Precise Control of Green to Blue Emission of Halide Perovskite Nanocrystals Using Terbium Chloride as Chlorine Source
Abstract
:1. Introduction
2. Experiments
2.1. Experimental Materials
2.2. Synthesis of CsPbClxBr3-x Nanocrystalline
2.3. Purifications
2.4. Characterizations
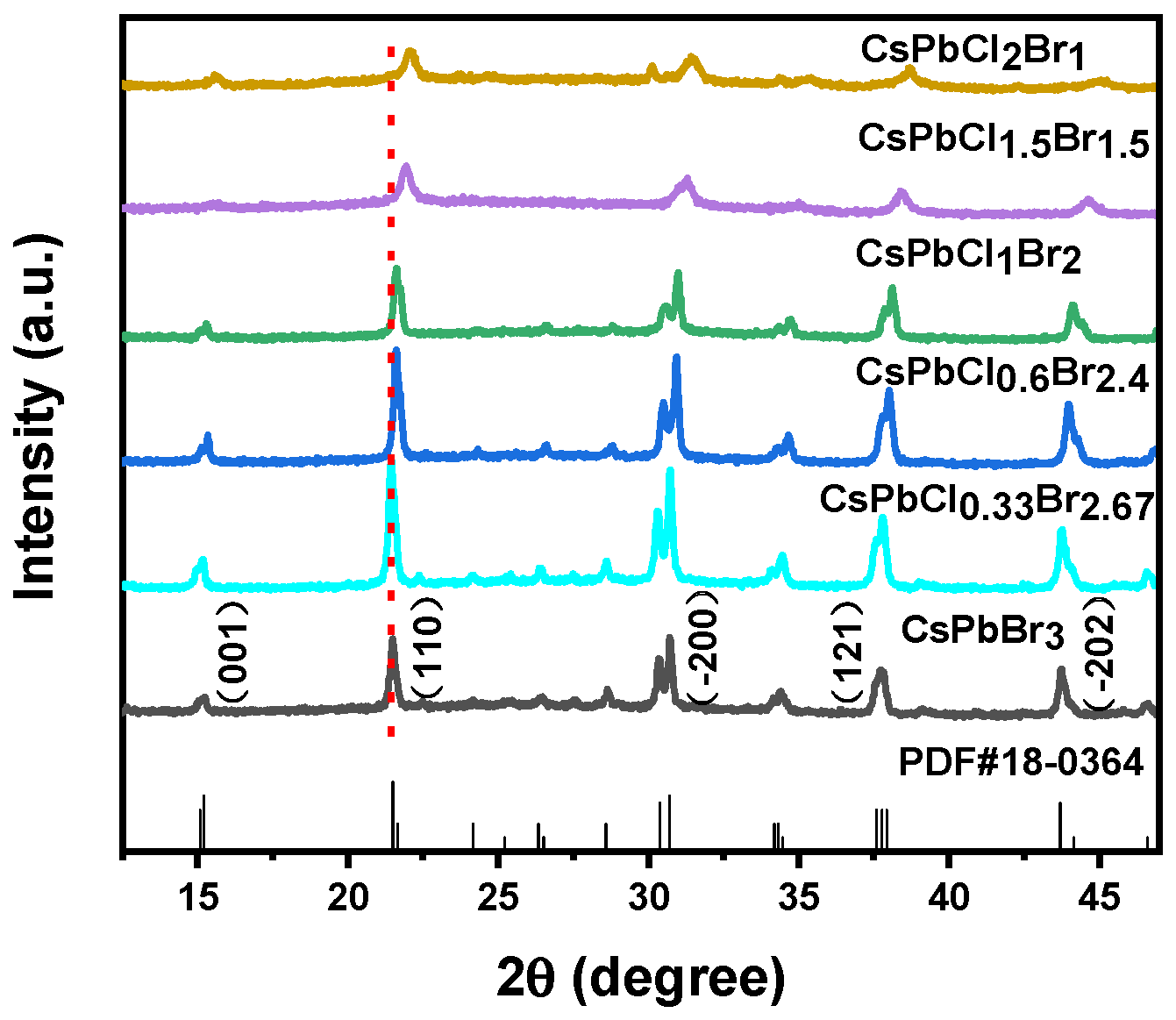
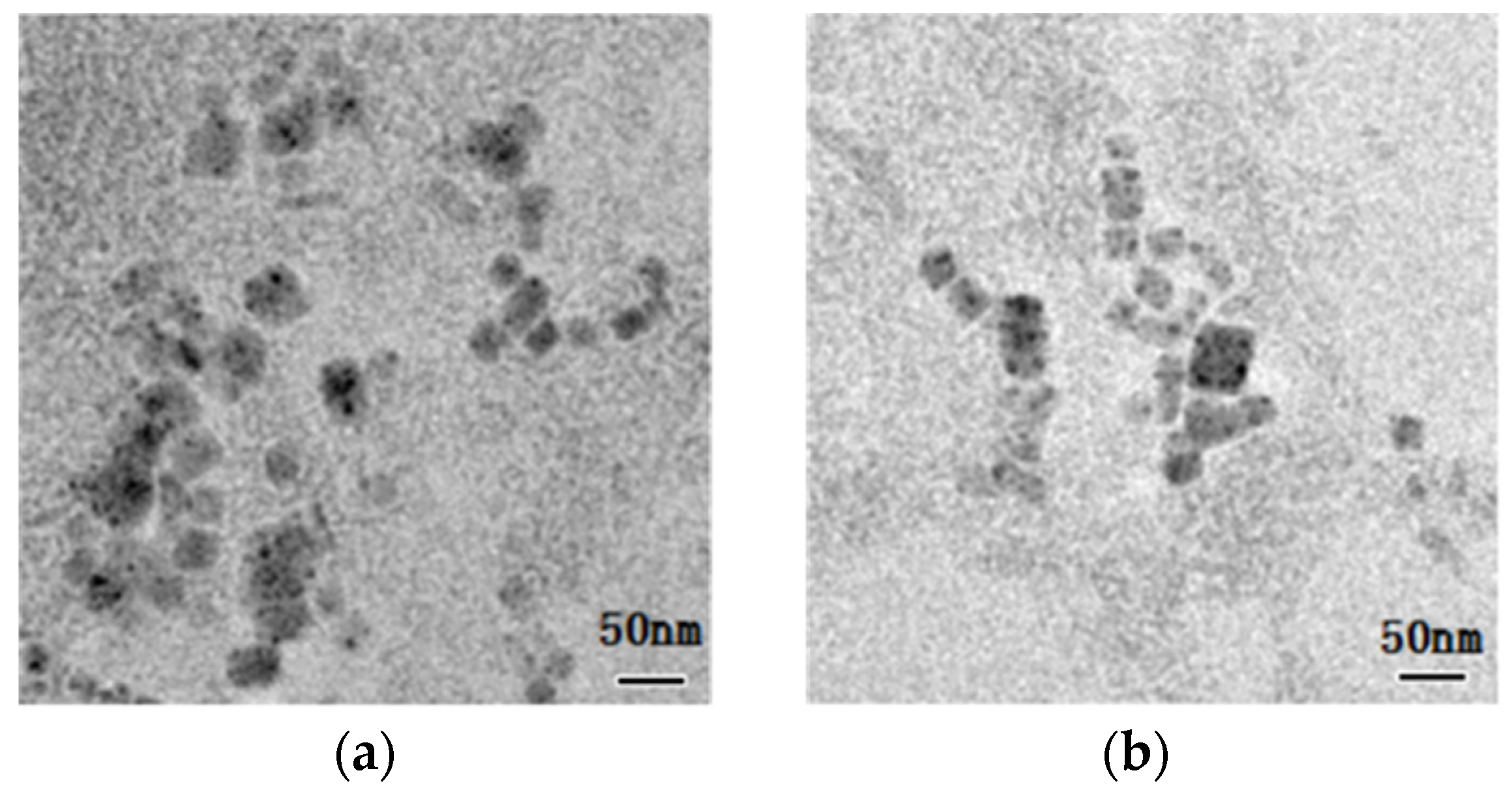
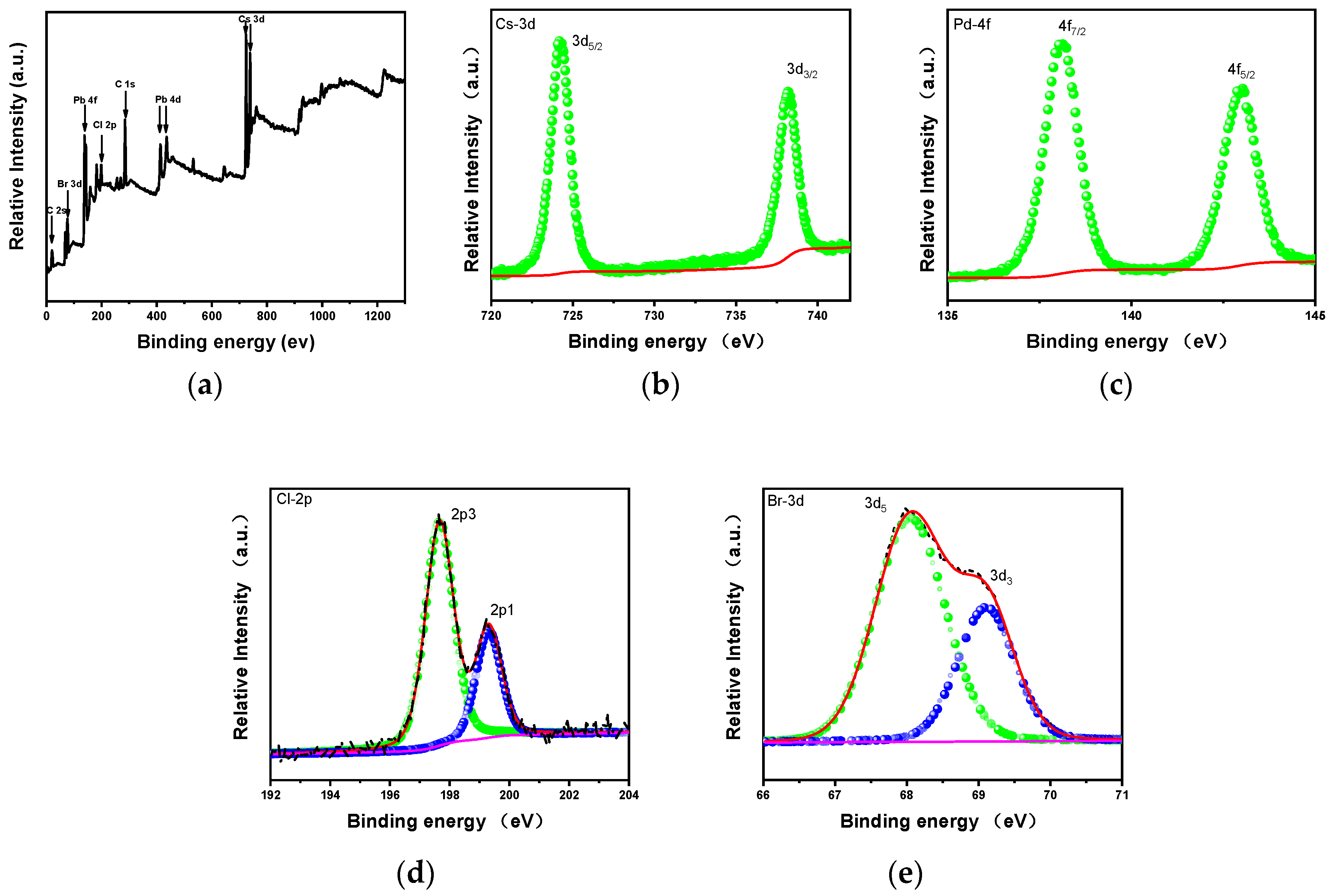
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef]
- Kumawat, N.K.; Dey, A.; Kumar, A.; Gopinathan, S.P.; Narasimhan, K.L.; Kabra, D. Band gap tuning of CH3NH3Pb(Br1-xClx)3 hybrid perovskite for blue electroluminescence. ACS Appl. Mater. Interfaces 2015, 7, 13110–13124. [Google Scholar] [CrossRef] [PubMed]
- Righetto, M.; Meggiolaro, D.; Rizzo, A.; Sorrentino, R.; He, Z.; Meneghesso, G.; Sum, T.C.; Gatti, T.; Lamberti, F. Coupling halide perovskites with different materials: From doping to nanocomposites, beyond photovoltaics. Prog. Mater. Sci. 2020, 110, 100639. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X= Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Lignos, I.; Stavrakis, S.; Nedelcu, G.; Protesescu, L.; deMello, A.J.; Kovalenko, M.V. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: Fast parametric space mapping. Nano Lett. 2016, 16, 1869–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amgar, D.; Stern, A.; Rotem, D.; Porath, D.; Etgar, L. Tunable length and optical properties of CsPbX3 (X = Cl, Br, I) nanowires with a few unit cells. Nano Lett. 2017, 17, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Liu, X.F.; Zhang, Q.; Ha, S.T.; Yuan, Y.W.; Shen, C.; Sum, T.C.; Xiong, Q. Vapor phase synthesis of organometal halide perovskite nanowires for tunable room-temperature nanolasers. Nano Lett. 2015, 15, 4571–4577. [Google Scholar] [CrossRef]
- Shamsi, J.; Dang, Z.; Bianchini, P.; Canale, C.; Stasio, F.D.; Brescia, R.; Prato, M.; Manna, L. Colloidal synthesis of quantum confined single crystal CsPbBr3 nanosheets with lateral size control up to the micrometer range. J. Am. Chem. Soc. 2016, 138, 7240–7243. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room- temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Shamsi, J.; Rastogi, P.; Caligiuri, V.; Abdelhady, A.L.; Spirito, D.; Manna, L.; Krahne, R. Bright-emitting perovskite films by large-scale synthesis and photoinduced solid-state transformation of CsPbBr3 nanoplatelets. ACS Nano 2017, 11, 10206–10213. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Kang, X.; Wang, L.; Huang, L.; Pan, D. Room-temperature and gram-scale synthesis of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with 50-85% photoluminescence quantum yields. Chem. Commun. 2016, 52, 7265–7268. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Wu, L.; Li, L.; Yao, H.-B.; Qian, H.-S.; Yu, S.-H. Large-scale synthesis of highly luminescent perovskite-related CsPb2Br5 nanoplatelets and their fast anion exchange. Angew. Chem. Int. Ed. 2016, 55, 8328–8332. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-free organic-inorganic hybrid perovskites for photovoltaic applications: Recent advances and perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Wu, G.; Dang, H.; Ma, K.; Chen, S. Multicolored mixed-organic-cation perovskite quantum dots (FAxMA1-xPbX3, X = Br and I) for white light-emitting diodes. Ind. Eng. Chem. Res. 2017, 56, 10053–10059. [Google Scholar] [CrossRef]
- Lignos, I.; Morad, V.; Shynkarenko, Y.; Bernasconi, C.; Maceiczyk, R.M.; Protesescu, L.; Bertolotti, F.; Kumar, S.; Ochsenbein, S.T.; Masciocchi, N.; et al. Exploration of near-infrared-emissive colloidal multinary lead halide perovskite nanocrystals using an automated microfluidic platform. ACS Nano 2018, 12, 5504–5517. [Google Scholar] [CrossRef]
- Yu, D.; Cao, F.; Gao, Y.; Xiong, Y.; Zeng, H. Room-temperature ion-exchange-mediated self-Assembly toward formamidinium perovskite nanoplates with finely tunable, ultrapure green emissions for achieving Rec. 2020 displays. Adv. Funct. Mater. 2018, 28, 1800248. [Google Scholar] [CrossRef]
- Fagiolari, L.; Bella, F. Carbon-based materials for stable, cheaper and large-scale processable perovskite solar cells. Energy Environ. Sci. 2019, 12, 3437–3472. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, J.S.; Shaikh, N.S.; Mali, S.S.; Patil, J.V.; Beknalkar, S.A.; Patil, A.P.; Tarwal, N.L.; Kanjanaboos, P.; Hong, C.K.; Patil, P.S. Quantum dot based solar cells: Role of nanoarchitectures, perovskite quantum dots, and charge-transporting layers. ChemSusChem 2019, 12, 4724–4753. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A review of perovskites solar cell stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Quan, L.N.; Yuan, M.; Comin, R.; Voznyy, O.; Beauregard, E.M.; Hoogland, S.; Buin, A.; Kirmani, A.R.; Zhao, K.; Amassian, A.; et al. Ligand-stabilized reduced-dimensionality perovskites. J. Am. Chem. Soc. 2016, 138, 2649–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippe, B.; Jacobsson, T.J.; Correa-Baena, J.-P.; Jena, N.K.; Banerjee, A.; Chakraborty, S.; Cappel, U.B.; Ahuja, R.; Hagfeldt, A.; Odelius, M.; et al. Valence level character in a mixed perovskite material and determination of the valence band maximum from photoelectron spectroscopy: Variation with photon energy. J. Phys. Chem. C 2017, 121, 26655–26666. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Bekenstein, Y.; Yu, Y.; Gibson, N.A.; Wong, A.B.; Eaton, S.W.; Kornienko, N.; Kong, Q.; Lai, M.; et al. Synthesis of composition tunable and highly luminescent cesium lead halide nanowires through anion-exchange reactions. J. Am. Chem. Soc. 2016, 138, 7236–7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Udayabhaskararao, T.; Houben, L.; Cohen, H.; Menahem, M.; Pinkas, I.; Avram, L.; Wolf, T.; Teitelboim, A.; Leskes, M.; Yaffe, O.; et al. A mechanistic study of phase transformation in perovskite nanocrystals driven by ligand passivation. Chem. Mater. 2018, 30, 84–93. [Google Scholar] [CrossRef]
- Balakrishnan, S.K.; Kamat, P.V. Ligand assisted transformation of cubic CsPbBr3 nanocrystals into two-dimensional CsPb2Br5 nanosheets. Chem. Mater. 2018, 30, 74–78. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl halides as alternative precursors for the colloidal synthesis of lead-based halide perovskite nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef] [Green Version]
- Creutz, S.E.; Crites, E.N.; De Siena, M.C.; Gamelin, D.R. Anion exchange in cesium lead halide perovskite nanocrystals and thin films using trimethylsilyl halide reagents. Chem. Mater. 2018, 30, 4887–4891. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Xu, W.; Yang, L.; Liu, Y.; Yao, D.; Zhang, D.; Zhang, H.; Yang, B. Engineering the Photoluminescence of CsPbX3 (X = Cl, Br, and I) Perovskite Nanocrystals Across the Full Visible Spectra with the Interval of 1 nm. ACS Appl. Mater. Interfaces 2019, 11, 14256–14265. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Xu, W.; Chen, X.; Zhai, Y.; Zhu, J.; Shao, H.; Ding, N.; Xu, L.; Dong, B.; et al. Bright blue light emission of Ni2+ ion-doped CsPbClxBr3-x perovskite quantum dots enabling efficient light-emitting devices. ACS Appl. Mater. Interfaces 2020, 12, 14195–14202. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, S.; Liu, J.; Yin, J.; Yuan, F.; Shen, W.-S.; Yao, K.; Wei, M.; Zhou, C.; Song, K.; et al. Chlorine vacancy passivation in mixed halide perovskite quantum dots by organic pseudohalides enables efficient rec. 2020 Blue Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 793–798. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals: From nanocube to ultrathin nanowire. Adv. Funct. Mater. 2017, 27, 17011–17021. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Ayguler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Highly Luminescent Cesium Lead Halide Perovskite Nanocrystals With Tunable Composition And Thickness By Ultrasonication. Angew. Chem. Int. Ed. Engl. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: The role of organic acid, base, and cesium precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Guo, A.; Gu, X.; Feng, M. Highly luminescent CsPbX3 (X=Cl, Br, I) perovskite nanocrystals with tunable photoluminescence properties. J. Alloys Compd. 2019, 789, 392–399. [Google Scholar] [CrossRef]
- Ravi, V.K.; Markad, G.B.; Nag, A. Band edge energies and excitonic transition probabilities of colloidal CsPbX3 (X = Cl, Br, I) perovskite nanocrystals. ACS Energy Lett. 2016, 1, 665–671. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Gao, H.; Shao, J.; Zou, H.; Yao, D.; Liu, Y.; Zhang, H.; Yang, B. One-step preparation of cesium lead halide CsPbX3 (X = Cl, Br, and I) perovskite nanocrystals by microwave irradiation. ACS Appl. Mater. Interfaces 2017, 9, 42919–42927. [Google Scholar] [CrossRef]
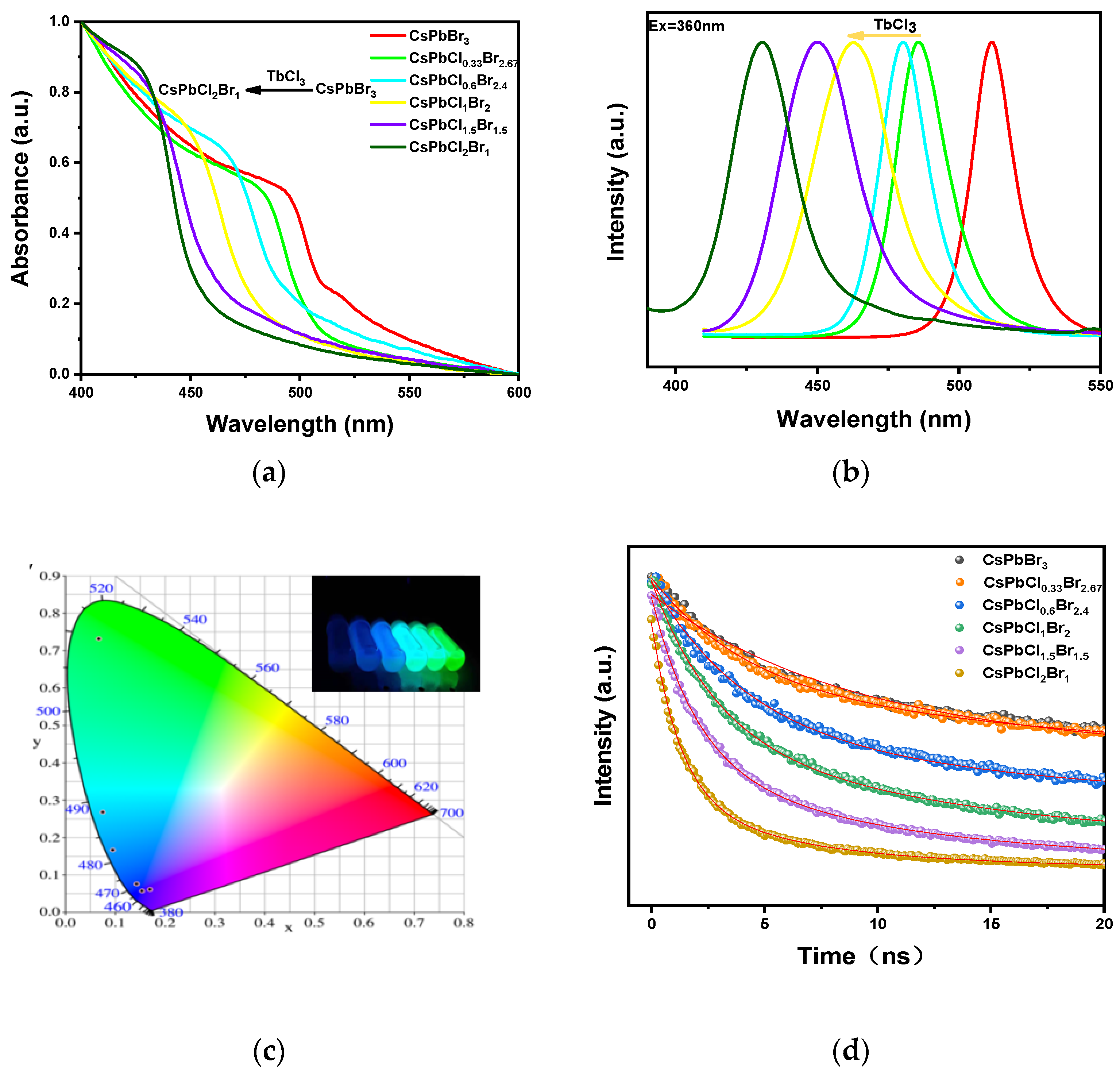
| The Sample | The Emission Peak | Full Width at Half Height | Mean Fluorescence Lifetime | CIE Coordinates |
|---|---|---|---|---|
| CsPbBr3 | 512 nm | 18.1 nm | 65.5 ns | (0.066, 0.732) |
| CsPbCl0.33Br2.67 | 486 nm | 23.1 nm | 56.9 ns | (0.075, 0.269) |
| CsPbCl0.6Br2.4 | 480 nm | 20.7 nm | 38.7 ns | (0.094, 0.166) |
| CsPbCl1Br2 | 463 nm | 34.1 nm | 17.9 ns | (0.142, 0.076) |
| CsPbCl1.5Br1.5 | 450 nm | 32.3 nm | 8.7 ns | (0.154, 0.057) |
| CsPbCl2Br1 | 431 nm | 27.3 nm | 4.3 ns | (0.169, 0.061) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, W.; Fan, T.; Lü, J.; Li, J.; Deng, T.; Liu, M. Precise Control of Green to Blue Emission of Halide Perovskite Nanocrystals Using Terbium Chloride as Chlorine Source. Nanomaterials 2021, 11, 2390. https://doi.org/10.3390/nano11092390
Deng W, Fan T, Lü J, Li J, Deng T, Liu M. Precise Control of Green to Blue Emission of Halide Perovskite Nanocrystals Using Terbium Chloride as Chlorine Source. Nanomaterials. 2021; 11(9):2390. https://doi.org/10.3390/nano11092390
Chicago/Turabian StyleDeng, Wenqiang, Ting Fan, Jiantao Lü, Jingling Li, Tingting Deng, and Mingqi Liu. 2021. "Precise Control of Green to Blue Emission of Halide Perovskite Nanocrystals Using Terbium Chloride as Chlorine Source" Nanomaterials 11, no. 9: 2390. https://doi.org/10.3390/nano11092390






