Synthesis of SAPO-34 Nanoplates with High Si/Al Ratio and Improved Acid Site Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SAPO-34 Particles
2.2. Characterization of SAPO-34 Particles
3. Results and Discussion
3.1. Effect of Template Concentration (x) on the Formation of SAPO-34 Particles
3.2. Effect of P2O5 Concentration (y) on the Formation of SAPO-34 Particles
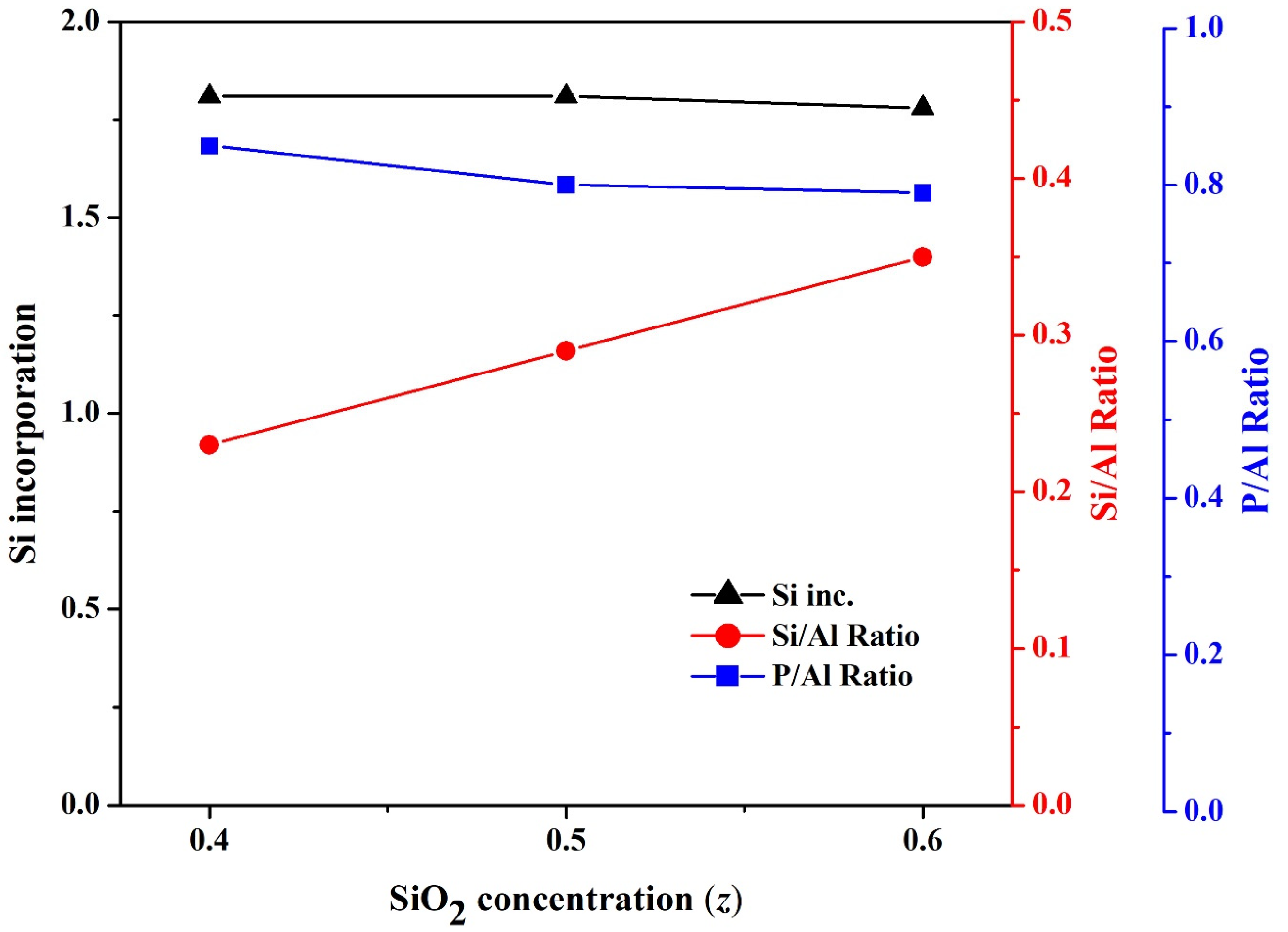
3.3. Effect of SiO2 Concentration (z) on the Formation of SAPO-34 Particles
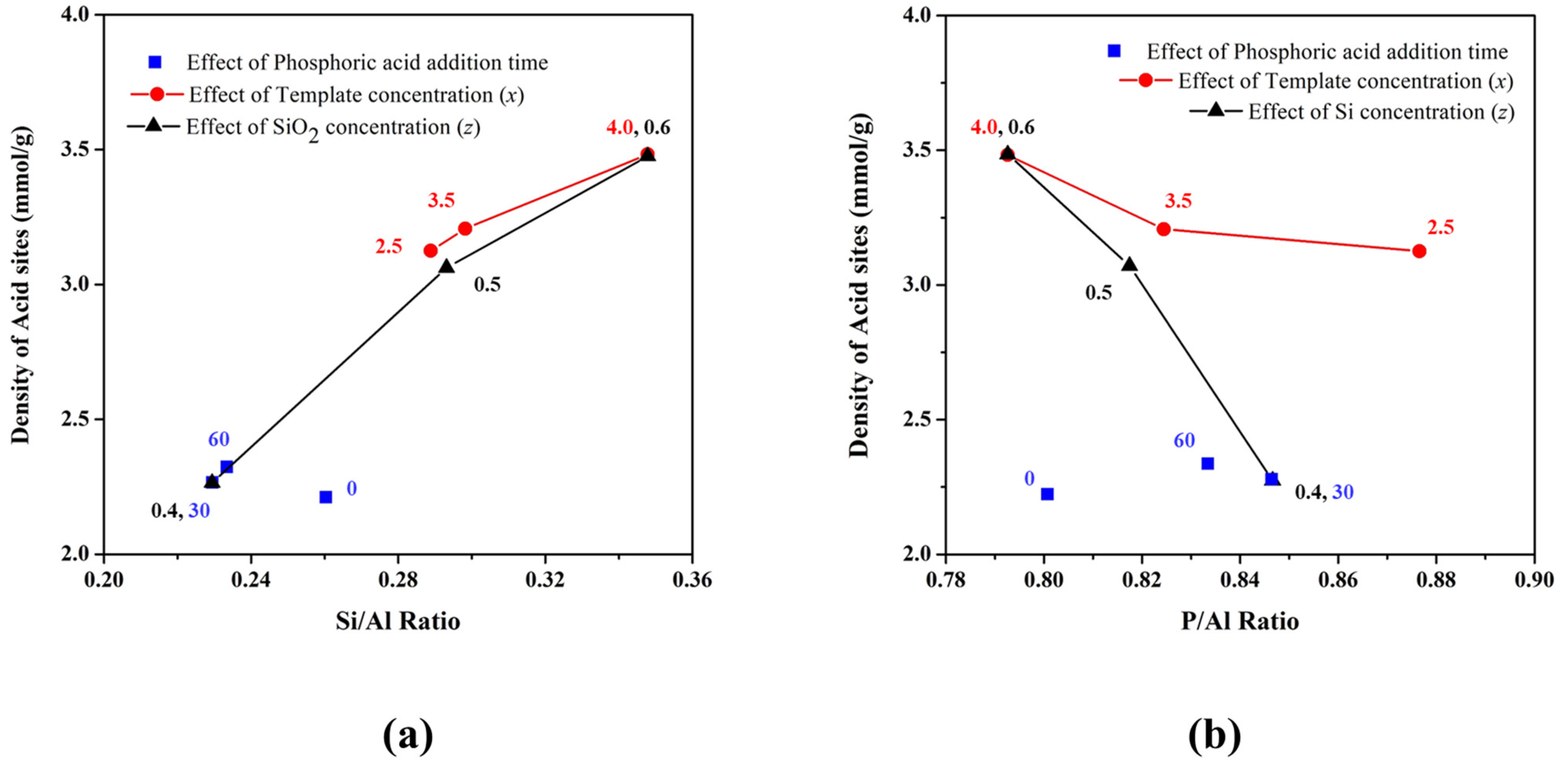
3.4. Density of Acid Sites in SAPO-34 Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Álvaro-Muñoz, T.; Marquez-Alvarez, C.; Sastre, E. Use of different templates on SAPO-34 synthesis: Effect on the acidity and catalytic activity in the MTO reaction. Catal. Today 2012, 179, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Pinilla-Herrero, I.; Márquez-Álvarez, C.; Sastre, E. Complex relationship between SAPO framework topology, content and distribution of Si and catalytic behaviour in the MTO reaction. Catal. Sci. Technol. 2017, 7, 3892–3901. [Google Scholar] [CrossRef] [Green Version]
- Carreon, M.A. (Ed.) Membranes for Gas Separations; World Scientific Pub Co Pte Ltd.: Hackensack, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Almeida, G.; Variani, Y.M.; Gómez-Hortigüela, L.; Mercury, J.M.R.; Rojas, A. Performance of three different cations based on imidazolium ring as structure directing agents in the synthesis of aluminophosphates and silicoaluminophosphates microporous materials. Microporous Mesoporous Mater. 2020, 294, 109861. [Google Scholar] [CrossRef]
- Kim, M.-Z.; Sharma, P.; Kim, Y.; Alam, S.F.; Lee, H.R.; Cho, C.H. One-step template-free hydrothermal synthesis of partially Sr-incorporated hierarchical K-CHA zeolite microspheres. Microporous Mesoporous Mater. 2019, 286, 65–76. [Google Scholar] [CrossRef]
- Alam, S.F.; Kim, M.-Z.; Kim, Y.J.; Rehman, A.U.; Devipriyanka, A.; Sharma, P.; Yeo, J.-G.; Lee, J.-S.; Kim, H.; Cho, C.-H. A new seeding method, dry rolling applied to synthesize SAPO-34 zeolite membrane for nitrogen/methane separation. J. Membr. Sci. 2020, 602, 117825. [Google Scholar] [CrossRef]
- Kim, M.-Z.; Alam, S.F.; Arepalli, D.; Rehman, A.U.; Choi, W.-Y.; Cho, C.-H. Prevention in Thermal Crack Formation in Chabazite (CHA) Zeolite Membrane by Developing Thin Top Zeolite and Thick Intermediate Layers. Nanomaterials 2021, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wei, Y.; Xu, S.; Chen, J.; Li, J.; Liu, Z.; Yu, J.; Xu, R. Nanosize-Enhanced Lifetime of SAPO-34 Catalysts in Methanol-to-Olefin Reactions. J. Phys. Chem. C 2013, 117, 8214–8222. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, G.; Wang, Z.; Jiang, R.; Wang, D.; Chen, J.; Huang, J. Tuning Hydrocarbon Pool Intermediates by the Acidity of SAPO-34 Catalysts for Improving Methanol-to-Olefins Reaction. ACS Sustain. Chem. Eng. 2018, 6, 16867–16875. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Restuccia, G.; Schmidt, T.; Py, X. The size of sorbents in low pressure sorption or thermochemical energy storage processes. Energy 2014, 77, 983–998. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Frazzica, A.; Freni, A.; Proverbio, E. Adsorption performance and thermodynamic analysis of SAPO-34 silicone composite foams for adsorption heat pump applications. Mater. Renew. Sustain. Energy 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Li, S.; Funke, H.F.; Falconer, J.L.; Noble, R.D. Ion-exchanged SAPO-34 membranes for light gas separations. Microporous Mesoporous Mater. 2007, 106, 140–146. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Amino-Functionalized SAPO-34 Membranes for CO2/CH4 and CO2/N2 Separation. Langmuir 2011, 27, 2888–2894. Available online: http://pubs.acs.org/doi/abs/10.1021/la105037n (accessed on 10 November 2021). [CrossRef] [PubMed]
- Zhou, L.; Yang, J.; Li, G.; Wang, J.; Zhang, Y.; Lu, J.; Yin, D. Highly H2 permeable SAPO-34 membranes by steam-assisted conversion seeding. Int. J. Hydrogen Energy 2014, 39, 14949–14954. Available online: http://www.sciencedirect.com/science/article/pii/S0360319914019028 (accessed on 20 November 2021). [CrossRef]
- Hong, M.; Li, S.; Falconer, J.L.; Noble, R.D. Hydrogen purification using a SAPO-34 membrane. J. Membr. Sci. 2008, 307, 277–283. Available online: http://ac.els-cdn.com/S0376738807006783/1-s2.0-S0376738807006783-main.pdf?_tid=cc8697ae-b523-11e6-80fa-00000aab0f6c&acdnat=1480307784_70fa6dc33c72ce43f7955cf7f4094ce4 (accessed on 20 November 2021). [CrossRef]
- Wu, T.; Lucero, J.; Crawford, J.; Sinnwell, M.A.; Thallapally, P.K.; Carreon, M.A. SAPO-34 membranes for xenon capture from air. J. Membr. Sci. 2019, 573, 288–292. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Azamat, J.; Pakdel, S.; Erfan-Niya, H.; Khataee, A. Separation of noble gases using CHA-type zeolite membrane: Insights from molecular dynamics simulation. Chem. Pap. 2020, 74, 3057–3065. [Google Scholar] [CrossRef]
- Salmasi, M.; Fatemi, S.; Hashemi, S. MTO reaction over SAPO-34 catalysts synthesized by combination of TEAOH and morpholine templates and different silica sources. Sci. Iran. 2012, 19, 1632–1637. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Zhu, J.; Liu, S.; Wang, Y. SAPO-34 with a low acidity outer layer by epitaxial growth and its improved MTO performance. RSC Adv. 2017, 7, 39889–39898. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Moljord, K.; Holmen, A. A methanol to olefins review: Diffusion, coke formation and deactivation on SAPO type catalysts. Microporous Mesoporous Mater. 2012, 164, 239–250. [Google Scholar] [CrossRef]
- Hajfarajollah, H.; Askari, S.; Halladj, R. Effects of micro and nano-sized SAPO-34 and SAPO-5 catalysts on the conversion of methanol to light olefins. React. Kinet. Mech. Catal. 2013, 111, 723–736. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Chen, H.; Wang, X.; Wang, C.; Zhang, X. Seed-assisted synthesis of hierarchical SAPO-18/34 intergrowth and SAPO-34 zeolites and their catalytic performance for the methanol-to-olefin reaction. Catal. Today 2020, 355, 188–198. [Google Scholar] [CrossRef]
- Gong, J.; Wang, C.; Zeng, C.; Zhang, L. Hydrothermal preparation of hierarchical SAPO-34 constructed by nano-sheets using rapeseed pollen extract as water and its CO2 adsorption property. Microporous Mesoporous Mater. 2016, 221, 128–136. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Sharma, R.P.; Yu, J.; Xu, R. Fabrication of SAPO-34 Crystals with Different Morphologies by Microwave Heating. Top. Catal. 2010, 53, 1304–1310. [Google Scholar] [CrossRef]
- Álvaro-Muñoz, T.; Sastre, E.; Márquez-Álvarez, C. Microwave-assisted synthesis of plate-like SAPO-34 nanocrystals with increased catalyst lifetime in the methanol-to-olefin reaction. Catal. Sci. Technol. 2014, 4, 4330–4339. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Lu, G.; Wang, Y. Synthesis of SAPO-34 nanoplates via hydrothermal method. Microporous Mesoporous Mater. 2016, 225, 144–153. [Google Scholar] [CrossRef]
- Di, C.-Y.; Li, X.-F.; Wang, P.; Li, Z.-H.; Fan, B.-B.; Dou, T. Green and efficient dry gel conversion synthesis of SAPO-34 catalyst with plate-like morphology. Pet. Sci. 2017, 14, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Sedighi, M.; Ghasemi, M.; Jahangiri, A. Catalytic performance of CeAPSO-34 molecular sieve with various cerium content for methanol conversion to olefin. Korean J. Chem. Eng. 2017, 34, 997–1003. [Google Scholar] [CrossRef]
- Bahrami, H.; Darian, J.T.; Sedighi, M. Simultaneous effects of water, TEAOH and morpholine on SAPO-34 synthesis and its performance in MTO process. Microporous Mesoporous Mater. 2018, 261, 111–118. [Google Scholar] [CrossRef]
- Doan, T.; Nguyen, K.; Dam, P.; Vuong, T.H.; Le, M.T.; Thanh, H.P. Synthesis of SAPO-34 Using Different Combinations of Organic Structure-Directing Agents. J. Chem. 2019, 2019, 6197527. [Google Scholar] [CrossRef] [Green Version]
- Sastre, G.; Lewis, A.D.W.; Catlow, C.R.A. Modeling of Silicon Substitution in SAPO-5 and SAPO-34 Molecular Sieves. J. Phys. Chem. B 1997, 101, 5249–5262. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Bao, X.; Liu, X.; Han, X.; He, C.; Zhai, R. Crystallization and Si incorporation mechanisms of SAPO-34. Microporous Mesoporous Mater. 2002, 53, 97–108. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishio, T.; Katada, N.; Sastre, G.; Niwa, M. Ammonia IRMS-TPD measurements on Brønsted acidity of proton-formed SAPO-34. Phys. Chem. Chem. Phys. 2011, 13, 3311–3318. [Google Scholar] [CrossRef]
- Li, M.; Zeng, C.; Zhang, L. Hydrothermal synthesis of SAPO-5 with novel morphologies from hydrogels containing acetic acid and high concentration of triethylamine under neutral or alkaline conditions. CrystEngComm 2012, 14, 3787–3792. [Google Scholar] [CrossRef]
- Terasaka, K.; Imai, H.; Li, X. Control of Morphology and Acidity of SAPO-5 for the Methanol-To- Olefins (MTO) Reaction. J. Adv. Chem. Eng. 2015, 5, 1000138. [Google Scholar] [CrossRef] [Green Version]
- Emrani, P.; Fatemi, S.; Ashraf, T.S. Effect of synthesis parameters on phase purity, crystallinity and particle size of SAPO-34. Iran. J. Chem. Chem. Eng. 2011, 30, 29–36. [Google Scholar]
- Denning, S.; Lucero, J.; Koh, C.A.; Carreon, M.A. Chabazite Zeolite SAPO-34 Membranes for He/CH4 Separation. ACS Mater. Lett. 2019, 1, 655–659. [Google Scholar] [CrossRef]
- Das, J.K.; Das, N.; Bandyopadhyay, S. Highly oriented improved SAPO 34 membrane on low cost support for hydrogen gas separation. J. Mater. Chem. A 2013, 1, 4966–4973. [Google Scholar] [CrossRef]
- Arstad, B.; Lind, A.; Cavka, J.H.; Thorshaug, K.; Akporiaye, D.; Wragg, D.; Fjellvag, H.; Grønvold, A.; Fuglerud, T. Structural changes in SAPO-34 due to hydrothermal treatment. A NMR, XRD, and DRIFTS study. Microporous Mesoporous Mater. 2016, 225, 421–431. [Google Scholar] [CrossRef]
- Zhong, J.; Han, J.; Wei, Y.; Tian, P.; Guo, X.; Song, C.; Liu, Z. Recent advances of the nano-hierarchical SAPO-34 in the methanol-to-olefin (MTO) reaction and other applications. Catal. Sci. Technol. 2017, 7, 4905–4923. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, Z.; Yu, J. The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion. Natl. Sci. Rev. 2017, 5, 542–558. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Han, D.; Wu, J. Facile synthesis of SAPO-34 with small crystal size for conversion of methanol to olefins. Powder Technol. 2014, 262, 177–182. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, W.; Xiao, S. Influence of phosphorous contents on Si incorporation mechanism and properties of SAPO-34. Adv. Powder Technol. 2016, 27, 625–630. [Google Scholar] [CrossRef]
- Fjermestad, T.; Svelle, S.; Swang, O. Mechanism of Si Island Formation in SAPO-34. J. Phys. Chem. C 2015, 119, 2086–2095. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Song, Z.; Li, S.; Yu, M. Growth of High-Quality, Thickness-Reduced Zeolite Membranes towards N2 /CH4 Separation Using High-Aspect-Ratio Seeds. Angew. Chem. Int. Ed. 2015, 54, 10843–10847. [Google Scholar] [CrossRef]
- Luo, M.; Liu, M.; Fu, Y.; Chen, W.; Wang, B.; Mao, G. TEAOH-Templated SAPO-34 Zeolite with Different Crystallization Processes and Silicon Sources: Crystallization Mechanism and MTO Performance. Eur. J. Inorg. Chem. 2020, 2020, 318–326. [Google Scholar] [CrossRef]
- Zhang, L.; Bates, J.; Chen, D.; Nie, H.-Y.; Huang, Y. Investigations of Formation of Molecular Sieve SAPO-34. J. Phys. Chem. C 2011, 115, 22309–22319. [Google Scholar] [CrossRef]
- Lyu, Y.; Liu, Y.; He, X.; Xu, L.; Liu, X.; Yan, Z. The regulation of Si distribution and surface acidity of SAPO-11 molecular sieve. Appl. Surf. Sci. 2018, 453, 350–357. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, J.; Fan, B. Synthesis and characterisation of SAPO-34 with high surface area. Micro Nano Lett. 2015, 10, 367–369. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Guo, J.-K.; Shen, S.; Au, C.-T.; Yin, S.-F. Synthesis of Submicron-Sized SAPO-34 as Efficient Catalyst for Olefin Generation from CH3Br. Ind. Eng. Chem. Res. 2019, 58, 18582–18589. [Google Scholar] [CrossRef]
- Soltanali, S.; Darian, J.T. Synthesis of mesoporous beta catalysts in the presence of carbon nanostructures as hard templates in MTO process. Microporous Mesoporous Mater. 2019, 286, 169–175. [Google Scholar] [CrossRef]
- Wang, C.; Yang, M.; Tian, P.; Xu, S.; Yang, Y.; Wang, D.; Yuan, Y.; Liu, Z. Dual template-directed synthesis of SAPO-34 nanosheet assemblies with improved stability in the methanol to olefins reaction. J. Mater. Chem. A 2015, 3, 5608–5616. [Google Scholar] [CrossRef]
- Gong, F.; Wang, X.; Wang, P.; Xuan, L.; Li, Z.; Zhu, Y. Facile synthesis of SAPO-34 with excellent methanol-to-olefin activity in a short time via a conventional hydrothermal method. New J. Chem. 2020, 44, 10410–10417. [Google Scholar] [CrossRef]
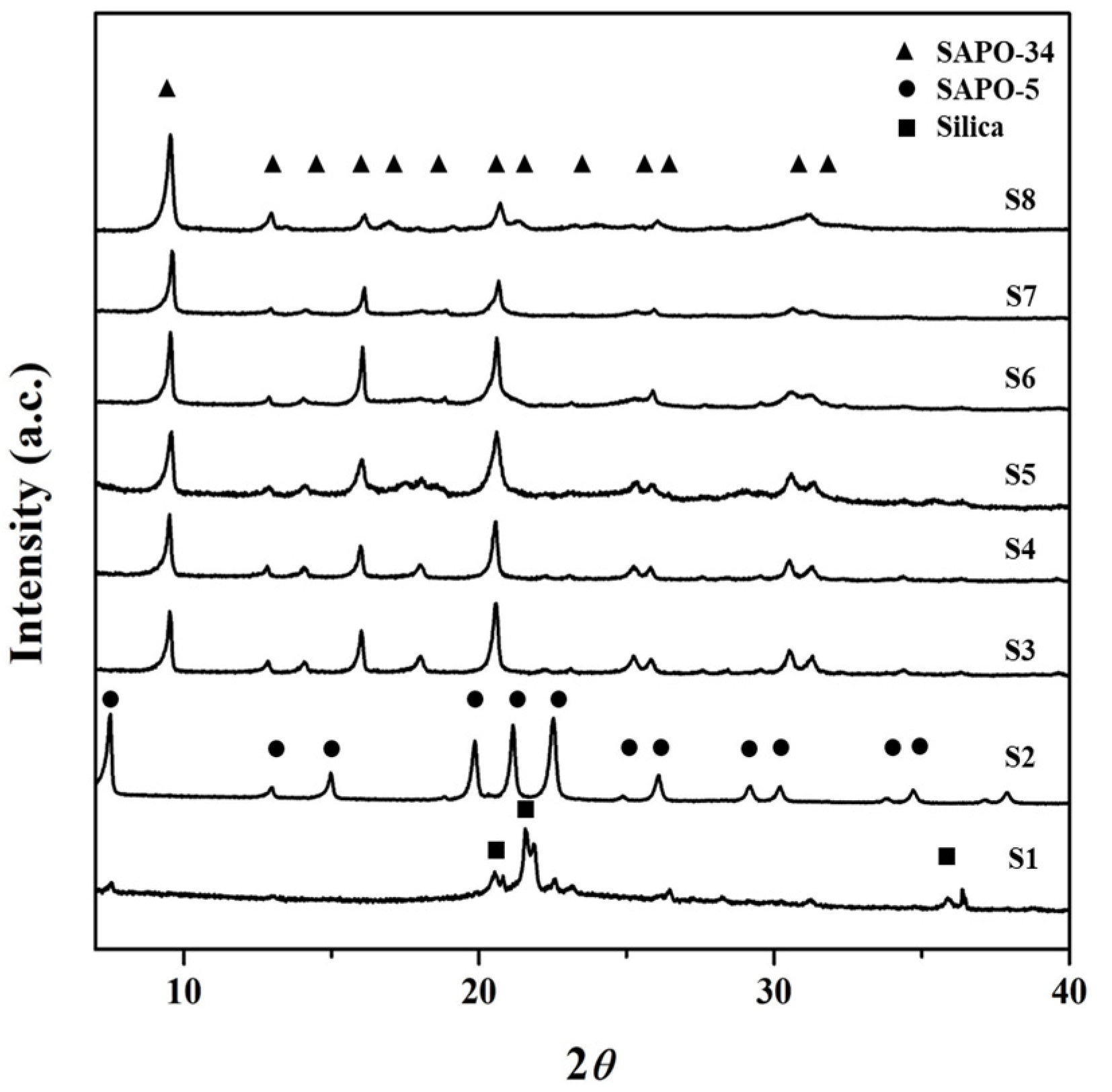

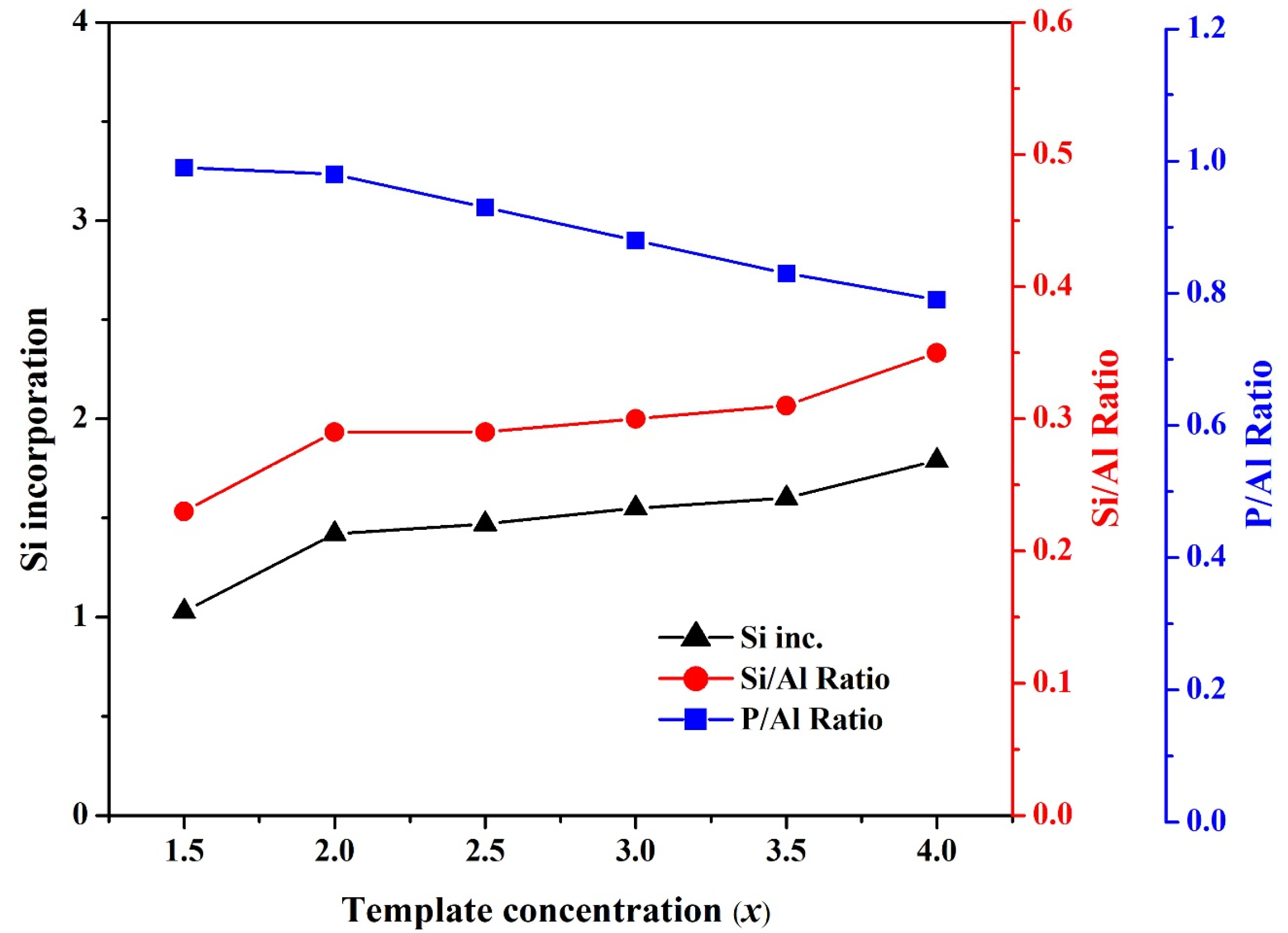
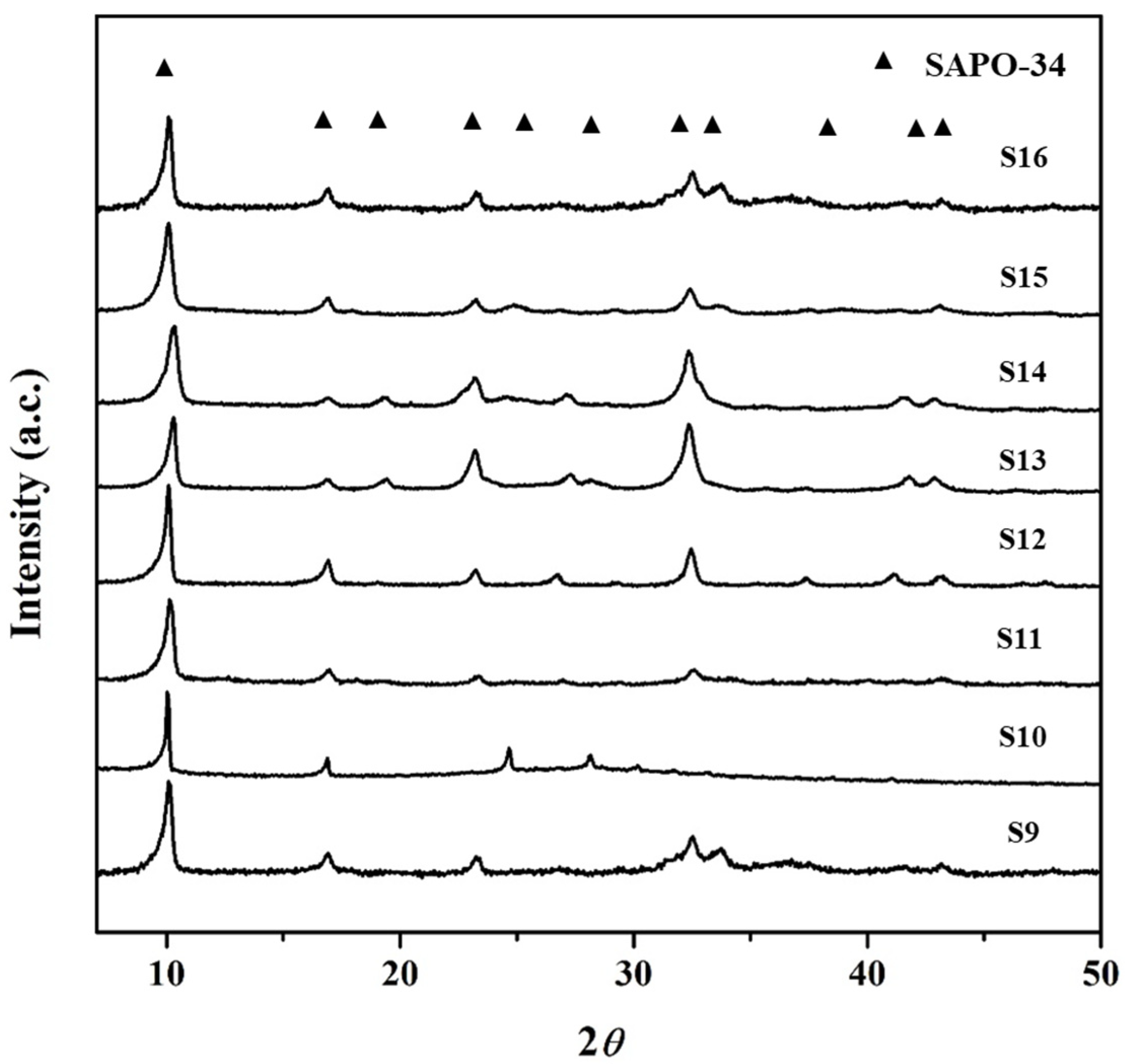
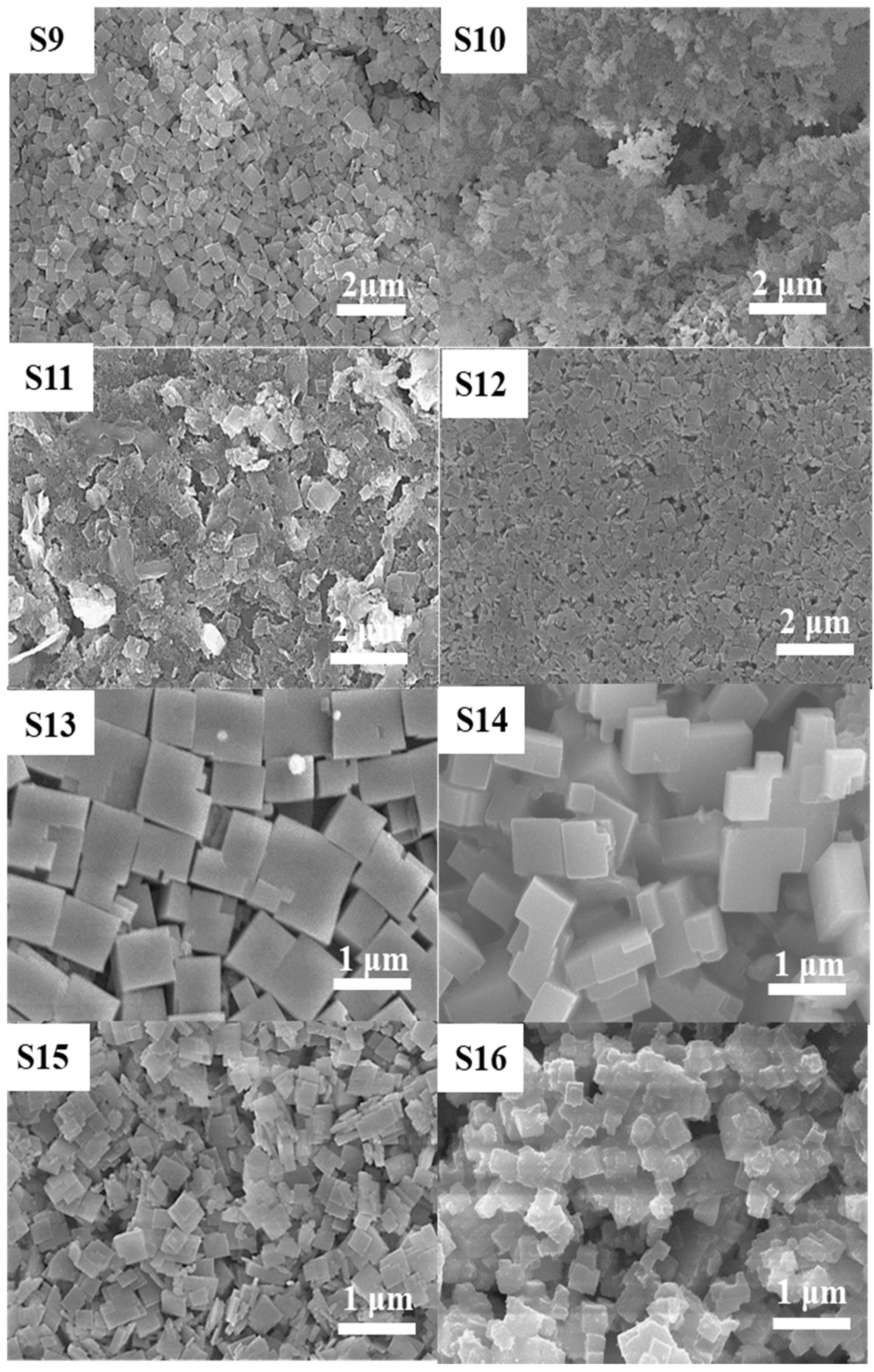


| Gel Composition (1Al2O3:xTEAOH:yP2O5:zSiO2:100H2O) | Time for Phosphoric Acid Addition | Synthesis | ||||
|---|---|---|---|---|---|---|
| x | y | z | min | T (K) | t (h) | |
| S1 | 0.0 | 2.0 | 0.6 | 30 | 453 | 7 |
| S2 | 1.0 | 2.0 | 0.6 | 30 | 453 | 7 |
| S3 | 1.5 | 2.0 | 0.6 | 30 | 453 | 7 |
| S4 | 2.0 | 2.0 | 0.6 | 30 | 453 | 7 |
| S5 | 2.5 | 2.0 | 0.6 | 30 | 453 | 7 |
| S6 | 3.0 | 2.0 | 0.6 | 30 | 453 | 7 |
| S7 | 3.5 | 2.0 | 0.6 | 30 | 453 | 7 |
| S8 | 4.0 | 2.0 | 0.6 | 30 | 453 | 7 |
| S9 | 3.5 | 1.5 | 0.6 | 30 | 453 | 7 |
| S10 | 3.5 | 1.0 | 0.6 | 30 | 453 | 7 |
| S11 | 3.5 | 1.0 | 0.6 | 30 | 453 | 12 |
| S12 | 3.5 | 1.0 | 0.6 | 30 | 453 | 24 |
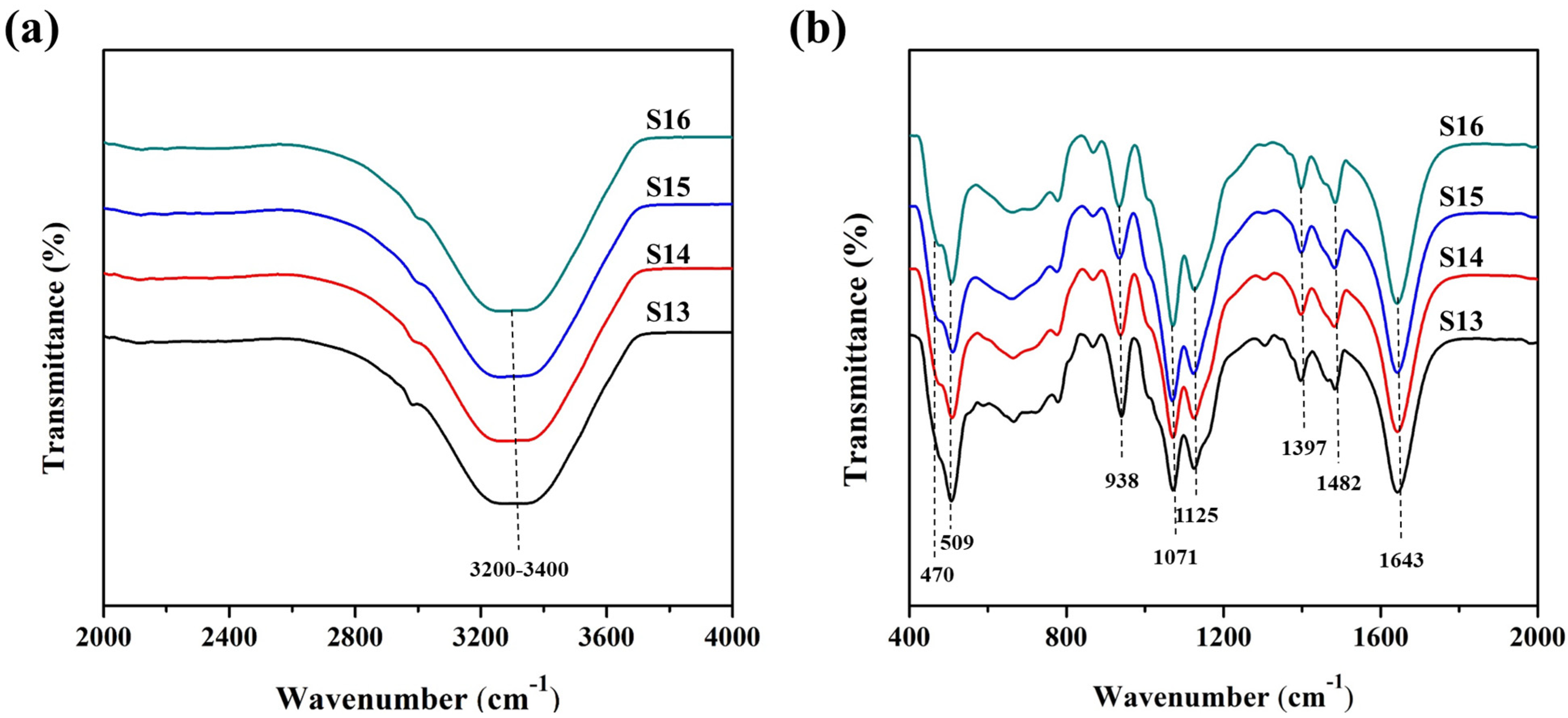
| S13 | 4.0 | 2.0 | 0.4 | 0 | 453 | 7 |
| S14 | 4.0 | 2.0 | 0.4 | 15 | 453 | 7 |
| S15 | 4.0 | 2.0 | 0.4 | 30 | 453 | 7 |
| S16 | 4.0 | 2.0 | 0.4 | 60 | 453 | 7 |
| S17 | 4.0 | 2.0 | 0.5 | 30 | 453 | 7 |
| Phase | Morphology | Particle Size (µm) | |
|---|---|---|---|
| S1 | Mixed Phase | Irregular aggregates | - |
| S2 | SAPO-5 | Hexagon | 3.91 |
| S3 | SAPO-34 | Cubic | 2.24 |
| S4 | SAPO-34 | Hyper rectangle | 1.92 |
| S5 | SAPO-34 | Nanoplate | 0.89 |
| S6 | SAPO-34 | Nanoplate | 0.22 |
| S7 | SAPO-34 | Nanoplate | 0.16 |
| S8 | SAPO-34 | Nanoplate | 0.13 |
| S9 | SAPO-34 | Nanoplate | 0.13 |
| S10 | Amorphous + SAPO-34 | Amorphous and nanoplate | - |
| S11 | SAPO-34 | Nanoplate | 0.18 |
| S12 | SAPO-34 | Nanoplate | 0.19 |
| S13 | SAPO-34 | Cubic | 1.10 |
| S14 | SAPO-34 | Hyper rectangle | 0.79 |
| S15 | SAPO-34 | Nanoplate | 0.33 |
| S16 | SAPO-34 | Nanoparticles | 0.25 |
| S17 | SAPO-34 | Nanoplate | 0.24 |
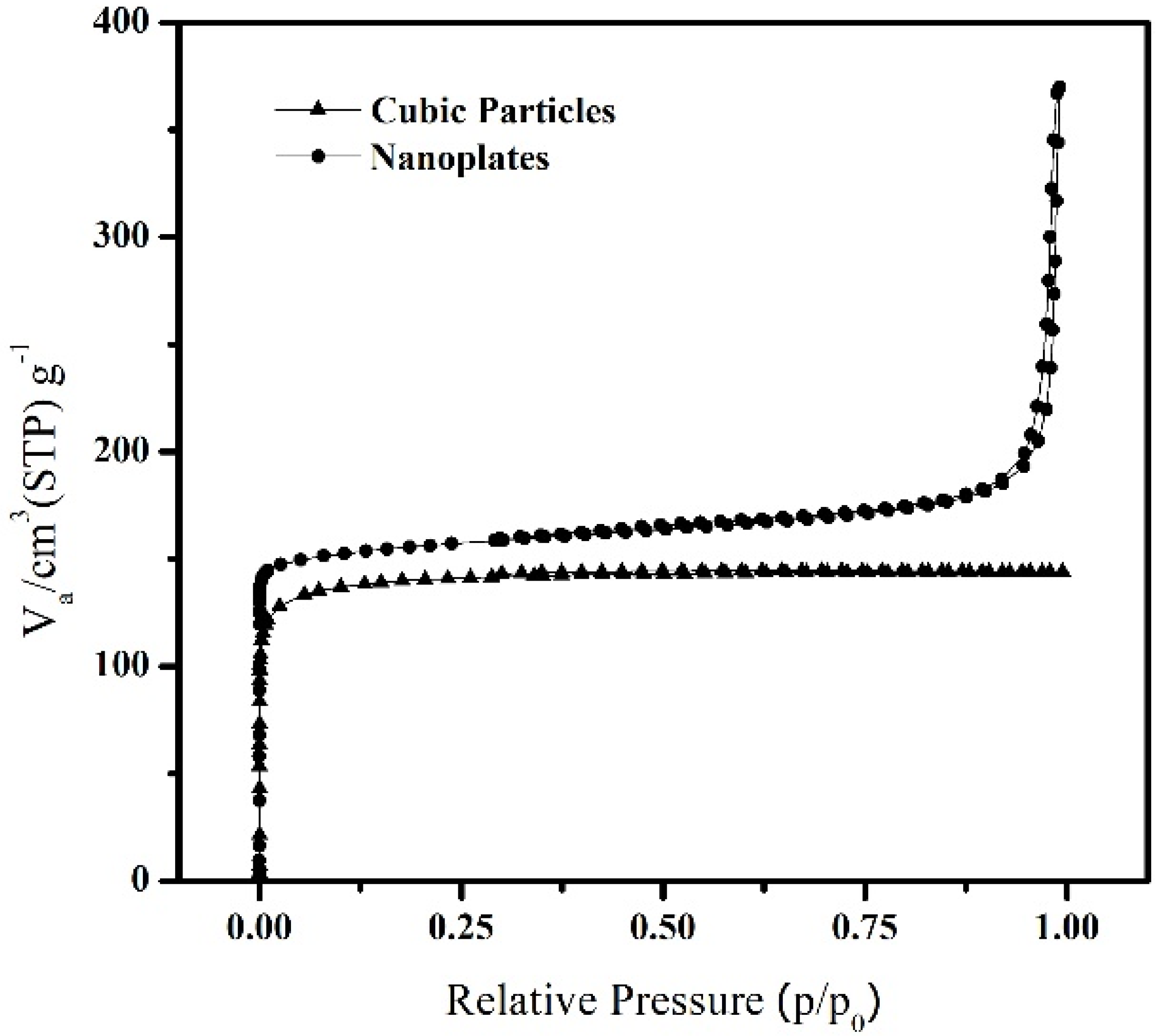
| SBET (m2 g−1) | Vm (cm3 (STP) g−1) | Total Pore Volume (cm3 g−1) | |
|---|---|---|---|
| Cubic | 471.59 | 108.35 | 0.2227 |
| Nanoplates | 601.67 | 138.24 | 0.2935 |
| Acid Sites | |||||
|---|---|---|---|---|---|
| A mmol/g | B mmol/g | Total Acid Sites (mmol/g) | T1 (K) | T2 (K) | |
| S5 | 2.197 | 0.929 | 3.126 | 432.5 | 669.2 |
| S7 | 1.666 | 1.541 | 3.207 | 445.8 | 675.6 |
| S8 | 2.276 | 1.206 | 3.482 | 452.5 | 683.1 |
| S13 | 1.061 | 1.155 | 2.216 | 427.1 | 657.5 |
| S15 | 1.611 | 0.675 | 2.286 | 445.8 | 675.6 |
| S16 | 1.491 | 0.838 | 2.329 | 430.8 | 675.9 |
| S17 | 1.671 | 1.398 | 3.069 | 449.4 | 672.8 |
| Author | Gel Composition | Morphology | Si/Al * | P/Al * | Density of Acid Sites (mmol/g) | Size (µm) | Ref. |
|---|---|---|---|---|---|---|---|
| Doan et al. 2019 | 1.0Al2O3:0.6SiO2:1.0P2O5:3TEAOH:110H2O | Cubic | 0.32 | 0.69 | 2.25 | 2.5 | [29] |
| Sedighi et al. 2017 | 1.0Al2O3:1.0P2O5:0.4SiO2:0.9Mor:1.1TEAOH:60H2O | Cubic | 0.23 | 0.84 | 1.04 | 0.5 | [27] |
| Zhang et al. 2018 | 1.0Al2O3:0.6SiO2:1.0P2O5:1.0Mor:1.0TEA:1.0TEAOH:100H2O | Cubic | 0.29 | 0.75 | 2.06 | 1–2 | [49] |
| Wang et al. 2019 | 1.0Al2O3:1.2P2O5:2.0TEAOH:0.6SiO2:40H2O | Cubic | 0.35 | 0.87 | 3.15 | 0.7 | [50] |
| Soltani et al. 2019 | Al2O3:0.6SiO2:0.5TEAOH:1.5Mor: P2O5:60H2O | Cubic | 3.26 | 4.8 | [51] | ||
| Wang et al. 2015 | 1.0Al2O3:1.0P2O5:0.5SiO2:2.3DEA:150H2O | Aggregates | 0.29 | 0.74 | 2.90 | 30 | [52] |
| Sun et al. 2019 | 7.0TEA:1.0Al2O3:1.0P2O5:0.3SiO2:15.7H2O | Hierarchical | 0.21 | 0.80 | 0.90 | 2.5 | [22] |
| Yang et al. 2013 | 1.0Al2O3:2.0P2O5:4.0TEAOH:0.6SiO2:140H2O | Sheet | 0.12 | 0.92 | 0.3 | [8] | |
| Gong et al. 2020 | 1.0Al2O3:1.0P2O5:1.1SiO2:1.9TEAOH:72H2O | Low crystalline sheets | 0.49 | 0.84 | 0.26 | 0.2–0.6 | [53] |
| Our study | 0.5Al2O3:1.0P2O5:0.3SiO2:2.0TEAOH:50H2O | Sheets | 0.35 | 0.79 | 3.48 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.F.; Kim, M.-Z.; Rehman, A.u.; Arepalli, D.; Sharma, P.; Cho, C.H. Synthesis of SAPO-34 Nanoplates with High Si/Al Ratio and Improved Acid Site Density. Nanomaterials 2021, 11, 3198. https://doi.org/10.3390/nano11123198
Alam SF, Kim M-Z, Rehman Au, Arepalli D, Sharma P, Cho CH. Synthesis of SAPO-34 Nanoplates with High Si/Al Ratio and Improved Acid Site Density. Nanomaterials. 2021; 11(12):3198. https://doi.org/10.3390/nano11123198
Chicago/Turabian StyleAlam, Syed Fakhar, Min-Zy Kim, Aafaq ur Rehman, Devipriyanka Arepalli, Pankaj Sharma, and Churl Hee Cho. 2021. "Synthesis of SAPO-34 Nanoplates with High Si/Al Ratio and Improved Acid Site Density" Nanomaterials 11, no. 12: 3198. https://doi.org/10.3390/nano11123198







