Graphene Family Nanomaterials (GFN)-TiO2 for the Photocatalytic Removal of Water and Air Pollutants: Synthesis, Characterization, and Applications
Abstract
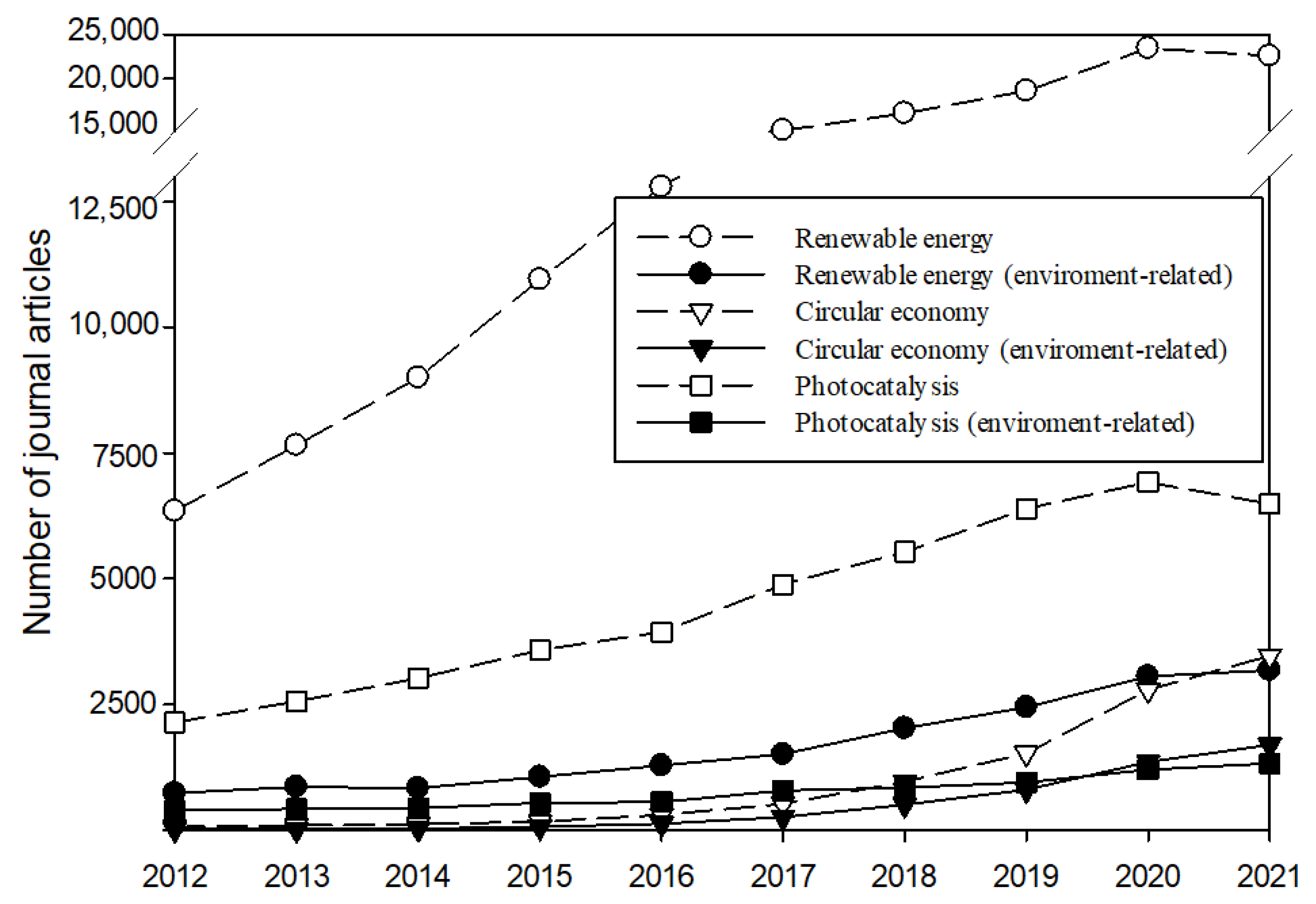
:1. Introduction
2. TiO2
2.1. Background
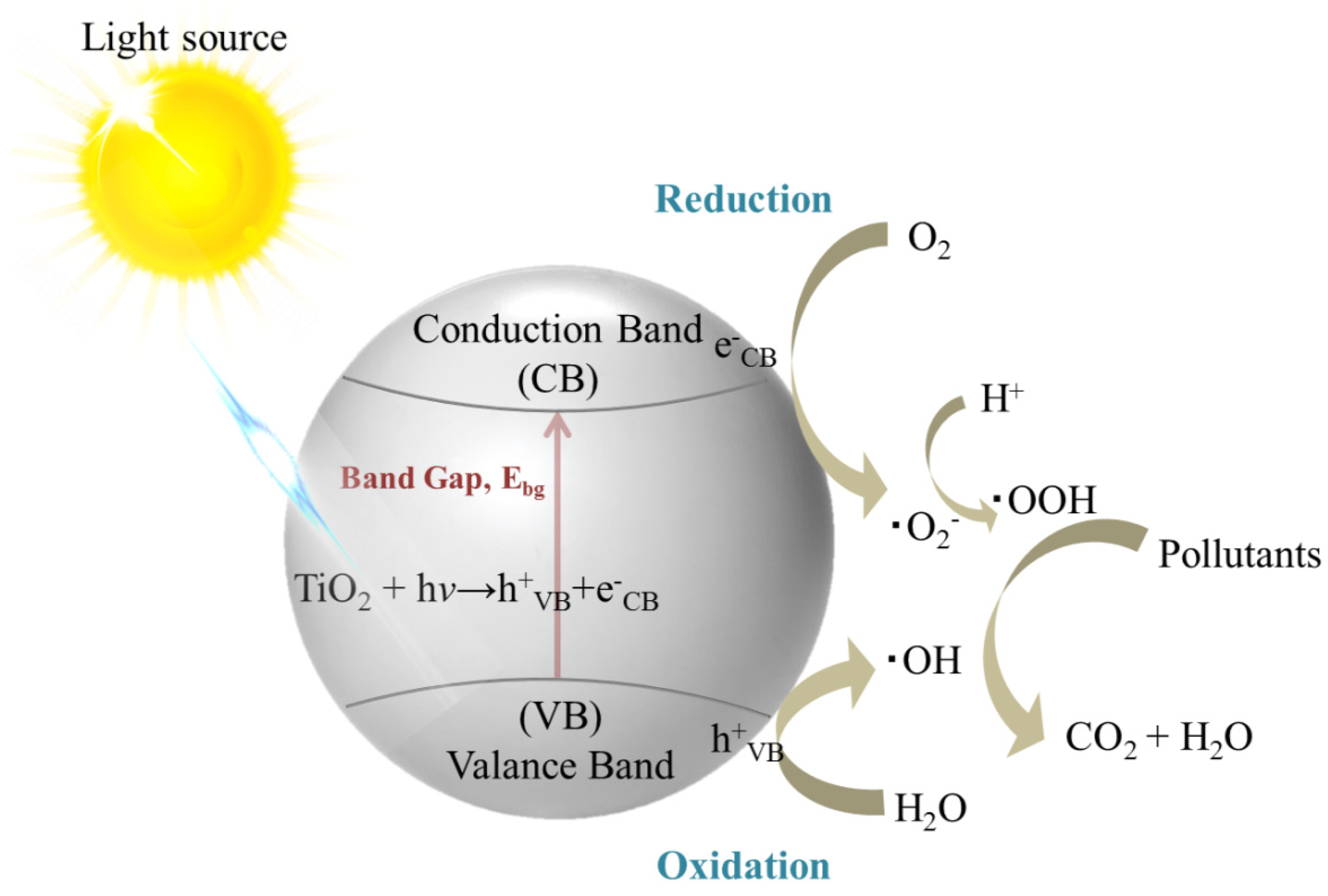
2.2. Photocatalysis
2.3. Synthesis
2.4. Properties between Different Polymorphs
3. Graphene Family Nanomaterials (GFN)
3.1. Graphene and Its Derivatives
3.2. Synthesis
3.3. Properties
| Properties | Graphene | GO | rGO | GQD |
|---|---|---|---|---|
| Functional group | No functional group | Epoxy, carboxyl, hydroxyl, and carboxyl | Epoxy, carboxyl, and hydroxyl | Epoxy, carbonyl, hydroxyl, and carboxyl |
| Nature | Hydrophobic | Hydrophilic | Hydrophilic | - |
| C:O ratio | No oxygen | 2-4 | 8-246 | 3 |
| d-spacing (nm) | 0.335 | 0.737 | 0.368 | 0.381 |
| Surface area (m2/g) | 2600 | 487 | 466 | - |
| Electron mobility (cm2V/s) | 10,000–50,000 | Insulator | 0.05–200 | - |
| Resistance (Ω) | 7200 | 0.514±0.236 | 2.01 ± 1.6 | - |
| Optics | 2.3% absorption(visible light) | - | ~20% adsorption (400–1800 nm) | - |
| Thermal conductivity (W/m·K) | ~5000 | 2.94 | 61.8 | - |
| Zeta potential (mV) | - | −33~−21.46 | −23.5~−26.5 | 8 |
| Young’s modulus | 1 | 0.2 | 0.25 | - |
| Reference | [79,103,104,105,106,107,108] | [103,104,109,110,111,112] | [77,103,104,110,113,114,115] | [103,104,113,116,117] |
4. GFN-TiO2
4.1. Synthesis
4.2. Characterization
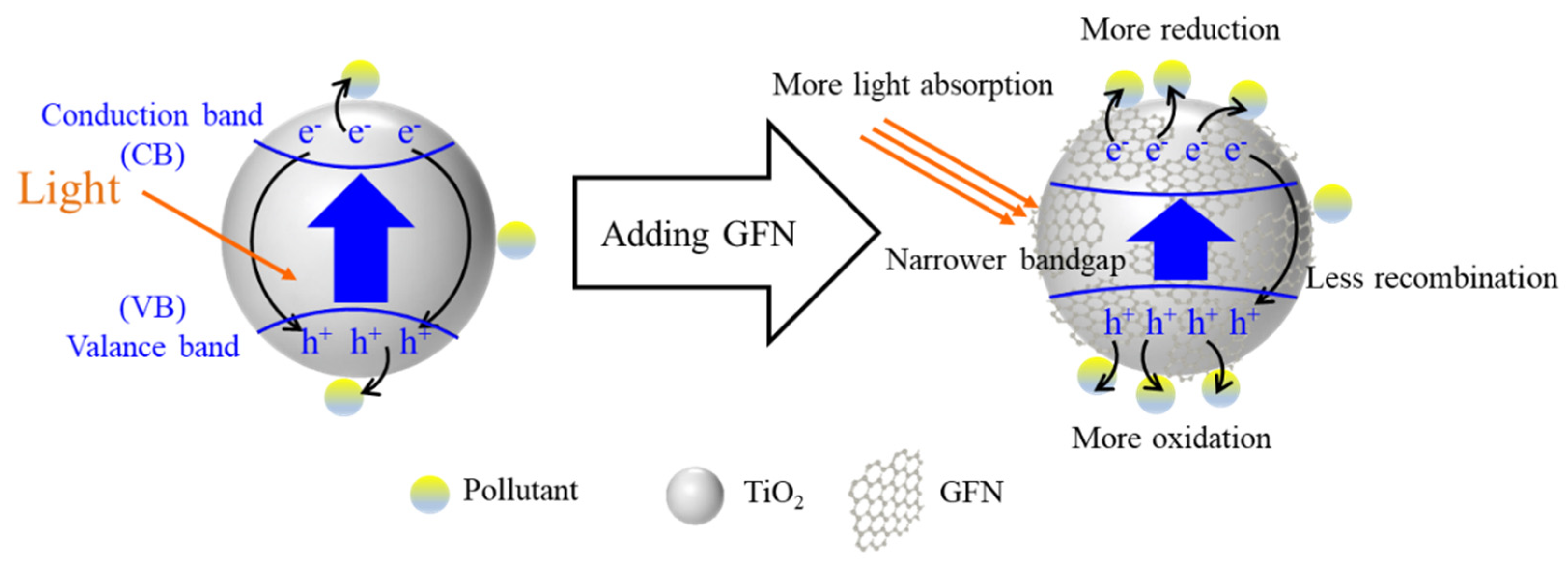
4.3. Photocatalysis Enhancement
5. Photocatalytic Removal of Pollutants
5.1. Water-Phase Pollutants
5.2. Air-Phase Pollutants
6. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasad, S.; Singh, A.; Joshi, H. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Motevali, A.; Ghobadian, B. Ethanol production form date wastes: Adapted technologies, challenges, and global potential. Renew. Energy 2019, 143, 1094–1110. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, L.; Ma, K.; Zhou, H.; Xin, Y. Study on catalytic performances and reaction mechanisms of graphene electroactive membrane in wastewater treatment. Sep. Purif. Technol. 2019, 226, 278–285. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Huang, C.-P.; Chan, Y.-H.; Huang, Y.-H. Electrochemical degradation of oxalic acid over highly reactive nano-textured γ-and α-MnO2/carbon electrode fabricated by KMnO4 reduction on loofah sponge-derived active carbon. J. Hazard. Mater. 2019, 379, 120759. [Google Scholar] [CrossRef] [PubMed]
- Wellia, D.V.; Xu, Q.C.; Sk, M.A.; Lim, K.H.; Lim, T.M.; Tan, T.T.Y. Experimental and theoretical studies of Fe-doped TiO2 films prepared by peroxo sol-gel method. Appl. Catal. A Gen. 2011, 401, 98–105. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, J.-R.; Lin, C.-H.; Huang, C.-P. Catalytic degradation of chlorpheniramine over GO-Fe3O4 in the presence of H2O2 in water: The synergistic effect of adsorption. Sci. Total Environ. 2020, 736, 139468. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, C.M.; Chen, C.H.; Chen, W.H. Removal of chlorpheniramine and variations of nitrosamine formation potentials in municipal wastewaters by adsorption onto the GO-Fe3O4. Environ. Sci. Pollut. Res. 2019, 26, 20701–20711. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor photocatalysis—Mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639. [Google Scholar] [CrossRef] [Green Version]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability In vivo in Mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [Green Version]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Carbon Black, Titanium Dioxide, and Talc; IARC: Lyon, France, 2010. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide and sulfite in aqueous solutions at semiconductor powders. J. Phys. Chem. 1977, 81, 1484–1488. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Keane, D.A.; McGuigan, K.G.; Ibáñez, P.F.; Polo-López, M.I.; Byrne, J.A.; Dunlop, P.S.; O’Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar photocatalysis for water disinfection: Materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A. Photocatalytic and self-cleaning functions of TiO2 coatings. In Sustainable Energy and Environmental Technologies; World Scientific: Singapore, 2001; pp. 1–5. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Singh, D.; Saini, K.; Kant, C.; Sharma, V.; Jain, S.; Sharma, C. Sol–gel-derived super-hydrophilic nickel doped TiO2 film as active photo-catalyst. Appl. Catal. A Gen. 2006, 314, 40–46. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Vecchio Ciprioti, S. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, R.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Boerio-Goates, J.; Woodfield, B.F. High purity anatase TiO2 nanocrystals: Near room-temperature synthesis, grain growth kinetics, and surface hydration chemistry. J. Am. Chem. Soc. 2005, 127, 8659–8666. [Google Scholar] [CrossRef]
- Hirano, M.; Nakahara, C.; Ota, K.; Tanaike, O.; Inagaki, M. Photoactivity and phase stability of ZrO2-doped anatase-type TiO2 directly formed as nanometer-sized particles by hydrolysis under hydrothermal conditions. J. Solid State Chem. 2003, 170, 39–47. [Google Scholar] [CrossRef]
- Wang, L.Q.; Yang, X.N.; Zhao, X.L.; Zhang, R.J.; Yang, Y.L. Preparation of TiO2 nanoparticles in the solvothermal method. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2011; pp. 1672–1677. [Google Scholar]
- Hong, S.-S.; Lee, M.S.; Park, S.S.; Lee, G.-D. Synthesis of nanosized TiO2/SiO2 particles in the microemulsion and their photocatalytic activity on the decomposition of p-nitrophenol. Catal. Today 2003, 87, 99–105. [Google Scholar] [CrossRef]
- Volz, H.G. Pigments, Inorganic Ullman’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Teleki, A.; Pratsinis, S.E.; Kalyanasundaram, K.; Gouma, P. Sensing of organic vapors by flame-made TiO2 nanoparticles. Sens. Actuators B Chem. 2006, 119, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.S.; Kim, H. Origin of Low Photocatalytic Activity of Rutile TiO2. Electron. Mater. Lett. 2009, 5, 73–76. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.G.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Gao, L.; Guo, J. Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl. Catal. B Environ. 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Kesselman, J.M.; Shreve, G.A.; Hoffmann, M.R.; Lewis, N.S. Flux-matching conditions at TiO2 photoelectrodes: Is interfacial electron transfer to O2 rate-limiting in the TiO2-catalyzed photochemical degradation of organics? J. Phys. Chem. 1994, 98, 13385–13395. [Google Scholar] [CrossRef]
- Hanaor, D.A.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Sclafani, A.; Herrmann, J. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 2000, 61, 7459. [Google Scholar] [CrossRef]
- Koelsch, M.; Cassaignon, S.; Minh, C.T.T.; Guillemoles, J.-F.; Jolivet, J.-P. Electrochemical comparative study of titania (anatase, brookite and rutile) nanoparticles synthesized in aqueous medium. Thin Solid Film. 2004, 451, 86–92. [Google Scholar] [CrossRef]
- Amtout, A.; Leonelli, R. Optical properties of rutile near its fundamental band gap. Phys. Rev. B 1995, 51, 6842. [Google Scholar] [CrossRef]
- Zhu, S.-C.; Xie, S.-H.; Liu, Z.-P. Nature of rutile nuclei in anatase-to-rutile phase transition. J. Am. Chem. Soc. 2015, 137, 11532–11539. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. Chem. Rev. 2014, 114, 9613–9644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.T.; Herrington, K. The structures of anatase and rutile. J. Am. Chem. Soc. 1955, 77, 4708–4709. [Google Scholar] [CrossRef]
- Mo, S.-D.; Ching, W. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys. Rev. B 1995, 51, 13023. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.L.; Liu, S.H.; Dai, Z.D.; He, Y.P.; Song, X.Z.; Tan, Z.Q. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Hamal, D.B.; Klabunde, K.J. Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. J. Colloid Interface Sci. 2007, 311, 514–522. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Li, C.M.; Chen, C.H.; Chen, W.H. Different influences of nanopore dimension and pH between chlorpheniramine adsorptions on graphene oxide-iron oxide suspension and particle. Chem. Eng. J. 2017, 307, 447–455. [Google Scholar] [CrossRef]
- Bo, Z.; Shuai, X.R.; Mao, S.; Yang, H.C.; Qian, J.J.; Chen, J.H.; Yan, J.H.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4, 4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.B.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wu, N.Q. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Jiang, C.B.; Cheng, H.M. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M. Synthesis of 2D materials in arc plasmas. J. Phys. D Appl. Phys. 2015, 48, 314007. [Google Scholar] [CrossRef] [Green Version]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Status Solidi 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Dato, A.; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 2008, 8, 2012–2016. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Wu, J.; Jiang, X.; Cao, P.; Hu, X.; Pan, T.; Qiu, C.; Yang, J.; Song, Y. Bottom-up fabrication of graphene on silicon/silica substrate via a facile soft-hard template approach. Sci. Rep. 2015, 5, 13480. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Dou, X.; Rouhanipour, A.; Zhi, L.; Räder, H.J.; Müllen, K. Two-dimensional graphene nanoribbons. J. Am. Chem. Soc. 2008, 130, 4216–4217. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1499. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, U.; König, E. Untersuchungen über Graphitoxyd. Z. Anorg. Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [Green Version]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zhao, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 5716. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen, D.; Ruoff, R.S. Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano 2010, 4, 1227–1233. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Fernandez-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solis-Fernandez, P.; Martinez-Alonso, A.; Tascon, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Pan, D.Y.; Zhang, J.C.; Li, Z.; Wu, M.H. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734. [Google Scholar] [CrossRef]
- Liang, W.X.; Bunker, C.E.; Sun, Y.P. Carbon Dots: Zero-Dimensional Carbon Allotrope with Unique Photoinduced Redox Characteristics. Acs Omega 2020, 5, 965–971. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yu, J.F.; Zhang, X.; Li, N.; Liu, B.; Li, Y.Y.; Wang, Y.H.; Wang, W.X.; Li, Y.Z.; Zhang, L.C.; et al. Large-Scale and Controllable Synthesis of Graphene Quantum Dots from Rice Husk Biomass: A Comprehensive Utilization Strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Van Tam, T.; Kang, S.G.; Babu, K.F.; Oh, E.-S.; Lee, S.G.; Choi, W.M. Synthesis of B-doped graphene quantum dots as a metal-free electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 10537–10543. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.-Q.; Li, N.; Zhu, J.-L.; Zhang, T.; Zhang, W.; Liu, B. Preparation of graphene quantum dots and their application in cell imaging. J. Nanomater. 2016, 2016, 9245865. [Google Scholar] [CrossRef]
- Li, Y.; Shu, H.; Niu, X.; Wang, J. Electronic and optical properties of edge-functionalized graphene quantum dots and the underlying mechanism. J. Phys. Chem. C 2015, 119, 24950–24957. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.-X.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xin, X.; Lin, Z. Cu2ZnSnS4 nanocrystals and graphene quantum dots for photovoltaics. Nanoscale 2011, 3, 3040–3048. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, Z.; Zheng, M.; Su, B.; Chen, X.; Chen, X. Electrochemical synthesis of phosphorus and sulfur co-doped graphene quantum dots as efficient electrochemiluminescent immunomarkers for monitoring okadaic acid. Sens. Actuators B Chem. 2020, 304, 127383. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Pan, D.; Wu, M. Room-temperature synthesis of graphene quantum dots via electron-beam irradiation and their application in cell imaging. Chem. Eng. J. 2017, 309, 374–380. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Singh, D.; Savu, R.; Moshkalev, S. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Hassan, M.; Haque, E.; Reddy, K.R.; Minett, A.I.; Chen, J.; Gomes, V.G. Edge-enriched graphene quantum dots for enhanced photo-luminescence and supercapacitance. Nanoscale 2014, 6, 11988–11994. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Jo, W.-K. FeWO4/g-C3N4 heterostructures decorated with N-doped graphene quantum dots prepared under various sonication conditions for efficient removal of noxious vapors. Ceram. Int. 2020, 46, 11346–11356. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Li, Z.; Shen, J.; Yang, Y.; Cui, X.; Yang, G. Bottom-up fabrication of single-layered nitrogen-doped graphene quantum dots through intermolecular carbonization arrayed in a 2D plane. Chem. Eur. J. 2016, 22, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Hai, X.; Chen, X.-W.; Wang, J.-H. Simultaneously fabrication of free and solidified N, S-doped graphene quantum dots via a facile solvent-free synthesis route for fluorescent detection. Talanta 2017, 168, 269–278. [Google Scholar] [CrossRef]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C 60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, J.T.; Ravishankar, N.; Shivakumara, C.; Rajamathi, M. Graphite oxide-intercalated anionic clay and its decomposition to graphene− inorganic material nanocomposites. Langmuir 2008, 24, 8240–8244. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, W.H. Influence of water, H2O2, H2SO4, and NaOH filtration on the surface characteristics of a graphene oxide-iron (GO-Fe) membrane. Sep. Purif. Technol. 2021, 262, 118317. [Google Scholar] [CrossRef]
- Carmalin, A.S.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—A review. Desalination Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Kudin, K.N.; McAllister, M.J.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Oxygen-driven unzipping of graphitic materials. Phys. Rev. Lett. 2006, 96, 176101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifvand, N.; Kowsari, E. Novel TiO2/graphene oxide functionalized with a cobalt complex for significant degradation of NOx and CO. RSC Adv. 2015, 5, 93706–93716. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly anisotropic thermal conductivity of free-standing reduced graphene oxide films annealed at high temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. New J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Ghosh, T.K.; Rana, D.; Roy, I.; Bhattacharyya, A.; Sarkar, G.; Chakraborty, M.; Chattopadhyay, D. Studies on synthesis of reduced graphene oxide (RGO) via green route and its electrical property. Mater. Res. Bull. 2016, 79, 41–51. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Zhao, D.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, Y.; Peng, B.; Li, W. NTCP-Reconstituted In Vitro HBV Infection System. In Hepatitis B Virus; Guo, H., Cuconati, A., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1540. [Google Scholar]
- Neikov, O.D.; Naboychenko, S.S.; Yefimov, N.A. Handbook of Non-Ferrous Metal Powders: Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Le, Q.V.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Jiang, L.C.; Zhang, W.D. Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 988–993. [Google Scholar]
- Cheng, Y.H.; Huang, Y.; Kanhere, P.D.; Subramaniam, V.P.; Gong, D.; Zhang, S.; Highfield, J.; Schreyer, M.K.; Chen, Z. Dual-Phase Titanate/Anatase with Nitrogen Doping for Enhanced Degradation of Organic Dye under Visible Light. Chem. Eur. J. 2011, 17, 2575–2578. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, Z.; Pourpoint, F.; Armstrong, A.R.; Grey, C.P.; Bruce, P.G. Nanoparticulate TiO2 (B): An anode for lithium-ion batteries. Angew. Chem. 2012, 124, 2206–2209. [Google Scholar] [CrossRef]
- Rongan, H.; Haijuan, L.; Huimin, L.; Difa, X.; Liuyang, Z. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; He, R.; Qi, L.; Zhang, L.; Cheng, B. Noble metal-free RGO/TiO2 composite nanofiber with enhanced photocatalytic H2-production performance. Appl. Surf. Sci. 2018, 434, 620–625. [Google Scholar] [CrossRef]
- Wu, M.-C.; Wu, P.-Y.; Lin, T.-H.; Lin, T.-F. Photocatalytic performance of Cu-doped TiO2 nanofibers treated by the hydrothermal synthesis and air-thermal treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Han, J.; Guo, R. TiO2 nanosheet/NiO nanorod hierarchical nanostructures: P–n heterojunctions towards efficient photocatalysis. J. Colloid Interface Sci. 2020, 562, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Safajou, H.; Khojasteh, H.; Salavati-Niasari, M.; Mortazavi-Derazkola, S. Enhanced photocatalytic degradation of dyes over graphene/Pd/TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles. J. Colloid Interface Sci. 2017, 498, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Wu, G.; Yuan, X.; Xie, T.; Zhang, L. Fabrication and optical properties of TiO2 nanowire arrays made by sol–gel electrophoresis deposition into anodic alumina membranes. J. Phys. Condens. Matter 2003, 15, 2917. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Self-doped TiO2 nanowires in TiO2-B single phase, TiO2-B/anatase and TiO2-anatase/rutile heterojunctions demonstrating individual superiority in photocatalytic activity under visible and UV light. Appl. Surf. Sci. 2018, 455, 1106–1115. [Google Scholar] [CrossRef]
- Al-Hajji, L.A.; Ismail, A.A.; Al-Hazza, A.; Ahmed, S.A.; Alsaidi, M.; Almutawa, F.; Bumajdad, A. Impact of calcination of hydrothermally synthesized TiO2 nanowires on their photocatalytic efficiency. J. Mol. Struct. 2020, 1200, 127153. [Google Scholar] [CrossRef]
- Dou, H.; Long, D.; Rao, X.; Zhang, Y.; Qin, Y.; Pan, F.; Wu, K. Photocatalytic Degradation Kinetics of Gaseous Formaldehyde Flow Using TiO2 Nanowires. ACS Sustain. Chem. Eng. 2019, 7, 4456–4465. [Google Scholar] [CrossRef]
- Lim, Y.W.L.; Tang, Y.; Cheng, Y.H.; Chen, Z. Morphology, crystal structure and adsorption performance of hydrothermally synthesized titania and titanate nanostructures. Nanoscale 2010, 2, 2751–2757. [Google Scholar] [CrossRef]
- Miao, L.; Tanemura, S.; Toh, S.; Kaneko, K.; Tanemura, M. Fabrication, characterization and Raman study of anatase-TiO2 nanorods by a heating-sol–gel template process. J. Cryst. Growth 2004, 264, 246–252. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Yang, J.; Zhao, X. Charge carrier interfacial transfer pathways from TiO2 and Au/TiO2 nanorod arrays to electrolyte and the association with photocatalysis. Appl. Surf. Sci. 2019, 464, 367–375. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Jiang, Z. Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2018, 445, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.-Z.; Li, S.-H.; Huang, J.-Y.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y.-K. TiO2 nanotube arrays loaded with reduced graphene oxide films: Facile hybridization and promising photocatalytic application. J. Mater. Chem. A 2015, 3, 3491–3499. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Huy, T.H.; Bui, D.P.; Kang, F.; Wang, Y.-F.; Liu, S.-H.; Thi, C.M.; You, S.-J.; Chang, G.-M.; Pham, V.V. SnO2/TiO2 nanotube heterojunction: The first investigation of NO degradation by visible light-driven photocatalysis. Chemosphere 2019, 215, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhou, J.; Liu, Y.; Yang, Y.; Zheng, S.; Wang, Q. Constructing Ag2O nanoparticle modified TiO2 nanotube arrays for enhanced photocatalytic performances. J. Alloys Compd. 2020, 849, 156493. [Google Scholar] [CrossRef]
- Parida, K.M.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Cho, T.-Y.; Han, C.-W.; Jun, Y.; Yoon, S.-G. Formation of artificial pores in nano-TiO 2 photo-electrode films using acetylene-black for high-efficiency, dye-sensitized solar cells. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wei, N.; Cui, H.; Wang, C.; Zhang, G.; Song, Q.; Sun, W.; Song, X.; Sun, M.; Tian, J. Bi2O3 nanoparticles incorporated porous TiO2 films as an effective p-n junction with enhanced photocatalytic activity. J. Am. Ceram. Soc. 2017, 100, 1339–1349. [Google Scholar] [CrossRef]
- Lv, X.; Wang, T.; Jiang, W. Preparation of Ag@AgCl/g-C3N4/TiO2 porous ceramic films with enhanced photocatalysis performance and self-cleaning effect. Ceram. Int. 2018, 44, 9326–9337. [Google Scholar] [CrossRef]
- Kim, S.; Chang, H.-K.; Kim, K.B.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Park, Y.M. Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition. Catalysts 2021, 11, 1144. [Google Scholar] [CrossRef]
- Low, F.W.; Lai, C.W.; Hamid, S.B.A. Surface modification of reduced graphene oxide film by Ti ion implantation technique for high dye-sensitized solar cells performance. Ceram. Int. 2017, 43, 625–633. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kang, H.-J. Titanium dioxide–graphene oxide composites with different ratios supported by Pyrex tube for photocatalysis of toxic aromatic vapors. Powder Technol. 2013, 250, 115–121. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Zhang, C.; Chaudhary, U.; Lahiri, D.; Godavarty, A.; Agarwal, A. Photocatalytic activity of spark plasma sintered TiO2–graphene nanoplatelet composite. Scr. Mater. 2013, 68, 719–722. [Google Scholar] [CrossRef]
- Liang, D.; Cui, C.; Hu, H.; Wang, Y.; Xu, S.; Ying, B.; Li, P.; Lu, B.; Shen, H. One-step hydrothermal synthesis of anatase TiO2/reduced graphene oxide nanocomposites with enhanced photocatalytic activity. J. Alloys Compd. 2014, 582, 236–240. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Shi, M.; Ma, H.; Li, N.; Ye, M. One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 2011, 21, 3415–3421. [Google Scholar] [CrossRef]
- Chang, B.Y.S.; Huang, N.M.; An’amt, M.N.; Marlinda, A.R.; Norazriena, Y.; Muhamad, M.R.; Harrison, I.; Lim, H.N.; Chia, C.H. Facile hydrothermal preparation of titanium dioxide decorated reduced graphene oxide nanocomposite. Int. J. Nanomed. 2012, 7, 3379. [Google Scholar]
- Li, W.; Wang, F.; Feng, S.; Wang, J.; Sun, Z.; Li, B.; Li, Y.; Yang, J.; Elzatahry, A.A.; Xia, Y. Sol–gel design strategy for ultradispersed TiO2 nanoparticles on graphene for high-performance lithium ion batteries. J. Am. Chem. Soc. 2013, 135, 18300–18303. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Liu, Y.; Wang, J.; Yang, J.; Zhang, L.; Elzatahry, A.A.; Al-Dahyan, D.; Xia, Y.; Zhao, D. General strategy to synthesize uniform mesoporous TiO2/graphene/mesoporous TiO2 sandwich-like nanosheets for highly reversible lithium storage. Nano Lett. 2015, 15, 2186–2193. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. Acs Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Li, X.; Wang, L.; Han, R.; Liu, B.; Zheng, W.; Li, X.; Liu, Y. Photo-assisted preparation and patterning of large-area reduced graphene oxide–TiO2 conductive thin film. Chem. Commun. 2010, 46, 3499–3501. [Google Scholar] [CrossRef]
- Cai, C.-J.; Xu, M.-W.; Bao, S.-J.; Ji, C.-C.; Lu, Z.-J.; Jia, D.-Z. A green and facile route for constructing flower-shaped TiO2 nanocrystals assembled on graphene oxide sheets for enhanced photocatalytic activity. Nanotechnology 2013, 24, 275602. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Grygar, T.M.; Bludská, J.; Kormunda, M. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J. 2013, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yu, J.; Xiang, Q.; Cheng, B. Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2–graphene composites for photodegradation of acetone in air. Appl. Catal. B Environ. 2012, 119, 109–116. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2—Graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2− graphene truly different from other TiO2—Carbon composite materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.N.; Chavez, C.A.; Hernandez-Sanchez, B.; Lu, P.; Bell, N.S.; Ambrosini, A.; Friedman, T.; Boyle, T.J.; Wheeler, D.R.; Huber, D.L. Synthesis and characterization of titania− graphene nanocomposites. J. Phys. Chem. C 2009, 113, 19812–19823. [Google Scholar] [CrossRef]
- Low, W.; Boonamnuayvitaya, V. Enhancing the photocatalytic activity of TiO2 co-doping of graphene–Fe3+ ions for formaldehyde removal. J. Environ. Manag. 2013, 127, 142–149. [Google Scholar] [CrossRef]
- Pallotti, D.; Gesuele, F.; Palomba, M.; Longo, A.; Carotenuto, G.; Maddalena, P.; Lettieri, S. Photoluminescence-based real-time monitoring of graphene oxide photoreduction: Demonstrations and application to graphene oxide/titanium dioxide composites. J. Lumin. 2017, 188, 129–134. [Google Scholar] [CrossRef]
- Li, K.; Xiong, J.; Chen, T.; Yan, L.; Dai, Y.; Song, D.; Lv, Y.; Zeng, Z. Preparation of graphene/TiO2 composites by nonionic surfactant strategy and their simulated sunlight and visible light photocatalytic activity towards representative aqueous POPs degradation. J. Hazard. Mater. 2013, 250, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tri, N.L.M.; Jitae, K.; Van Thuan, D.; Huong, P.T.; Al Tahtamouni, T. Improved photocatalytic decomposition of methyl ethyl ketone gas from indoor air environment by using TiO2/graphene oxide. Mater. Res. Express 2019, 6, 105509. [Google Scholar]
- Najafi, M.; Kermanpur, A.; Rahimipour, M.R.; Najafizadeh, A. Effect of TiO2 morphology on structure of TiO2-graphene oxide nanocomposite synthesized via a one-step hydrothermal method. J. Alloys Compd. 2017, 722, 272–277. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; Pastrana-Martínez, L.M.; Likodimos, V.; Falaras, P.; Silva, A.N.M.; Hiskia, A. Photocatalytic Degradation of Microcystin-LR and Off-Odor Compounds in Water under UV-A and Solar Light with a Nanostructured Photocatalyst Based on Reduced Graphene Oxide–TiO2 Composite. Identification of Intermediate Products. Ind. Eng. Chem. Res. 2013, 52, 13991–14000. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Wang, H.; Cai, X.; Hu, X.; Tang, C.; Liu, Y.; Yang, Y. Decontamination of Cr (VI) by graphene oxide@ TiO2 in an aerobic atmosphere: Effects of pH, ferric ions, inorganic anions, and formate. J. Chem. Technol. Biotechnol. 2018, 93, 2226–2233. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2009, 4, 380–386. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Tang, Y.; Feng, X. Graphene encapsulated hollow TiO2 nanospheres: Efficient synthesis and enhanced photocatalytic activity. J. Mater. Chem. A 2013, 1, 3752–3756. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132, 452–459. [Google Scholar] [CrossRef]
- Ng, Y.H.; Lightcap, I.V.; Goodwin, K.; Matsumura, M.; Kamat, P.V. To what extent do graphene scaffolds improve the photovoltaic and photocatalytic response of TiO2 nanostructured films? J. Phys. Chem. Lett. 2010, 1, 2222–2227. [Google Scholar] [CrossRef]
- Fernandez-Ibanez, P.; Polo-López, M.; Malato, S.; Wadhwa, S.; Hamilton, J.; Dunlop, P.; D’sa, R.; Magee, E.; O’shea, K.; Dionysiou, D. Solar photocatalytic disinfection of water using titanium dioxide graphene composites. Chem. Eng. J. 2015, 261, 36–44. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Jiang, Y.; Li, R.; Li, Y.; Tang, H. Bifunctional TiO2/Ag3 PO4/graphene composites with superior visible light photocatalytic performance and synergistic inactivation of bacteria. Rsc Adv. 2014, 4, 18627–18636. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Ou, X.-M.; Zeng, G.-M.; Gong, J.-L.; Deng, C.-H.; Jiang, Y.; Liang, J.; Yuan, G.-Q.; Liu, H.-Y.; He, X. Synthesis of magnetic graphene oxide—TiO2 and their antibacterial properties under solar irradiation. Appl. Surf. Sci. 2015, 343, 1–10. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.; Pablos, C.; Díaz-Jiménez, L.; Cortés-Hernández, D.A.; Sharma, P.K.; Castro-Alférez, M.; Fernández-Ibañez, P.; Dunlop, P.S.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. B Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Xu, M.; Hu, X.; Wang, S.; Yu, J.; Zhu, D.; Wang, J. Photothermal effect promoting CO2 conversion over composite photocatalyst with high graphene content. J. Catal. 2019, 377, 652–661. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Wang, X.; Tong, S.; Xie, X. Polarity on adsorption and photocatalytic performances of N-GR/TiO2 towards gaseous acetaldehyde and ethylene. Appl. Surf. Sci. 2019, 485, 255–265. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Pelizzo, M.G.; Lauria, A.; Modesti, M.; Lorenzetti, A. Nanostructured photocatalysts based on different oxidized graphenes for VOCs removal. Ind. Eng. Chem. Res. 2017, 56, 9980–9992. [Google Scholar] [CrossRef]

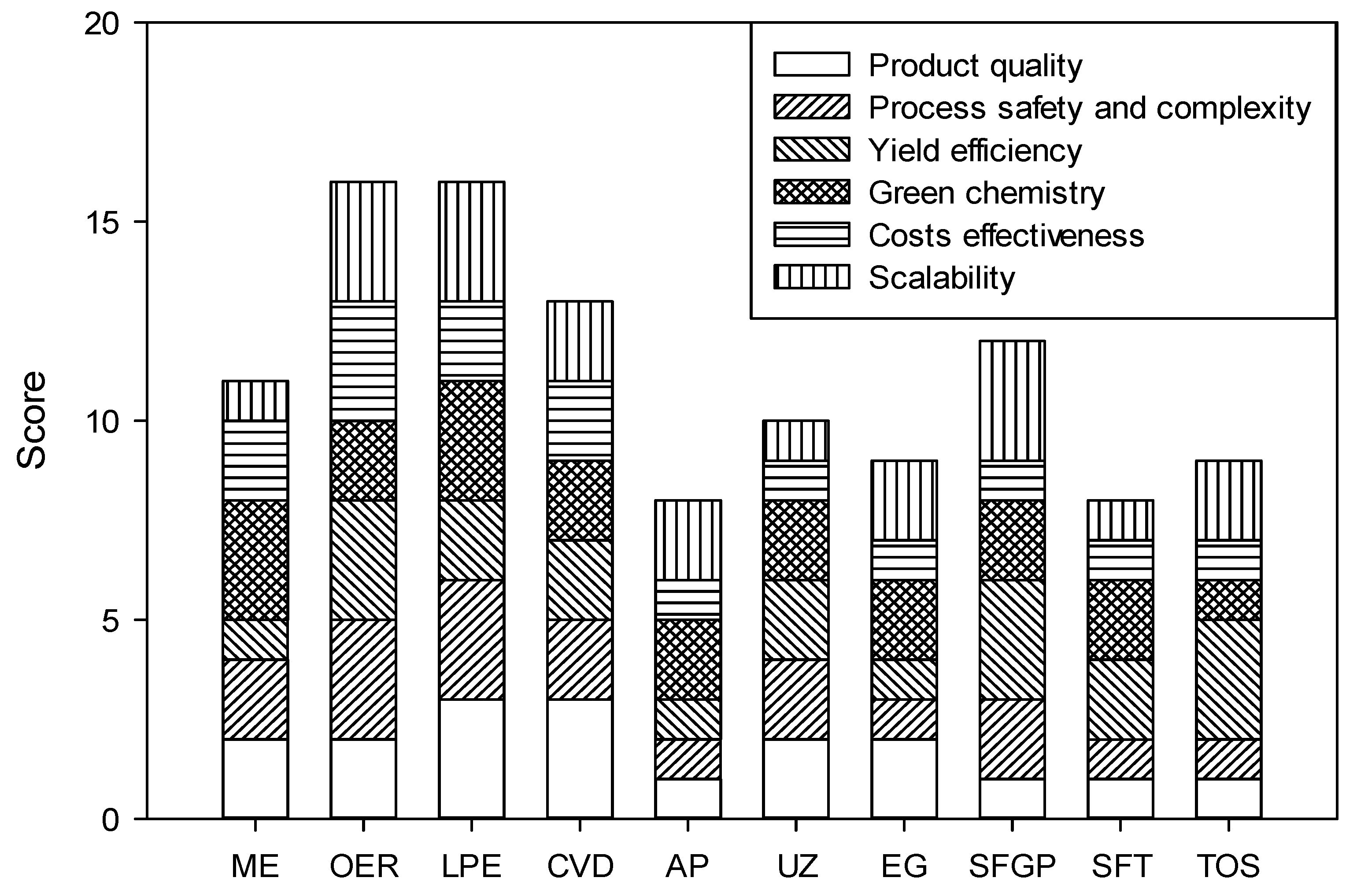
| Method | Mechanism | Phase of Formation | Pros and Cons | Reference |
|---|---|---|---|---|
| Sol-gel | Hydrolysis and condensation of TiCl4 or an organometallic compound | Amorphous and rutile | High purity, fine particle sizes, good size distribution, high surface areas, but the ease of agglomeration and long reaction time | [25,26,27,28] |
| Hydrothermal | Precipitation of TiO2 from aqueous solution at elevated temperature and pressure | Anatase and rutile | High crystallinity, low defects, fine particle size, good size distribution, limited agglomeration, control of crystal shape by temperature adjustment, but relatively higher costs | [25,29,30] |
| Solvothermal | Precipitation of TiO2 from organic solution at elevated temperature and pressure | Anatase and rutile | High crystallinity, low defects, suitability for materials unstable at high temperature, but organic solvents needed | [25,31] |
| Micelle and inverse micelle | Aggregation of TiO2 in a liquid colloid | Amorphous | High crystallinity, low defects, fine particle sizes, but relatively high costs and high crystallization temperatures | [25,32] |
| Flame pyrolysis | Combustion of TiCl4 with oxygen; used in industrial processes | Anatase and rutile | Rapid and mass production, but high energy needed and ease of rutile formation | [25,33,34] |
| Properties | Anatase | Brookite | Rutile |
|---|---|---|---|
| Crystal structure | Tetragonal | Orthorhombic | Tetragonal |
| Density (g/cm3) | 3.79 | 3.99 | 4.13 |
| Band gap (eV) | 3.2 a | ~3.2 b | 3.0 c |
| Light absorption (nm) | <390 | - | <415 |
| Dielectric constant | 6.04 | 7.89 | 6.62 |
| Lattice energy (kJ/mol) d | 24.75 | 18.53 | 0 |
| Surface enthalpy (J/m2) e | 1.34 | 1.66 | 1.93 |
| Photocatal. activity (mol/h) f | 3.5 × 10−5 | - | 1.1 × 10−5 |
| Effective electron mass (me*/m0) g | 0.0948 | 0.0949 | 1.4640 |
| Effective hole mass (mh*/m0) g | 0.1995 | 0.5620 | 0.4345 |
| Ti-O bond length (Å) h | 1.94 (shorter); 1.97 (longer) | 1.87–2.04 | 1.95 (shorter); 1.98 (longer) |
| O-Ti-O bond angle (degree) | 77.7; 92.6 | 77.0–105 | 81.2; 90.0 |
| Method | Major Approach | Pros and Cons | Cost | |
|---|---|---|---|---|
| Graphene | Mechanical exfoliation | Micro-mechanical cleavage, sonication, ball milling, and fluid dynamics | Straightforward and eco-friendly processes, fine product qualities, but relatively higher costs and limits of scalable production | High |
| Oxidative exfoliation-reduction | Chemical reduction, thermal reduction, and electrochemical reduction | Straightforward processes, cost-effectiveness, scalable production, but possible structural damage due to mal exfoliation, and potential use of hazardous chemicals | Low | |
| Liquid phase exfoliation | Sonication with proper solvents | Straightforward and eco-friendly processes (solvents recyclable), fine product qualities, scalable production, but parameters (e.g., solvent and ultra-sonication) critical to avoid physical deformation and defects | Moderate | |
| Chemical vapor deposition (CVD) | Thermal CVD, plasma-enhanced CVD, and thermal decomposition | Highly connected products with low defects and high surface areas, but relatively higher costs, limited yields, and high technical thresholds | Moderate | |
| Graphene oxide | Brodie | Graphite + H2CO3 (C/O ratio = 2.23) | Adjustable oxidation states, but potentials of long reaction time and production of explosive ClO2 and acid fog | Low |
| Staudenmaier | Graphite + HNO3 (fuming) + H2SO4 + KClO3 (C/O ratio = 2.52) | Adjustable oxidation state, but long reaction time and low temperatures to avoid exothermic reactions | Low | |
| Hofmann | Graphite + HNO3 + H2SO4 + KClO3 (C/O ratio = 2.52) | Low | ||
| Hummers | Graphite+NaNO3 +H2SO4+ KMnO4 (C/O ratio = 2.1-2.9) | Safe and fast reactions, but more parameters to control | Low | |
| Reduced graphene oxide | Chemical reduction | Various reductants | Fine product qualities, scalable production, but the potential of using hazardous reductants. Lower product qualities and removal of excess chemicals with the use of green reductants | Low |
| Thermal reduction | 1000–1100 °C for 30–45 s in the absence of air | Straightforward and eco-friendly processes, cost-effectiveness, but high capital costs and energy needed | Moderate | |
| Electrochemical reduction | The cathodic potential of 1–1.5 V | Low-defect products, rapid and eco-friendly processes, cost-effectiveness, but lower reduction levels and limited scalable production | Low | |
| Microwave and photo-reduction | Microwave reaction with visible or UV light | Fast reactions, no chemicals needed, and high yield efficiencies | Low | |
| Graphene quantum dot | Top-down | Hydrothermal synthesis, solvent thermal method, chemical oxidation, electrochemical exfoliation, electron beam lithography, microwave-assisted method, and ultra-sonication exfoliation | Scalable production, but difficulty of effective size control | High |
| Bottom-up | Soft template method, acid- and solvent-free synthesis, and metal catalysis | Effective size control, but long reaction time and limited scalable production | High |
| Dimension | Structure | Surface Area | Light Absorption Wavelength | Current Density | Reference |
|---|---|---|---|---|---|
| 0 | Nanoparticle (less than 100 nm) | 180–250 m2/g | Ultraviolet to infrared radiation | Not available | [121,122,123,124] |
| 1 | Nanofiber | 52–55 m2/g | <510 nm | 0.06 mA/cm2 | [125,126,127] |
| Nanowire | 61.5–92.6 m2/g | 250–540 nm | 1.6 mA/cm2 | [130,131,132,133,134] | |
| Nanorod | 104.6 m2/g | ~380 nm | 0.8 mA/cm2 | [135,136,137,138] | |
| Nanotube | 400 m2/g | <500 nm | 0.02 mA/cm2 | [139,140,141,142] | |
| 2 | Nanosheet | 31–146 m2/g | 200–900 nm | 0.03 mA/cm2 | [128,129] |
| 3 | Porous film | 36.4–70.8 m2/g | 200–700 nm | 18.54 mA/cm2 | [146,147,148,149] |
| Methods | Crystal Form | GFN Ratio | Pros and Cons | Reference |
|---|---|---|---|---|
| Ion implantation | Anatase | Not available | Fast production, few interfacial defects, great optical character, but high energy costs | [150] |
| Colloidal blending process | Anatase or rutile | adjustable | Aging at room temperature and vacuum drying needed | [151,152] |
| Spark plasma sintering | Rutile | 1% v/v | Fast production, but high energy costs and increased rutile form | [153] |
| Hydrothermal method | Anatase | adjustable | Adjustable doping ratio, but high pressure needed | [154,155,156] |
| Sol-gel method | Anatase | 48% w/w | Aging at room temperature, long reaction time, and calcination needed | [157] |
| Hydrolysis | Anatase | 16% w/w | Great heterogeneous nucleation, but longer reaction time and calcination needed | [158] |
| UV-assisted photo-reduction | Not available | Not available | Fast production and few collapses during reduction, but extra light source needed | [159,160] |
| In-situ assembly | Anatase | Not available | No calcination and full anatase formation, but long synthesis time | [161,162] |
| Category | Technology | Description | Ref. |
|---|---|---|---|
| Morphology | SEM | Spherical and non-spherical (platelet- or flower-like) shapes were observed with low and high GFN contents, respectively. | [151,163,164,165,166,167] |
| TEM | A fine dispersion of TiO2 in GFN with low- and nano-dimensions was reported. | [163,165,166,167] | |
| AFM | The thickness of GFN-TiO2 was increased to a scale of μm after preparation. | [164] | |
| Chemical constitution | FTIR | The peak of Ti-O-Ti at 400–900 cm−1 was broadened or shifted by the influence of Ti-O-C. The signals of carbonyl and epoxy groups were reduced. | [151,165,168] |
| XPS | The formation of C-Ti, O=C-O-Ti, and C-O-Ti bonds in GFN-TiO2 was observed. | [163] [164] | |
| XRD | The signals due to the presence of anatase and rutile were reported. | [151,163,164,165,166,168] | |
| Raman | The signals of both TiO2 and GFN were reported. The D/G intensity ratio of GFN-TiO2 was higher than that of GFN. | [163,164,165] | |
| EPR | The formation of hydroxyl and superoxide radical species was observed in GFN-TiO2. | [166] | |
| Physicochemical properties | Zeta potential | The zeta potential of GFN-TiO2 ranged between those of GFN and TiO2. | [164] |
| TGA | The irregular mass loss occurred at high temperatures. | [164] | |
| BET | The surface area of GFN-TiO2 was significantly increased at a certain ratio of GFN to TiO2. | [151,163,164,165,168] | |
| ACM | The current density of GFN-TiO2 was significantly increased at a certain ratio of GFN to TiO. | [168] | |
| PL | The time dynamics of the TiO2-induced photoreduction of GO were observed. | [169] | |
| UV-Vis | A shift to larger wavelengths in the absorption edge was observed, indicating bandgap narrowing. | [151,164,165,166,168] |
| Materials | Average Size (nm) | Functional Group | Bandgap (eV) | Wavelength (nm) | Surface Area (m2/g) | Reference |
|---|---|---|---|---|---|---|
| Graphene-TiO2 | 3.8 | C-O, C=O, O=C-O, and O-Ti | NA 1 | 600 | 176 | [170] |
| Graphene-TiO2 | ~6 | C-O and O-C=O | NA | NA | 252 | [158] 2 |
| GO-TiO2 | NA | C-O, Ti-O-Ti, Ti-O-C, and OH | NA | ~800 | 69.2 | [151] |
| GO-Co-TiO2 | NA | C-O, C-N, O-C=O | 2.77 | 421 | 206 | [109] |
| GO-Ti | NA | NA | 2.9 | ~550 | 68.9 | [171] |
| rGO-TiO2 | 35 | NA | NA | ~360 | 212.75 | [172] |
| rGO-TiO2 | ~8 | NA | NA | NA | 229 | [157] 2 |
| Pollutant | Catalyst | Light Source | Removal | Ref. | |
|---|---|---|---|---|---|
| Inorganic | Cr(VI) (0.2 mM) | GO-TiO2 (0.5 g/L) | 254 nm, 20 W, UV lamp | 90% | [164] |
| Cr(VI)(10 mg/L) | GO-TiO2 (0.5 g/L) | 365 nm, 8 W, UV lamp | 99% | [174] | |
| Organic | Methylene blue (0.01 g/L) | Graphene-TiO2 (0.75 g/L) | 365 nm, 100 W, high-pressure Hg lamp >400 nm, 500W, Xe lamp | 85% 65% | [175] |
| Rhodamine B (20 mg/L) | Graphene-TiO2 (0.1 g/L) | 11 W, low-pressure Hg lamp | 91% | [176] | |
| Rhodamine B (20 mg/L) Norfloxacin (20 mg/L) Aldicarb (10.5 mg/L) | Graphene-TiO2 (1 g/L) | >400 nm, Xe lamp | 79.7% 86.2% 36.8% | [170] | |
| Malachite green oxalate (13.1 mg/L) | GO-TiO2 (0.2 g/L) | 450 W, water-cooled Hg lamp | 80% | [145] | |
| Phenol (10 mg/L) | rGO-TiO2 (5 g/L) | 310-400 nm, UV lamp | Not given | [177] | |
| 2,4-D (15 mM) | rGO-TiO2 (film) | <320 nm, 450 W, Xe lamp | ~87% | [178] | |
| Biological | E. coli (106 CFU/mL), F. solani spores (103 CFU/mL) | rGO-TiO2 (0.5 g/L) | Sunlight | ~100% | [179] |
| E. coli, S.aureus, S.typhi, P. aeruginosa, B. subtilis, B. pumilus (106 CFU/mL) | Graphene-Ag3PO4-TiO2 | >420 nm, 350 W, Xe lamp | ~100% | [180] | |
| E. coli (105–106 CFU/mL) | GO-TiO2 (0.2 g/L) | Xe lamp | ~100% | [181] | |
| E. coli (106 CFU/mL) | rGO-TiO2 (18 mg/L) | >285 nm, UV-visible light; >420 nm, visible light | ~100% | [182] |
| Pollutant | Catalyst | Light Source | Humidity or Flow Rate | Removal | Ref. | |
|---|---|---|---|---|---|---|
| Inorganic | NOx (1 ppm) | Graphene-TiO2 rGO-TiO2 | 15 W, UVA 8 W, visible light | 50% humidity, 3 L/min | 42% 49% | [165] |
| NOx (200 ppb) | Graphene-TiO2 | 280–780 nm, 300 W, solar lamp | 1 L/min | 77% | [183] | |
| CO (50 ppm) NOx (1 ppm) | Graphene-TiO2 | 8 W, UV lamp | 0.2 L/min | 46% 51% | [109] | |
| Organic | Acetone (300 ± 20 ppm) | Graphene-TiO2 | 365 nm, 15 W, UV lamp | 1 L/min | ~60% | [163] |
| Acetaldehyde (500 ppm) Ethylene (50 ppm) | Graphene-TiO2 | 260 W, fluorescent lamp 500 W, Xenon lamp | 20 cm3/min | ~82% ~90% | [185] | |
| Benzene (250 ppm) | Graphene-TiO2 | 254 nm, 4 W, UV lamp | 20 mL/min | 6.4% | [166] | |
| Formaldehyde (3000 ppm) | Graphene-TiO2 | 365 nm, 8 W, black light blue lamp >420 nm, 8 W, fluorescent lamp | Not specified | 50.3% 25.5% | [168] | |
| Methanol (4,000 ppm) | Graphene-TiO2 GO-TiO2 rGO-TiO2 | 254 nm, 16 W, UV lamp | 155 cm3/min | 80% 99% 99% | [186] | |
| BTEX (1 ppm) | GO-TiO2 | 400–720 nm, 8 W, daylight lamp | 55% humidity, 1 L/min | 96% | [151] | |
| MEKT (30 ppm) | GO-TiO2 | 80 W, Xe lamp | 40% humidity, 50 mL/min | 96.8% | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chen, W.-H. Graphene Family Nanomaterials (GFN)-TiO2 for the Photocatalytic Removal of Water and Air Pollutants: Synthesis, Characterization, and Applications. Nanomaterials 2021, 11, 3195. https://doi.org/10.3390/nano11123195
Lin C-H, Chen W-H. Graphene Family Nanomaterials (GFN)-TiO2 for the Photocatalytic Removal of Water and Air Pollutants: Synthesis, Characterization, and Applications. Nanomaterials. 2021; 11(12):3195. https://doi.org/10.3390/nano11123195
Chicago/Turabian StyleLin, Chih-Hsien, and Wei-Hsiang Chen. 2021. "Graphene Family Nanomaterials (GFN)-TiO2 for the Photocatalytic Removal of Water and Air Pollutants: Synthesis, Characterization, and Applications" Nanomaterials 11, no. 12: 3195. https://doi.org/10.3390/nano11123195






