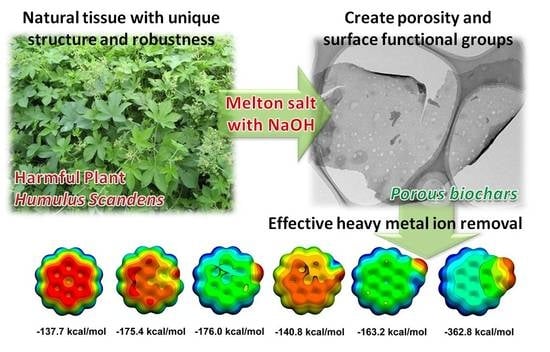
Humulus scandens-Derived Biochars for the Effective Removal of Heavy Metal Ions: Isotherm/Kinetic Study, Column Adsorption and Mechanism Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Biochar
2.3. Characterization of the Biochar
2.4. Heavy Metal Sorption Experiments
2.4.1. Adsorption Experiment
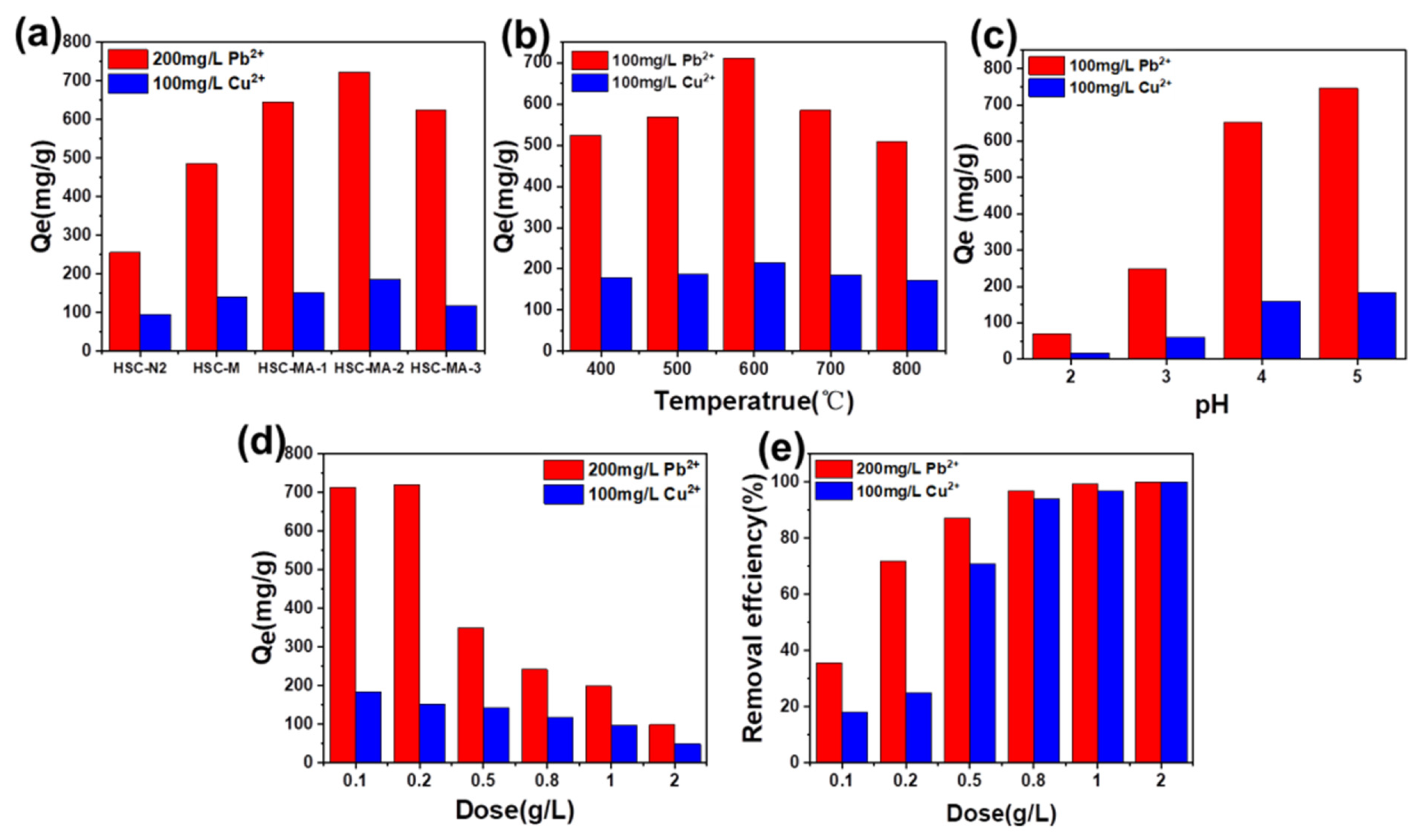
2.4.2. Influence of Initial pH Value
2.4.3. Influence of Adsorbent Dosage
2.4.4. Adsorption Kinetics and Isothermal
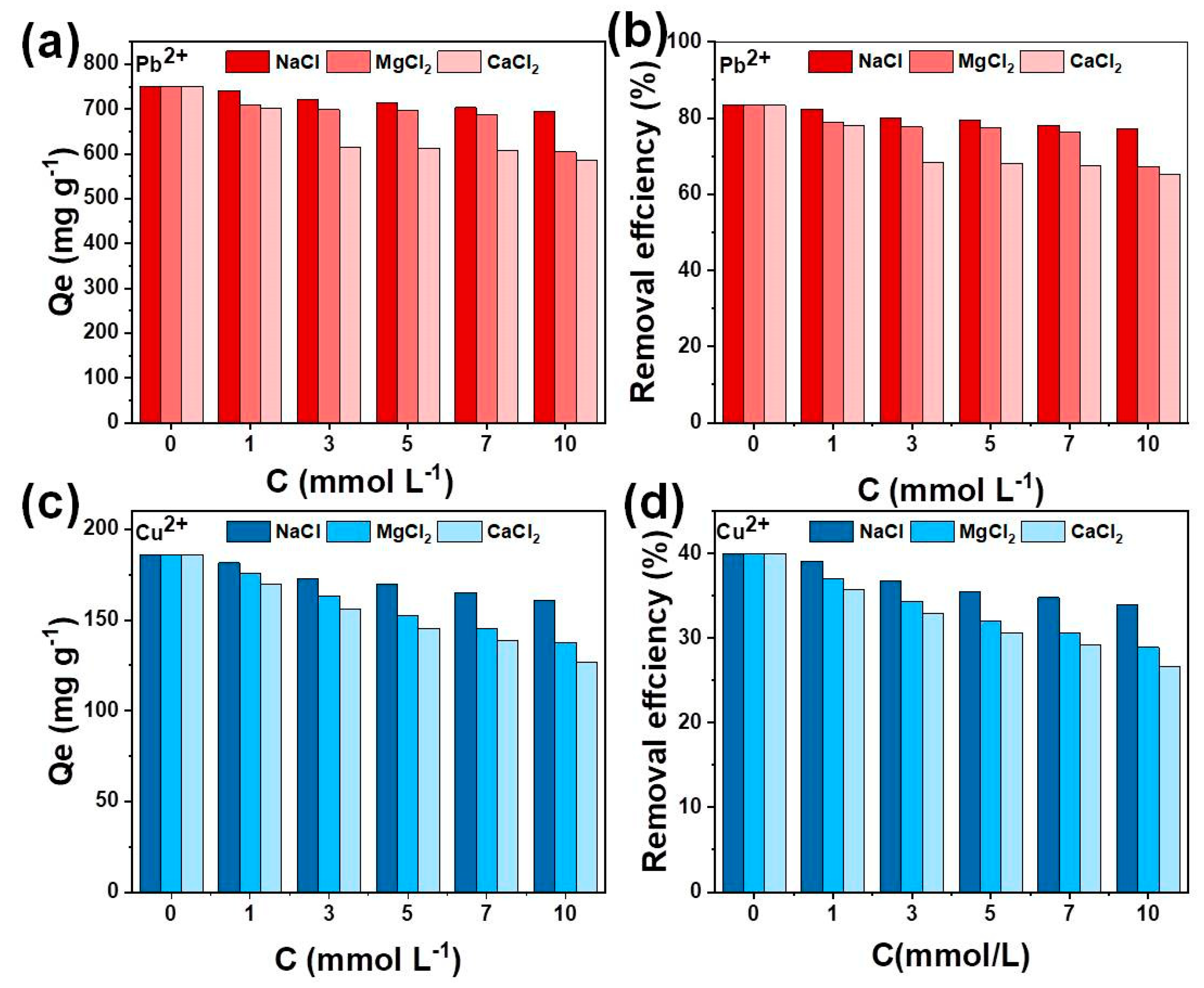
2.5. The Effect of Background Ions
2.6. Multi-Metal Adsorption
2.7. Column Sorption
2.8. Gaussian Computations
3. Results and Discussion
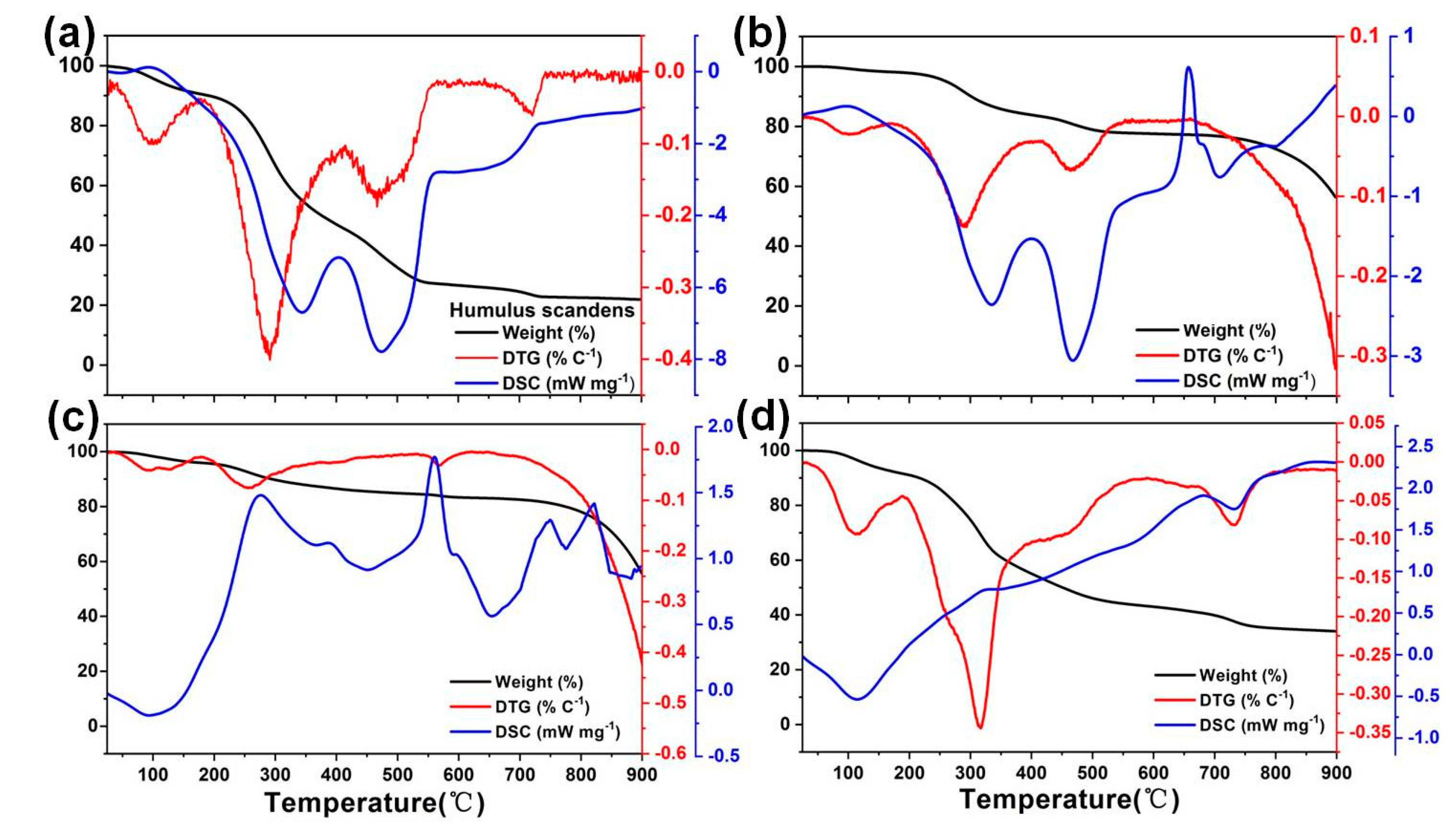
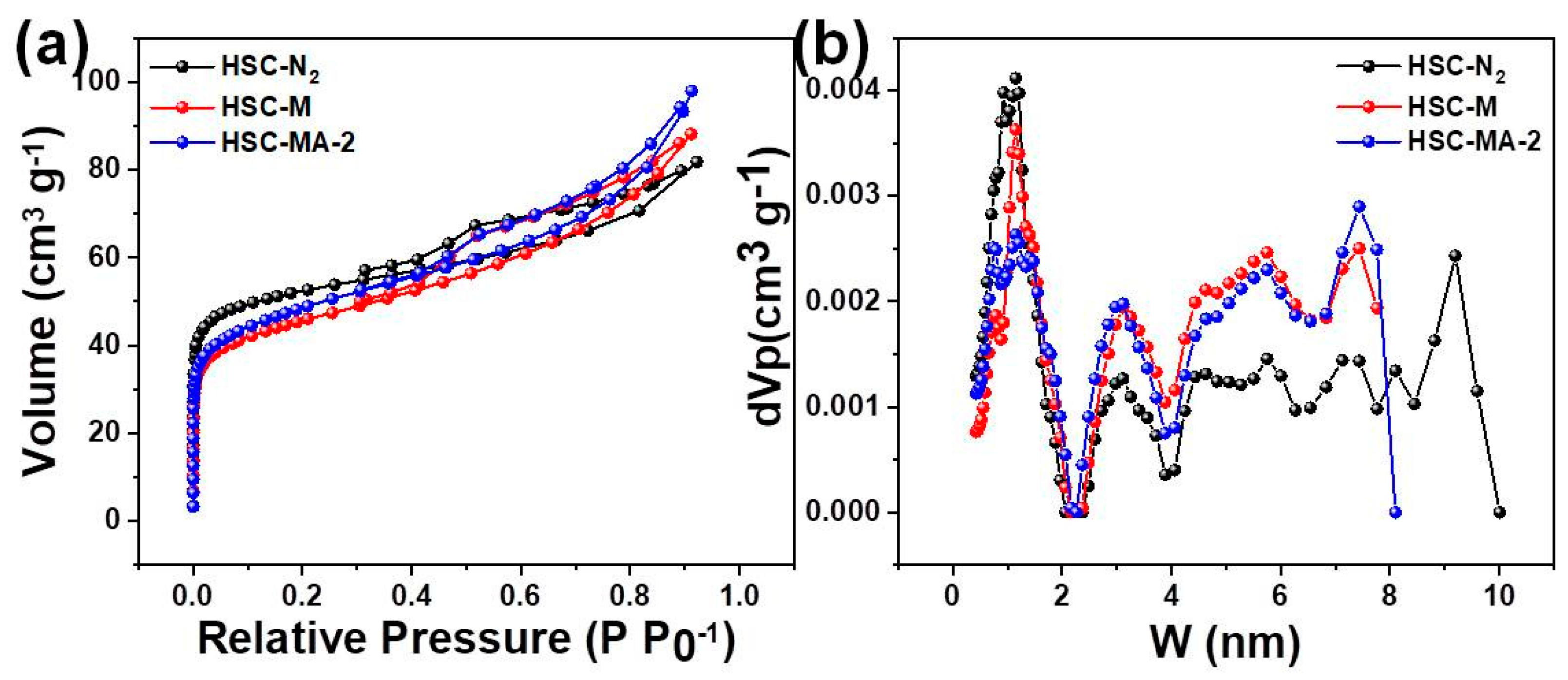
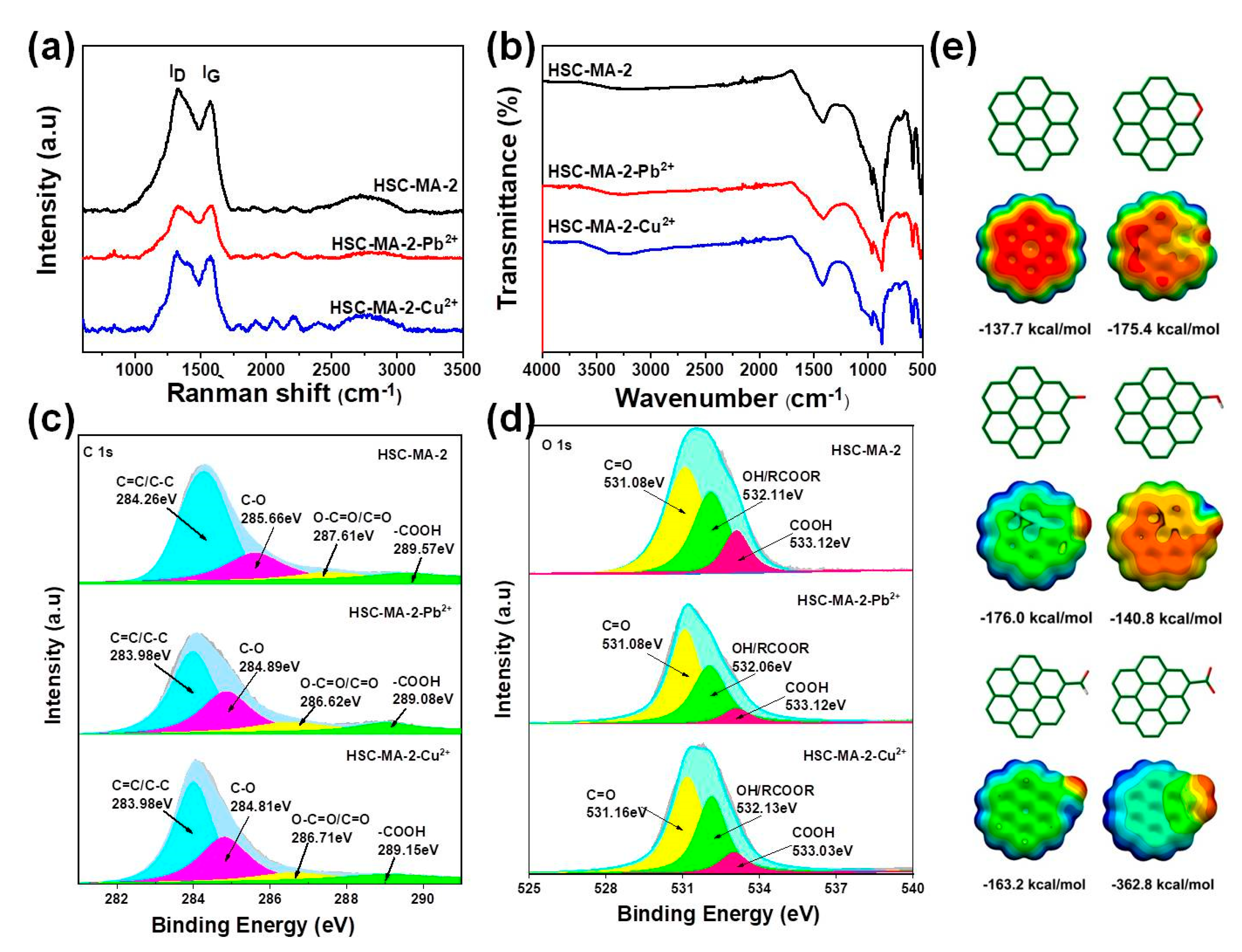
3.1. Material Characterization
Surface Area and Pore Structure
3.2. Adsorption Tests
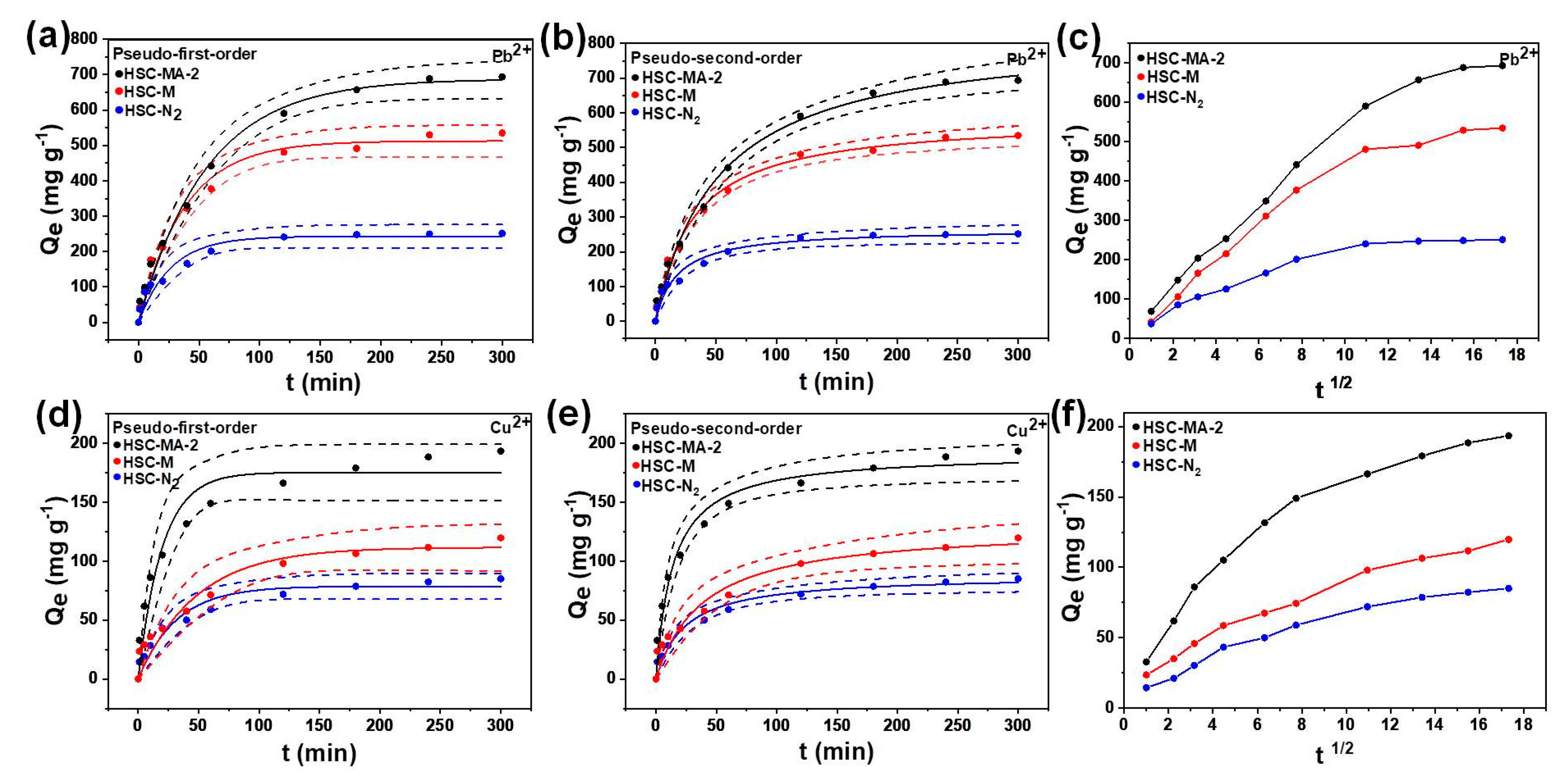
3.2.1. Adsorption Kinetics
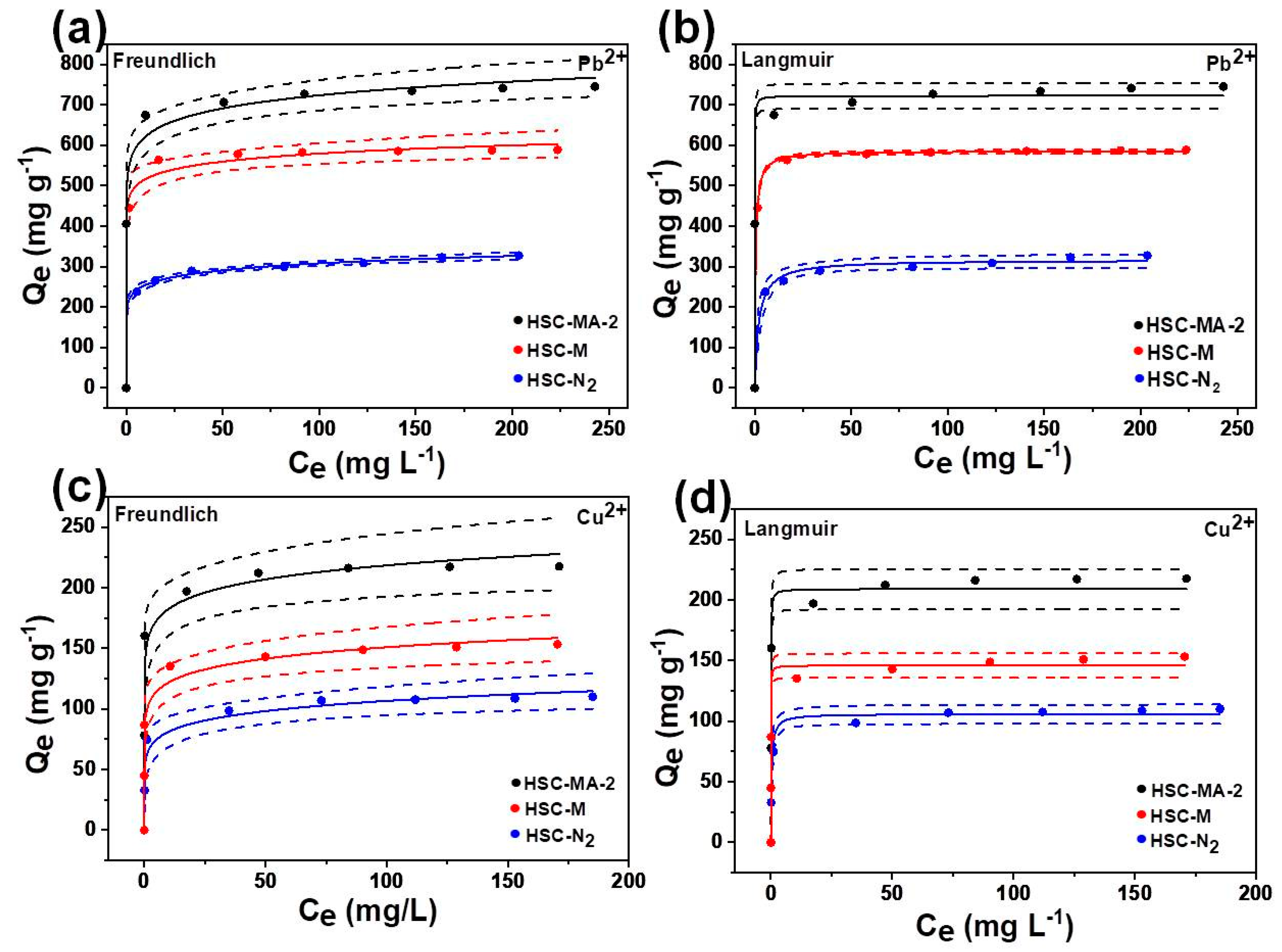
3.2.2. Adsorption Isotherm
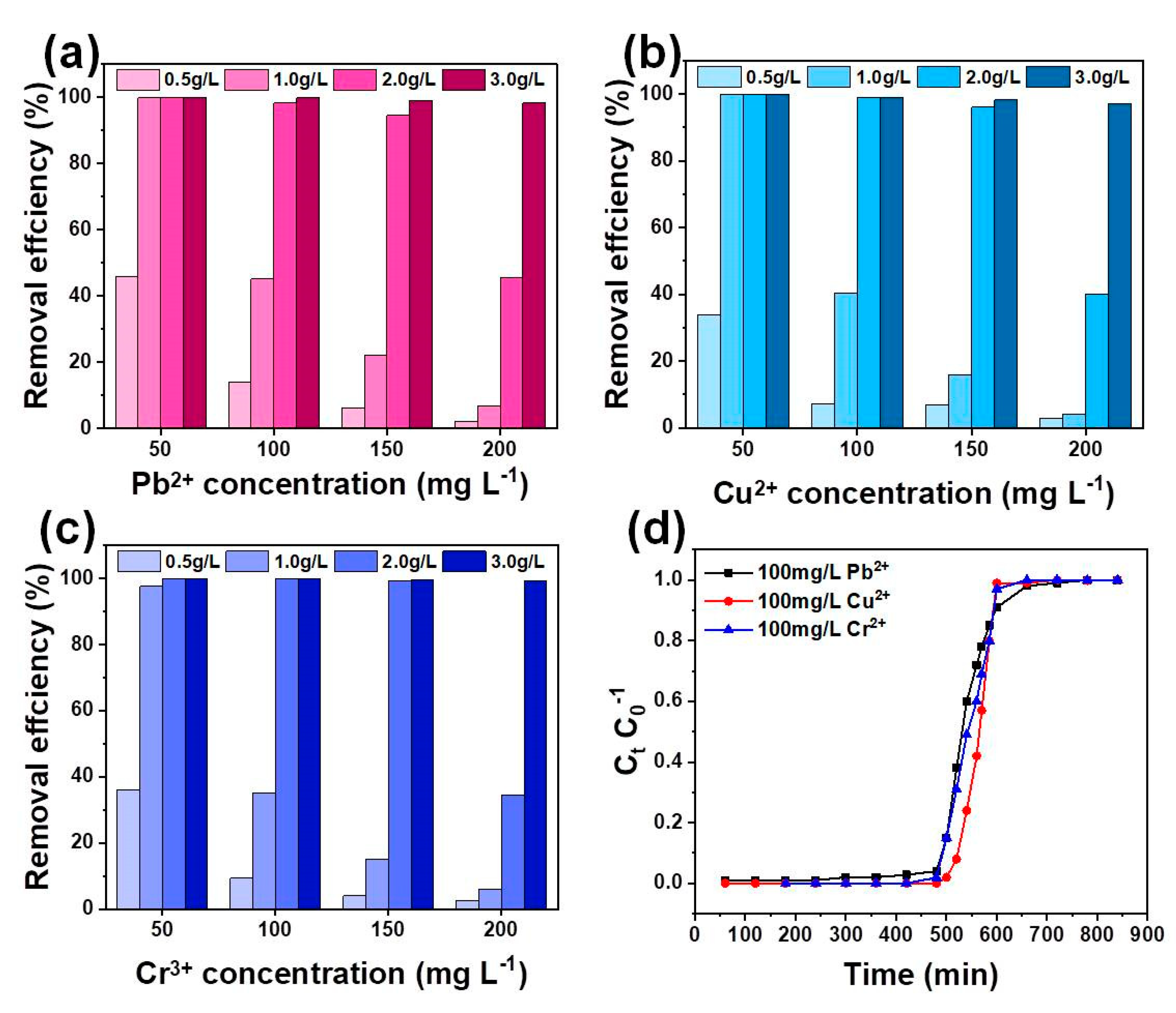
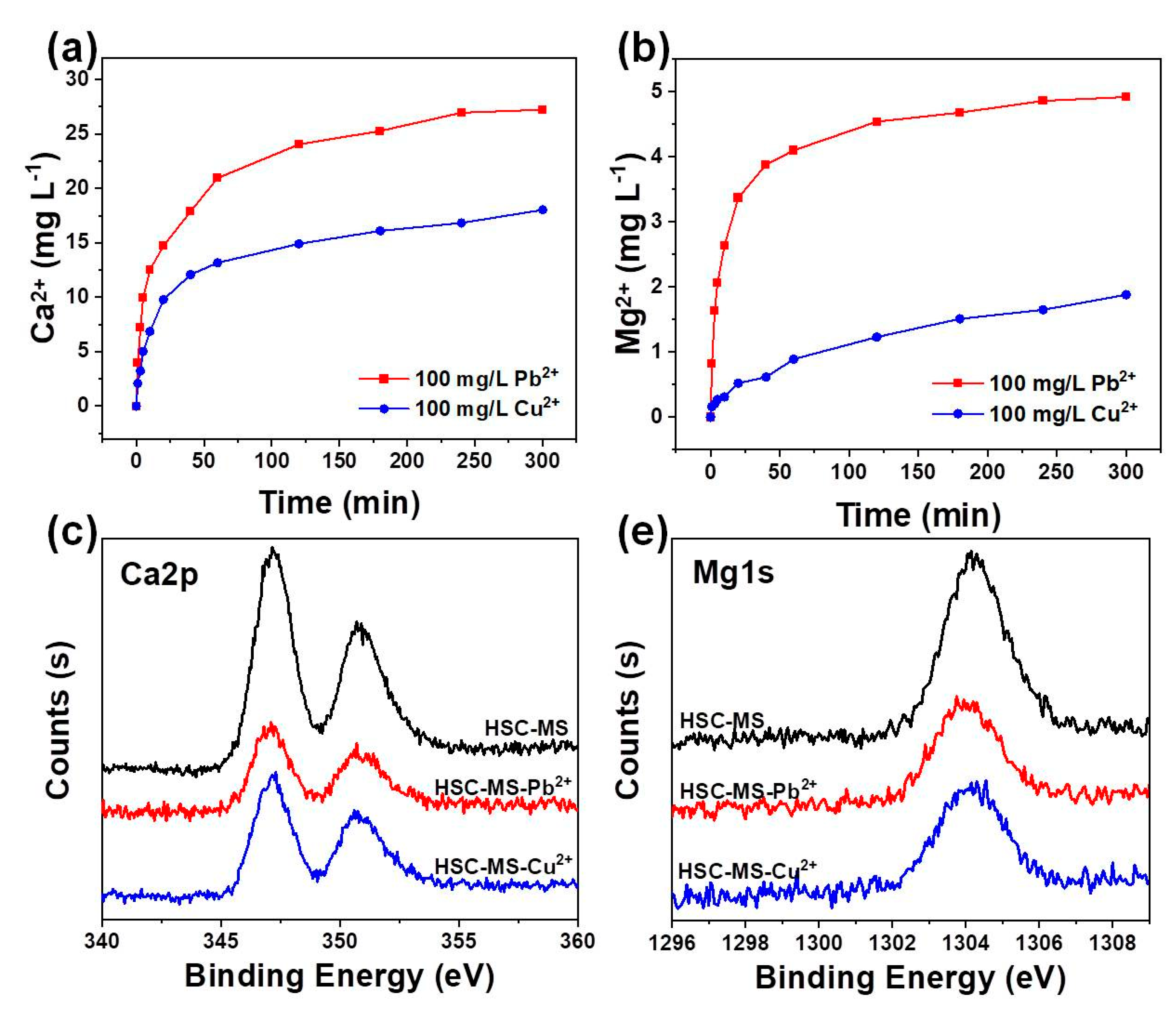
3.2.3. Effects of Competition Ions
3.2.4. Multi-Metal Adsorption and Column Test
3.3. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Chen, S.; Zhang, H.; Wang, X. Removal behaviors and mechanisms of hexavalent chromiun from aqueous solution by cephalosporin residue and derived chars. Bioresour. Technol. 2017, 238, 484–491. [Google Scholar] [CrossRef]
- Liu, P.; Rao, D.; Zou, L.; Teng, Y.; Yu, H. Capacity and potential mechanisms of Cd(II) adsorption from aqueous solution by blue algae-derived biochars. Sci. Total Environ. 2021, 767, 145447. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Biochar-supported nanomaterials for environmental applications. J. Ind. Eng. Chem. 2019, 78, 21–33. [Google Scholar] [CrossRef]
- Meseldžija, S.; Petrovic, J.; Onjia, A.; Volkov-Husovic, T.; Nesic, A.; Vukelic, N. Utilization of agro-industrial waste for removal of copper ions from aqueous solutions and mining-wastewater. J. Ind. Eng. Chem. 2019, 75, 246–252. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Hong, N.; Cheng, Q.; Goonetilleke, A.; Bandala, E.R.; Liu, A. Assessing the effect of surface hydrophobicity/hydrophilicity on pollutant leaching potential of biochar in water treatment. J. Ind. Eng. Chem. 2020, 89, 222–232. [Google Scholar] [CrossRef]
- Hopkins, D.; Hawboldt, K. Biochar for the removal of metals from solution: A review of lignocellulosic and novel marine feedstocks. J. Environ. Chem. Eng. 2020, 8, 103975. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Adi, V.S.K.; Huang, H.-L.; Lin, H.-P.; Huang, Z.-H. Adsorption of metal ions with biochars derived from biomass wastes in a fixed column: Adsorption isotherm and process simulation. J. Ind. Eng. Chem. 2019, 76, 240–244. [Google Scholar] [CrossRef]
- Zhang, L.X.; Tang, S.Y.; He, Y.; Liu, Y.; Mao, W.; Guan, Y.T. Highly efficient and selective capture of heavy metals by poly(acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Chem. Eng. J. 2019, 378, 122215. [Google Scholar] [CrossRef]
- Wang, J.; Lei, S.; Liang, L. Preparation of porous activated carbon from semi-coke by high temperature activation with KOH for the high-efficiency adsorption of aqueous tetracycline. Appl. Surf. Sci. 2020, 530, 147187. [Google Scholar] [CrossRef]
- Takdastan, A.; Samarbaf, S.; Tahmasebi, Y.; Alavi, N.; Babaei, A.A. Alkali modified oak waste residues as a cost-effective adsorbent for enhanced removal of cadmium from water: Isotherm, kinetic, thermodynamic and artificial neural network modeling. J. Ind. Eng. Chem. 2019, 78, 352–363. [Google Scholar] [CrossRef]
- Jia, X.X.; Zhang, Y.; He, Z.; Chang, F.; Zhang, H.; Wågberg, T.; Hu, G. Mesopore-rich badam-shell biochar for efficient adsorption of Cr(VI) from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105634. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, H.J.; Kim, H.W.; Lim, H. Iron-impregnated spent coffee ground biochar for enhanced degradation of methylene blue during cold plasma application. J. Ind. Eng. Chem. 2021, 98, 383–388. [Google Scholar] [CrossRef]
- Kim, B.-S.; Lee, H.W.; Park, S.H.; Baek, K.; Jeon, J.-K.; Cho, H.J.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. Removal of Cu2+ by biochars derived from green macroalgae. Environ. Sci. Pollut. Res. 2015, 23, 985–994. [Google Scholar] [CrossRef]
- Lang, J.; Matejova, L.; Cuentas-Gallegos, A.K.; Lobato-Peralta, D.R.; Ainassaari, K.; Gomez, M.M.; Solis, J.L.; Mondal, D.; Keiski, R.L.; Cruz, G.J.F. Evaluation and selection of biochars and hydrochars derived from agricultural wastes for the use as adsorbent and energy storage materials. J. Environ. Chem. Eng. 2021, 9, 105979. [Google Scholar] [CrossRef]
- Wang, S.W.; Zhong, S.; Zheng, X.Y.; Xiao, D.; Zheng, L.L.; Yang, Y.; Zhang, H.D.; Ai, B.L.; Sheng, Z.W. Calcite modification of agricultural waste biochar highly improves the adsorption of Cu(II) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106215. [Google Scholar] [CrossRef]
- Leng, L.J.; Xiong, Q.; Yang, L.H.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2020, 763, 144204. [Google Scholar] [CrossRef]
- Cho, H.J.; Baek, K.; Jeon, J.-K.; Park, S.H.; Suh, D.J.; Park, Y.-K. Removal characteristics of copper by marine macro-algae-derived chars. Chem. Eng. J. 2013, 217, 205–211. [Google Scholar] [CrossRef]
- Güzel, F.; Cumali, Y. Synthesis, characterization, and lead (II) sorption performance of a new magnetic separable composite: MnFe2O4@ wild plants-derived biochar. J. Environ. Chem. Eng. 2021, 9, 104567. [Google Scholar]
- Zhao, H.-T.; Ma, S.; Zheng, S.-Y.; Han, S.-W.; Yao, F.-X.; Wang, X.-Z.; Wang, S.-S.; Feng, K. β–cyclodextrin functionalized biochars as novel sorbents for high-performance of Pb2+ removal. J. Hazard. Mater. 2018, 362, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Dai, D.; Hou, J.; Wang, S.; Wu, X.; Wang, X. Hierarchical porous biochar-based functional materials derived from biowaste for Pb(II) removal. Appl. Surf. Sci. 2018, 465, 297–302. [Google Scholar] [CrossRef]
- Cruz, G.J.F.; Mondal, D.; Rimaycuna, J.; Soukup, K.; Gómez, M.M.; Solis, J.L.; Lang, J. Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J. Environ. Chem. Eng. 2020, 8, 103800. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, T.; Liu, Y.; Yan, L.; Wei, Q.; Du, B.; Xu, W. EDTA functionalized magnetic biochar for Pb(II) removal: Adsorption performance, mechanism and SVM model prediction. Sep. Purif. Technol. 2019, 227, 115696. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Wang, X.; Yang, X.; Lv, Y. Key factors and microscopic mechanisms controlling adsorption of cadmium by surface oxidized and aminated biochars. J. Hazard. Mater. 2019, 382, 121002. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jin, M.; Ye, L.; Cao, Y.; Yan, Y.; Du, R.; Yoshiie, R.; Ueki, Y.; Naruse, I.; Lin, C.; et al. Removal of gaseous elemental mercury by hydrogen chloride non-thermal plasma modified biochar. J. Hazard. Mater. 2019, 377, 132–141. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Dosche, C. Cu(II) adsorption on 2-thiouracil-modified Luffa cylindrica biochar fibres from artificial and real samples, and competition reactions with U(VI). J. Hazard. Mater. 2020, 383, 120950. [Google Scholar] [CrossRef]
- Li, J.; Tian, L.; Liang, F.; Wang, J.; Han, L.; Zhang, J.; Ge, S.; Dong, L.; Zhang, H.; Zhang, S. Molten salt synthesis of hierarchical porous N-doped carbon submicrospheres for multifunctional applications: High performance supercapacitor, dye removal and CO2 capture. Carbon 2018, 141, 739–747. [Google Scholar] [CrossRef]
- Li, K.; Tang, D.; Zhang, W.; Qiao, Z.; Liu, Y.; Huo, Q.; Liang, D.; Zhu, J.; Zhao, Z. Molten salt synthesis of Co-entrapped, N-doped porous carbon as efficient hydrogen evolving electrocatalysts. Mater. Lett. 2017, 209, 256–259. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. A green and scalable route to yield porous carbon sheets from biomass for supercapacitors with high capacity. J. Mater. Chem. A 2017, 6, 1244–1254. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Wang, J.; Li, S.; Zhang, H.; Zhang, S. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Chen, J.; Li, H.; Cao, R.; Zhang, W.; Gao, Z.; Jiang, K. Promoting sulphur conversion chemistry with tri-modal porous N, O-codoped carbon for stable Li–S batteries. J. Mater. Chem. A 2021, 9, 5497–5506. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, H.; Niu, B.; Zhang, W.; Wu, D. Synergistic mediation of polysulfide immobilization and conversion by a catalytic and dual-adsorptive system for high performance lithium-sulfur batteries. Chem. Eng. J. 2020, 406, 126802. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total. Environ. 2020, 744, 140653. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhu, H.; Zhang, C. Enhanced heavy metals sorption by modified biochars derived from pig manure. Sci. Total. Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Kılıç, M.; Kırbıyık, C.; Çepelioğullar, Ö.; Pütün, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Liu, Y.; Guo, J.; Ren, J.; Zhou, F. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water. Fuel 2018, 233, 469–479. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J. Environ. Chem. Eng. 2018, 6, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, W.; Cao, H.; Hu, D.; Chen, X.; Guan, Y.; Tang, J.; Gao, H. Ultra-efficient sorption of Cu2+ and Pb2+ ions by light biochar derived from Medulla tetrapanacis. Bioresour. Technol. 2019, 291, 121818. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Song, S.; Han, J.; Chen, H.; Wang, X.; Sun, R.; Cheng, J. Adsorption of copper(II) and lead(II) from seawater using hydrothermal biochar derived from Enteromorpha. Mar. Pollut. Bull. 2019, 149, 110586. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, W.; Yang, X.; Bao, Y. Modification of biochar with silicon by one-step sintering and understanding of adsorption mechanism on copper ions. Sci. Total. Environ. 2019, 704, 135252. [Google Scholar] [CrossRef] [PubMed]
- Dinh Viet, C.; Liu, N.-L.; Viet Anh, N.; Hou, C.-H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar]
- Abdelhafez, A.A.; Li, J. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(ii) and Pb(ii) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D.; Seo, D.-C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.; Shen, D.; Shen, F.; Wu, C.; Xiao, R. Low cost earthworm manure-derived carbon material for the adsorption of Cu 2+ from aqueous solution: Impact of pyrolysis temperature. Ecol. Eng. 2016, 98, 189–195. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.-S.; Tsang, D.C. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]

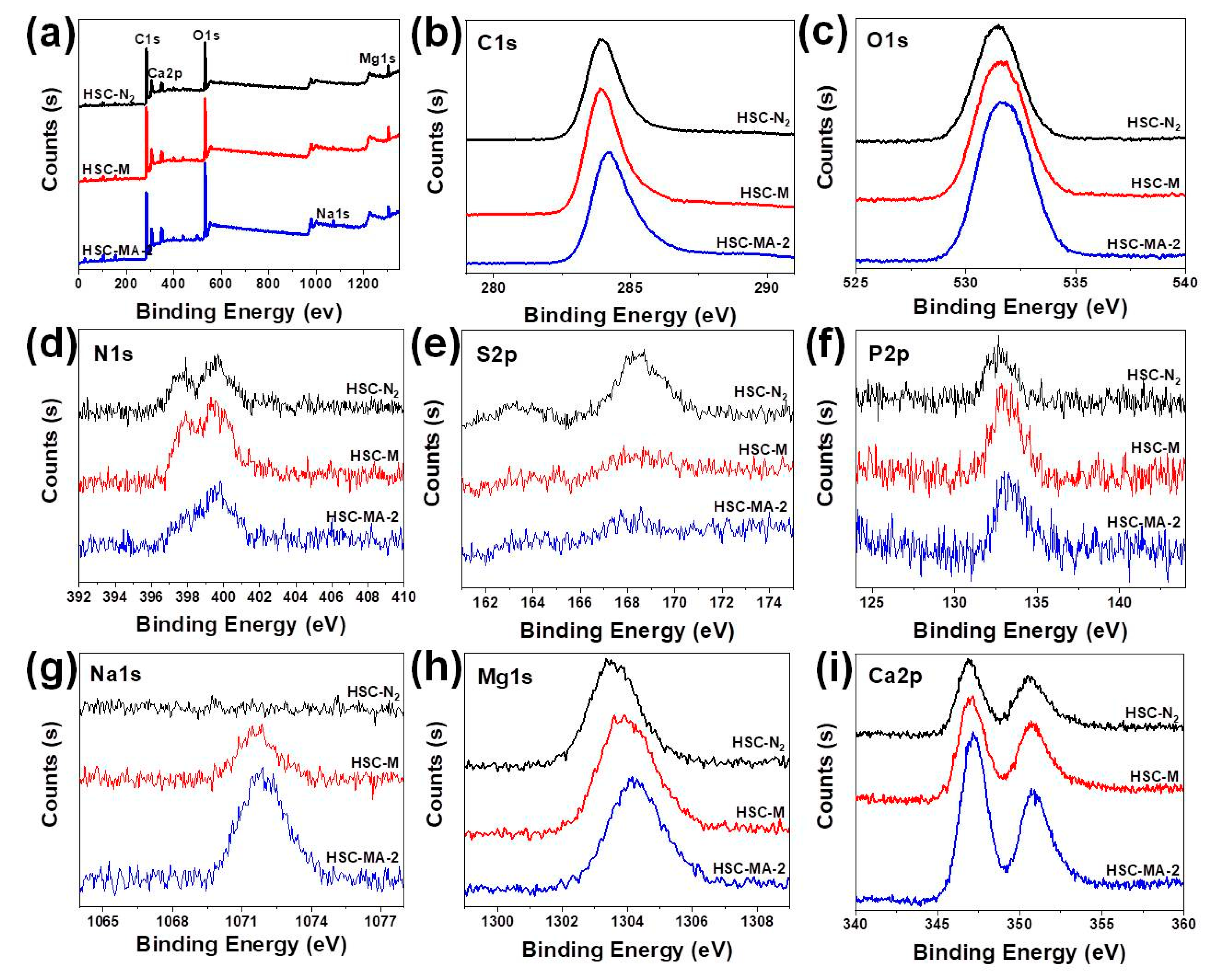

| Element | C (%) | O (%) | N (%) | P (%) | S (%) | Na (%) | Mg(%) | Ca (%) |
|---|---|---|---|---|---|---|---|---|
| HSC-N2 | 60.23 | 24.53 | 3.69 | 0.79 | 1.28 | 0 | 2.82 | 3.28 |
| HSC-M | 60.67 | 24.45 | 3.65 | 1.12 | 0 | 0.71 | 2.93 | 3.36 |
| HSC-MA-2 | 54.48 | 28.75 | 2.02 | 1.38 | 0 | 1.63 | 5.25 | 5.22 |
| Element (mg/g) | Na23 | Mg24 | Ca43 |
|---|---|---|---|
| HS | 0.89 | 8.45 | 9.26 |
| HSC-N2 | 3.48 | 22.60 | 17.00 |
| HSC-M | 3.19 | 26.40 | 23.70 |
| HSC-MA-2 | 3.33 | 41.50 | 30.10 |
| Sample | SSA (m2/g) | Mean Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| HSC-N2 | 182.9 | 2.77 | 0.13 |
| HSC-M | 161.4 | 3.38 | 0.14 |
| HSC-MA-2 | 171.9 | 3.52 | 0.15 |
| Sample | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| R2 | Qe | K1 | R2 | Qe | K2 | ||
| Pb2+ | HSC-MS | 0.9870 | 512.5411 | 0.2627 | 0.9943 | 578.1483 | 0.0100 |
| HSC-MS-2 | 0.9940 | 687.8283 | 0.2798 | 0.9946 | 729.8656 | 0.0100 | |
| HSC-N2 | 0.9625 | 242.9751 | 0.1138 | 0.9757 | 266.1626 | 0.0100 | |
| Cu2+ | HSC-MS | 0.9525 | 111.7617 | 0.0208 | 0.9822 | 129.4631 | 0.0100 |
| HSC-MS-2 | 0.9609 | 161.0000 | 0.0655 | 0.9664 | 192.0009 | 0.0100 | |
| HSC-N2 | 0.9636 | 78.6980 | 0.0319 | 0.9819 | 88.1899 | 0.0100 | |
| Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| R2 | Qm | KL | R2 | Nf | Kf | ||
| Pb2+ | HSC-MS | 0.9999 | 594.4691 | 1.0000 | 0.9916 | 20.2338 | 460.9877 |
| HSC-MS-2 | 0.9932 | 748.0998 | 1.0000 | 0.9900 | 13.0424 | 502.6869 | |
| HSC-N2 | 0.9913 | 310.6050 | 0.4741 | 0.9984 | 12.0606 | 210.2611 | |
| Cu2+ | HSC-MS | 0.9814 | 155.0275 | 1.0000 | 0.9622 | 9.8353 | 96.2226 |
| HSC-MS-2 | 0.9879 | 221.0786 | 1.0000 | 0.9696 | 12.7665 | 152.2614 | |
| HSC-N2 | 0.9856 | 110.0282 | 1.0000 | 0.9656 | 8.4288 | 61.7383 | |
| Biomass | Target Ions | Qmax | SSA | References |
|---|---|---|---|---|
| soybean cake | Pb2+ | 133.60 mg/g | 32.7 m2/g | [22] |
| Shell | Pb2+ | 100.25 mg/g | 499.2 m2/g | [24] |
| Medulla tetrapanacis | Cu2+, Pb2+ | Cu2+ 458.72 mg/g Pb2+ 1031.23 mg/g | 246.85 m2/g | [39] |
| Enteromorpha | Cu2+, Pb2+ | Cu2+ 254 mg/g Pb2+ 98 mg/g | 29.7 m2/g | [40] |
| corn stalks | Cu2+ | 152.61 mg/g | 4.46 m2/g | [41] |
| Rice husks | Cu2+ | 265 mg/g | 2330 m2/g | [42] |
| Palm | Pb2+ | 118.08 mg/g | Not given | [21] |
| sugar cane | Pb2+ | 86.96 mg/g | 92.30 m2/g | [43] |
| water hyacinths | Pb2+ | 128.95 mg/g | 51.15 m2/g | [44] |
| Enteromorpha compressa | Cu2+ | 137 mg/g | 52 m2/g | [15] |
| sesame straw | Cu2+, Pb2+ | Cu2+ 55 mg/kg Pb2+ 102 mg/kg | Not given | [45] |
| fresh banana peels | Cu2+, Pb2+ | Cu2+ 24.27 mg/g Pb2+ 193 mg/g | 82.4 m2/g | [46,47] |
| corn stalk | Cu2, Pb2+ | Cu2+ 161.9 mg/g Pb2+ 195.1 mg/g | 603.4 m2/g | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Xing, L.; Liu, N.; Ma, N.; Huang, K.; Wu, D.; Yin, M.; Jiang, K. Humulus scandens-Derived Biochars for the Effective Removal of Heavy Metal Ions: Isotherm/Kinetic Study, Column Adsorption and Mechanism Investigation. Nanomaterials 2021, 11, 3255. https://doi.org/10.3390/nano11123255
Bai X, Xing L, Liu N, Ma N, Huang K, Wu D, Yin M, Jiang K. Humulus scandens-Derived Biochars for the Effective Removal of Heavy Metal Ions: Isotherm/Kinetic Study, Column Adsorption and Mechanism Investigation. Nanomaterials. 2021; 11(12):3255. https://doi.org/10.3390/nano11123255
Chicago/Turabian StyleBai, Xingang, Luyang Xing, Ning Liu, Nana Ma, Kexin Huang, Dapeng Wu, Mengmeng Yin, and Kai Jiang. 2021. "Humulus scandens-Derived Biochars for the Effective Removal of Heavy Metal Ions: Isotherm/Kinetic Study, Column Adsorption and Mechanism Investigation" Nanomaterials 11, no. 12: 3255. https://doi.org/10.3390/nano11123255