Handbook of Analytical Techniques in Concrete Science and Technology; Ramachandran, V.S., Beaudoin, J.J., Eds.; William Andrew Publishing: Norwich, NY, USA, 2001; pp. 629–657. [Google Scholar]
- Rodrigues, J.M.M.; Farinha, A.S.F.; Lin, Z.; Cavaleiro, J.A.S.; Tome, A.C.; Tome, J.P.C. Phthalocyanine-functionalized magnetic silicananoparticles as anionchemosensors. Sensors 2021, 21, 1632. [Google Scholar] [CrossRef]









| Ligand | LOD | Limitations | Reference |
|---|---|---|---|
| Solid phase peptide | 7.59 nM | Tedious reaction procedure | [45] |
| Azobenzene | 50 µM | Tedious reaction procedure | [46] |
| Anthracen-9(-ylmethylene)hydrazine | 220 nM | pH specificity, Effective over pH 5–8 only | [47] |
| Merocyanin dye | 0.27 µM | Molecule disrupted by UV light | [48] |
| Pyrazole based derivative | 0.473 µM | Detection limited to pH 6–11 | [49] |
| 2-Hydroxy-5(-4-nitrophenyl) diazonyl benzaldehyde | 6.1 µM | Solvent specificity, ligand worked in presence of DMSO only | [50] |
| 3-(4-Dimethylamino-phenyl) allylidene-3-nitro benzohydrazide | 0.11 µM | pH specificity, effective pH > 7 only | [51] |
| 8-Hydroxy-7-(4-nbutyl phenzlazo)quinoline (Solid phase) | 0.18 µg/L | pH dependent worked between 5–7 pH only | [52] |
| 1-Benzothiazole-2-yl-3(pyridine-3-ylazo)-nathalen-2-ol | 8.5 µM | Lack of specificity, selective for AcO−, Hg2+, Cu2+, and electrochemically for Zn2+ | [53] |
| Ninhydrin-thiosemicarbazone | 1 µM | Dual sensing for Hg2+ and F− | [54] |
| 2-(Anthracen-9-ylmethylene-hydrazonomethyl)-quinolin-8-ol | 220 nM | pH dependent, worked between 6–8 pH only | [55] |
| Antipyrene Schiff base Silatranes | 100 µM | Dual selectivity for Fe3+ and Hg2+ | [56] |
| Silica nano-composite (11) | 41.2 nM | Present work | |
| Titania nano-composite (12) | 18.8 nM | Present work |
| Entry | Elemental Analysis | BET Surface Area (m2/g) | ||
|---|---|---|---|---|
| C% | H% | N% | ||
| SiO2 (5) | - | - | - | 201.81 |
| APTES@SiO2 (9) | 2.53 | 0.84 | 0.96 | 113.21 |
| (3)@APTES@SiO2 (11) | 4.52 | 0.98 | 1.32 | 77.56 |
| TiO2 (6) | - | - | - | 210.21 |
| APTES@TiO2 (10) | 5.65 | 1.29 | 1.87 | 95.35 |
| (3)@APTES@TiO2(12) | 19.24 | 1.05 | 1.42 | 60.43 |
| Nature of Sample Studied # | Amount of Hg2+ Ions Added (nM) | Amount of Hg2+ Ions Found (nM) (n = 3 *) | Recovery %Age of Hg2+ Ions | Relative Error %Age |
|---|---|---|---|---|
| Nano-composite (11) | ||||
| Tap water | 100 | 98.12 | 98.12 | 0.0010 |
| 150 | 148.50 | 99.00 | 0.0028 | |
| 200 | 198.65 | 99.325 | 0.0024 | |
| Distilled water | 100 | 99.30 | 99.30 | 0.0062 |
| 150 | 149.48 | 99.65 | 0.0103 | |
| 200 | 199.24 | 99.62 | 0.0194 | |
| Bottled water | 100 | 98.97 | 98.97 | 0.0085 |
| 150 | 147.36 | 98.24 | 0.0056 | |
| 200 | 199.34 | 99.67 | 0.0064 | |
| Nano-composite (12) | ||||
| Tap water | 100 | 99.67 | 99.67 | 0.0058 |
| 150 | 149.28 | 99.52 | 0.0056 | |
| 200 | 199.21 | 99.61 | 0.0107 | |
| Distilled water | 100 | 99.96 | 99.96 | 0.0124 |
| 150 | 149.53 | 99.69 | 0.0091 | |
| 200 | 199.93 | 99.97 | 0.0105 | |
| Bottled water | 100 | 99.67 | 99.67 | 0.0095 |
| 150 | 149.42 | 99.61 | 0.0098 | |
| 200 | 199.89 | 99.94 | 0.0091 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekta; Utreja, D. Fluorescence Based Comparative Sensing Behavior of the Nano-Composites of SiO2 and TiO2 towards Toxic Hg2+ Ions. Nanomaterials 2021, 11, 3082. https://doi.org/10.3390/nano11113082
Ekta, Utreja D. Fluorescence Based Comparative Sensing Behavior of the Nano-Composites of SiO2 and TiO2 towards Toxic Hg2+ Ions. Nanomaterials. 2021; 11(11):3082. https://doi.org/10.3390/nano11113082
Chicago/Turabian StyleEkta, and Divya Utreja. 2021. "Fluorescence Based Comparative Sensing Behavior of the Nano-Composites of SiO2 and TiO2 towards Toxic Hg2+ Ions" Nanomaterials 11, no. 11: 3082. https://doi.org/10.3390/nano11113082






