Homing Peptide-Based Targeting of Tenascin-C and Fibronectin in Endometriosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Phage Binding to Cultured 12Z and HESC Cells
2.4. Synthesis and Functionalization of AgNPs
2.5. Characterization of AgNPs
2.6. Expression Analysis of Peptide Receptors in Cultured 12Z and HESC Cells
2.7. Luminescence-Based Cellular Viability Assay
2.8. AgNP Interaction with Cells in 2D and 3D Cultures
2.9. Treatment of 12Z Spheroids with Cytotoxic AgNPs
2.10. Clinical Endometriosis Sample Collection and Immunostaining
2.11. Statistical Analysis
3. Results and Discussion
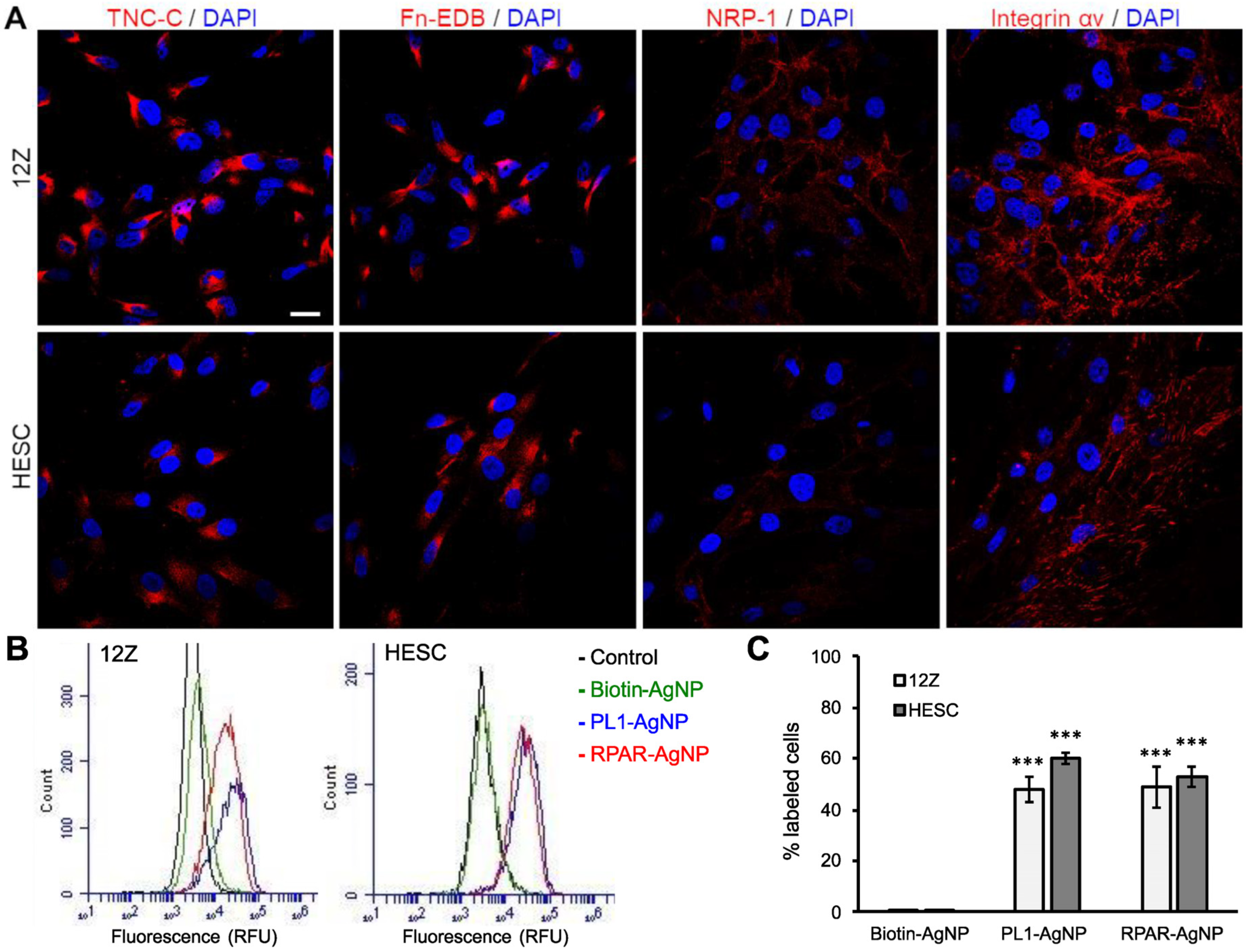
3.1. TNC-C and Fn-EDB Targeting Peptide-Displaying Phages Bind to 12Z and HESC Cells
3.2. PL1 Receptors Are Expressed in Cultured 12Z and HESC Cells
3.3. PL1-Targeted Nanoparticles Internalize in 12Z and HESC Cells
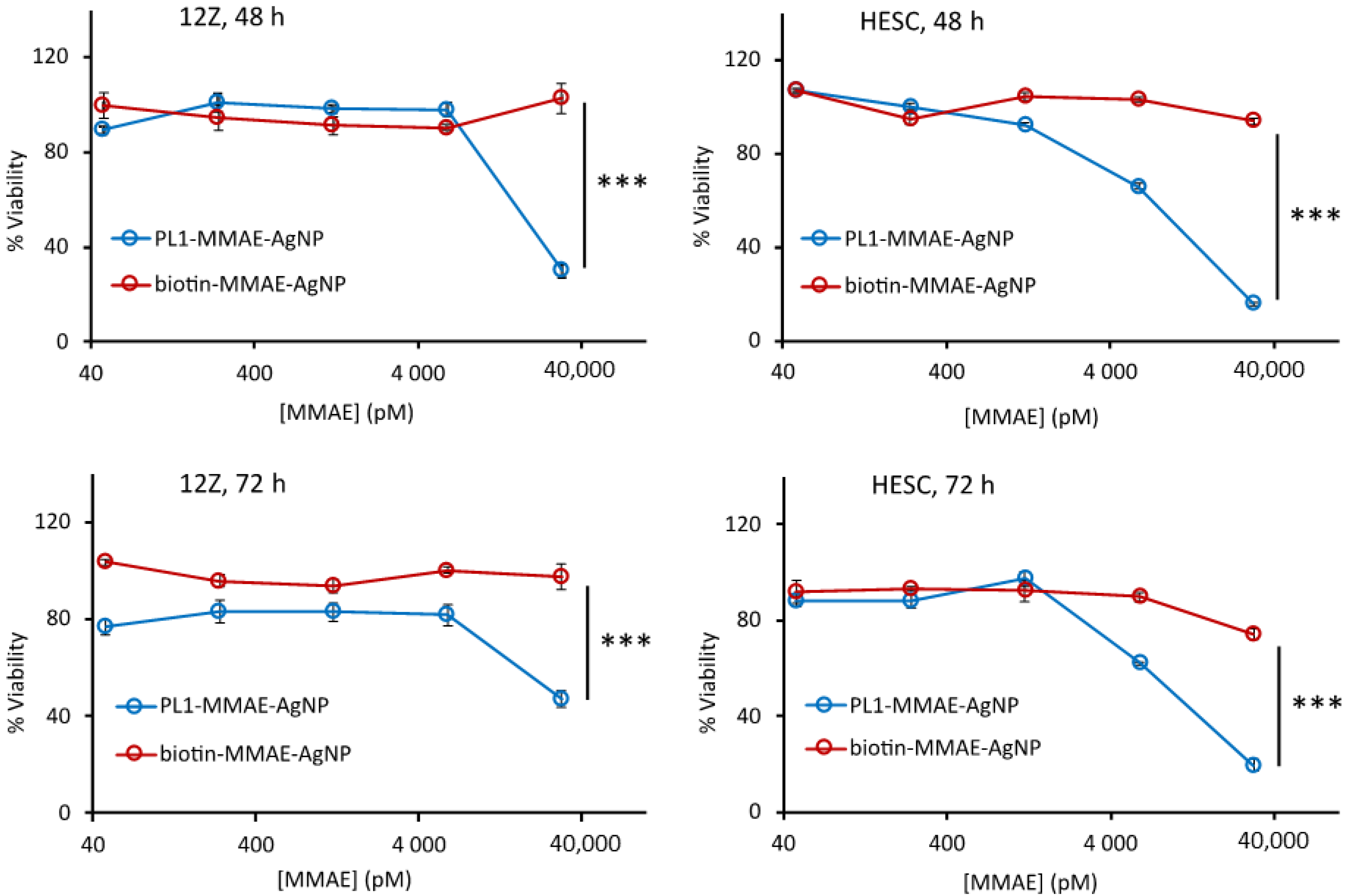
3.4. PL1 Conjugation Increases the Effect of Cytotoxic Nanoparticles in 12Z and HESC Cells
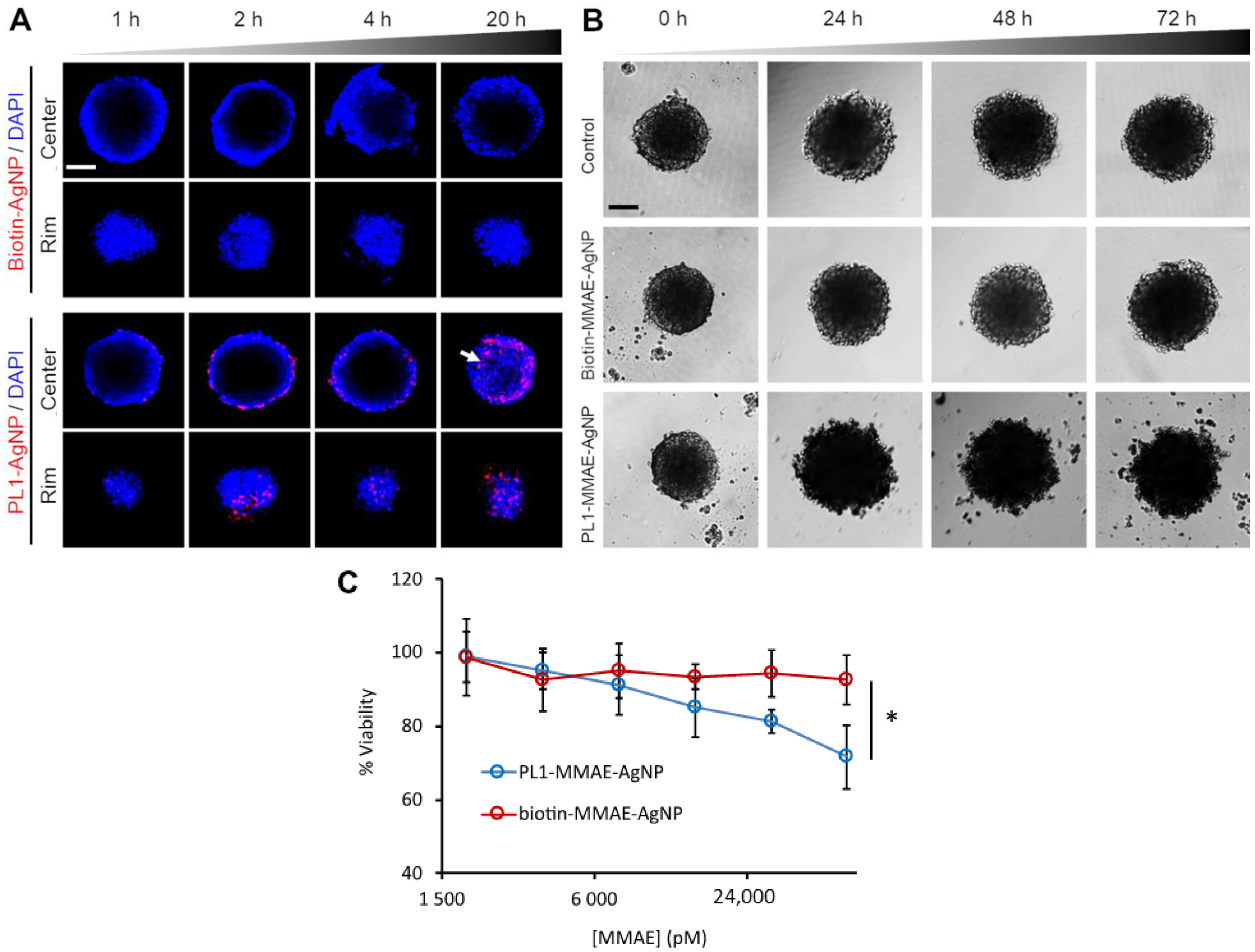
3.5. PL1 Promotes the Penetration and Cytotoxicity of AgNPs in Endometriotic Spheroids
3.6. PL1-AgNPs Bind to TNC-C- and Fn-EDB-Positive Areas of Human Peritoneal Endometriotic Lesions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mason, B.R.; Chatterjee, D.; Menias, C.O.; Thaker, P.H.; Siegel, C.L.; Yano, M. Encyclopedia of endometriosis: A pictorial rad-path review. Abdom. Radiol. 2020, 45, 1587–1607. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharmacother. 2018, 19, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Crosignani, P.; Abbiati, A.; Somigliana, E.; Vigano, P.; Fedele, L. The effect of surgery for symptomatic endometriosis: The other side of the story. Hum. Reprod. Update 2009, 15, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Fernandez, M.L.; Anglesio, M.; Yong, P.J.; Carey, M.S. Endometriosis and endometriosis-associated cancers: New insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018, 12, 803. [Google Scholar] [CrossRef] [Green Version]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemmt, P.A.; Carver, J.G.; Koninckx, P.; McVeigh, E.J.; Mardon, H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007, 22, 3139–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Schwenzer, A.; Rupp, T.; Murdamoothoo, D.; Vegliante, R.; Lefebvre, D.; Klein, A.; Hussenet, T.; Orend, G. Tenascin-C promotes tumor cell migration and metastasis through integrin α9β1–mediated YAP inhibition. Cancer Res. 2018, 78, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Tan, O.; Ornek, T.; Seval, Y.; Sati, L.; Arici, A. Tenascin is highly expressed in endometriosis and its expression is upregulated by estrogen. Fertil. Steril. 2008, 89, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.J.; Lessey, B.A.; Rai, V.; Bergqvist, A.; Kennedy, S.; Manek, S.; Barlow, D.H.; Mardon, H.J. Tenascin is differentially expressed in endometrium and endometriosis. J. Pathol. 1999, 187, 242–248. [Google Scholar] [CrossRef]
- Schwager, K.; Bootz, F.; Imesch, P.; Kaspar, M.; Trachsel, E.; Neri, D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum. Reprod. 2011, 26, 2344–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.A.; Chan, B.M.; Uniyal, S.; Barbin, Y.P.; Farhangkhoee, H.; Chen, S.; Chakrabarti, S. EDB fibronectin and angiogenesis–a novel mechanistic pathway. Angiogenesis 2005, 8, 183–196. [Google Scholar] [CrossRef]
- Kumra, H.; Reinhardt, D.P. Fibronectin-targeted drug delivery in cancer. Adv. Drug Deliv. Rev. 2016, 97, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Xu, X.; Kang, B.R.; Lee, J.; Lee, Y.S.; Kim, C.; Kim, H.; Kang, S.-H.; Na, Y.J.; Moon, H.J.; et al. Extra-domain B of fibronectin as an alternative target for drug delivery and a cancer diagnostic and prognostic biomarker for malignant glioma. Theranostics 2021, 11, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Lingasamy, P.; Tobi, A.; Haugas, M.; Hunt, H.; Paiste, P.; Asser, T.; Rätsep, T.; Kotamraju, V.R.; Bjerkvig, R.; Teesalu, T. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials 2019, 219, 119373. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Lingasamy, P. Bi-Specific Extracellular Matrix Binding Peptides and Methods of Use Thereof. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020161602 (accessed on 24 November 2021).
- Lingasamy, P.; Tobi, A.; Kurm, K.; Kopanchuk, S.; Sudakov, A.; Salumäe, M.; Rätsep, T.; Asser, T.; Bjerkvig, R.; Teesalu, T. Tumor-penetrating peptide for systemic targeting of Tenascin-C. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tobi, A.; Willmore, A.A.; Kilk, K.; Sidorenko, V.; Braun, G.B.; Soomets, U.; Sugahara, K.N.; Ruoslahti, E.; Teesalu, T. Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug. Adv. Ther. 2021, 4, 2000097. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwaldd, R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptdei Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, K.; Kobayashi, Y.; Suzuki, A.; Tamura, N.; Motamedchaboki, K.; Huang, C.-T.; Akama, T.; Pecotte, J.; Frost, P.; Bauer, C.; et al. Development of pro-apoptotic peptides as potential therapy for peritoneal endometriosis. Nat. Commun. 2014, 5, 4478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linzhi, Y.; Dan, C.; Arhin, S.K.; Ledan, W.; Wenju, L.; Jieqiang, L. Screening for novel peptides specifically binding to the surface of ectopic endometrium cells by phage display. Cell. Mol. Biol. 2018, 64, 36–40. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Mapping of Vascular ZIP Codes by Phage Display. Methods Enzymol. 2012, 503, 35–56. [Google Scholar] [PubMed]
- Põšnograjeva, K.; Pleiko, K.; Haugas, M.; Teesalu, T. New tools for streamlined in vivo homing peptide identification. Cell Penetrating Pept. 2022, 2383, 385–412. [Google Scholar]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Braun, G.B.; Friman, T.; Pang, H.-B.; Pallaoro, A.; De Mendoza, T.H.; Willmore, A.-M.A.; Kotamraju, V.R.; Mann, A.P.; She, Z.-G.; Sugahara, K.N.; et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat. Mater. 2014, 13, 904–911. [Google Scholar] [CrossRef] [Green Version]
- Čeponytė, U.; Paškevičiūtė, M.; Petrikaitė, V. Comparison of NSAIDs activity in COX-2 expressing and non-expressing 2D and 3D pancreatic cancer cell cultures. Cancer Manag. Res. 2018, ume 10, 1543–1551. [Google Scholar] [CrossRef] [Green Version]
- Lingasamy, P.; Teesalu, T. Homing Peptides for Cancer Therapy. Adv. Exp. Med. Biol. 2021, 1295, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.P.; Scodeller, P.; Hussain, S.; Braun, G.B.; Mölder, T.; Toome, K.; Ambasudhan, R.; Teesalu, T.; Lipton, S.A.; Ruoslahti, E. Identification of a peptide recognizing cerebrovascular changes in mouse models of Alzheimer’s disease. Nat. Commun. 2017, 8, 1403. [Google Scholar] [CrossRef]
- Dietze, R.; Starzinski-Powitz, A.; Scheiner-Bobis, G.; Tinneberg, H.-R.; Meinhold-Heerlein, I.; Konrad, L. Lysophosphatidic acid triggers cathepsin B-mediated invasiveness of human endometriotic cells. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1369–1377. [Google Scholar] [CrossRef]
- Ferella, L.; Bastón, J.I.; Bilotas, M.A.; Singla, J.J.; González, A.M.; Olivares, C.N.; Meresman, G. Active compounds present inRosmarinus officinalis leaves andScutellaria baicalensis root evaluated as new therapeutic agents for endometriosis. Reprod. Biomed. Online 2018, 37, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Reis, F.M.; Taylor, R.N. Angiogenesis and Endometriosis. Obstet. Gynecol. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, O.; Yamada-Nomoto, K.; Kobayashi, M.; Andoh, T.; Hongo, M.; Ono, Y.; Hasegawa-Idemitsu, A.; Sakai, A.; Osuga, Y.; Saito, S. Bradykinin system is involved in endometriosis-related pain through endothelin-1 production. Eur. J. Pain 2017, 22, 501–510. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Lingasamy, P.; Põšnograjeva, K.; Kopanchuk, S.; Tobi, A.; Rinken, A.; General, I.J.; Asciutto, E.K.; Teesalu, T. PL1 Peptide Engages Acidic Surfaces on Tumor-Associated Fibronectin and Tenascin Isoforms to Trigger Cellular Uptake. bioRxiv 2021. [Google Scholar] [CrossRef]
- Moses, A.S.; Demessie, A.A.; Taratula, O.; Korzun, T.; Slayden, O.D.; Taratula, O. Nanomedicines for Endometriosis: Lessons Learned from Cancer Research. Small 2021, 17, e2004975. [Google Scholar] [CrossRef] [PubMed]
- Simón-Gracia, L.; Hunt, H.; Teesalu, T. Peritoneal Carcinomatosis Targeting with Tumor Homing Peptides. Molecules 2018, 23, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Säälik, P.; Lingasamy, P.; Toome, K.; Mastandrea, I.; Rousso-Noori, L.; Tobi, A.; Simón-Gracia, L.; Hunt, H.; Paiste, P.; Kotamraju, V.R.; et al. Peptide-guided nanoparticles for glioblastoma targeting. J. Control. Release 2019, 308, 109–118. [Google Scholar] [CrossRef]
- Simón-Gracia, D.L.; Sidorenko, V.; Uustare, A.; Ogibalov, I.; Tasa, A.; Tshobrik, O.; Teesalu, T. Novel Anthracycline Utorubicin for Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 17018. [Google Scholar] [CrossRef] [PubMed]
- Simón-Gracia, L.; Hunt, H.; Scodeller, P.; Gaitzsch, J.; Kotamraju, V.R.; Sugahara, K.N.; Tammik, O.; Ruoslahti, E.; Battaglia, G.; Teesalu, T. iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials 2016, 104, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simón-Gracia, L.; Scodeller, P.; Fuentes, S.S.; Vallejo, V.G.; Ríos, X.; Sebastián, E.S.; Sidorenko, V.; Di Silvio, D.; Suck, M.; De Lorenzi, F.; et al. Application of polymersomes engineered to target p32 protein for detection of small breast tumors in mice. Oncotarget 2018, 9, 18682–18697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wannasarit, S.; Wang, S.; Figueiredo, P.; Trujillo, C.; Eburnea, F.; Simon-Gracia, L.; Correia, A.; Ding, Y.; Liu, D.; Wiwattanapatapee, R.; et al. A Virus-Mimicking pH-Responsive Acetalated Dextran-Based Membrane-Active Polymeric Nanoparticle for Intracellular Delivery of Antitumor Therapeutics. Adv. Funct. Mater. 2019, 29, 1905352. [Google Scholar] [CrossRef]
- Ikemoto, H.; Lingasamy, P.; Willmore, A.-M.A.; Hunt, H.; Kurm, K.; Tammik, O.; Scodeller, P.; Gracia, L.S.; Kotamraju, V.R.; Lowy, A.M.; et al. Hyaluronan-binding peptide for targeting peritoneal carcinomatosis. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, H.; Gracia, L.S.; Tobi, A.; Kotamraju, V.R.; Sharma, S.; Nigul, M.; Sugahara, K.N.; Ruoslahti, E.; Teesalu, T. Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles. J. Control. Release 2017, 260, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Wonder, E.; Simón-Gracia, L.; Scodeller, P.; Majzoub, R.N.; Kotamraju, V.R.; Ewert, K.K.; Teesalu, T.; Safinya, C.R. Competition of charge-mediated and specific binding by peptide-tagged cationic liposome–DNA nanoparticles in vitro and in vivo. Biomaterials 2018, 166, 52–63. [Google Scholar] [CrossRef]
- Scodeller, P.; Gracia, L.S.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Säälik, P.; Kurm, K.; Squadrito, M.L.; Kotamraju, V.R.; Rinken, A.; et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bessone, M.I.D.; Simon-Gracia, L.; Scodeller, P.; de los Angeles Ramirez, M.; Huvelle, M.A.L.; Soler-Illia, G.J.A.A.; Simian, M. IRGD-guided tamoxifen polymersomes inhibit estrogen receptor transcriptional activity and decrease the number of breast cancer cells with self-renewing capacity. J. Nanobiotechnol. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Lepland, A.; Asciutto, E.K.; Malfanti, A.; Simón-Gracia, L.; Sidorenko, V.; Vicent, M.J.; Teesalu, T.; Scodeller, P. Targeting Pro-Tumoral Macrophages in Early Primary and Metastatic Breast Tumors with the CD206-Binding mUNO Peptide. Mol. Pharm. 2020, 17, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, Y.; Zhao, Y.; Xu, C.; Zhang, A.; Zhang, Q.; Wang, D.; He, J.; Hua, W.; Duan, P. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Liu, B.; Liang, L.; Wu, Y.; Xie, H.; Huang, J.; Guo, X.; Tan, J.; Zhan, X.; Liu, Y.; et al. Antiangiogenesis Therapy of Endometriosis Using PAMAM as a Gene Vector in a Noninvasive Animal Model. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cheng, J.-L.; Yan, J.-J.; Chen, F.-Y.; Sheng, J.-Z.; Sun, D.-L.; Chen, J.; Miao, J.; Zhang, R.-J.; Zheng, C.; et al. Hyaluronic acid reagent functional chitosan-PEI conjugate with AQP2-siRNA suppressed endometriotic lesion formation. Int. J. Nanomed. 2016, 11, 1323–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Li, W.; Zhou, J.; Hou, W.; Wen, X.; Zhang, H.; Kong, F.; Luo, L.; Li, Q.; Du, Y.; et al. Specific Photothermal Ablation Therapy of Endometriosis by Targeting Delivery of Gold Nanospheres. Small 2017, 13, 1603270. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.S.; Taratula, O.R.; Lee, H.; Luo, F.; Grenz, T.; Korzun, T.; Lorenz, A.S.; Sabei, F.Y.; Bracha, S.; Alani, A.W.G.; et al. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020, 16, e1906936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, P.; Liu, L.; Ma, G.; Ma, J.; Liu, X.; Liu, Y.; Lin, W.; Zhu, Y. Evaluation of PLGA containing anti -CTLA4 inhibited endometriosis progression by regulating CD4 + CD25 + Treg cells in peritoneal fluid of mouse endometriosis model. Eur. J. Pharm. Sci. 2017, 96, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Chakravarty, B.; Chaudhury, K. Letrozole and curcumin loaded-PLGA nanoparticles: A therapeutic strategy for endometriosis. J. Nanomed. Biotherap. Discov. 2014, 4, 10.4172. [Google Scholar]
- Kiisholts, K.; Kurrikuff, K.; Arukuusk, P.; Porosk, L.; Peters, M.; Salumets, A.; Langel, U. Cell-Penetrating Peptide and siRNA-Mediated Therapeutic Effects on Endometriosis and Cancer In Vitro Models. Pharmaceutics 2021, 13, 1618. [Google Scholar] [CrossRef] [PubMed]
- Rekker, K.; Tasa, T.; Saare, M.; Samuel, K.; Ülle, K.; Karro, H.; Götte, M.; Salumets, A.; Peters, M. Differentially-Expressed miRNAs in Ectopic Stromal Cells Contribute to Endometriosis Development: The Plausible Role of miR-139-5p and miR-375. Int. J. Mol. Sci. 2018, 19, 3789. [Google Scholar] [CrossRef] [Green Version]
- Lavogina, D.; Samuel, K.; Lavrits, A.; Meltsov, A.; Sõritsa, D.; Kadastik, Ü.; Peters, M.; Rinken, A.; Salumets, A. Chemosensitivity and chemoresistance in endometriosis–differences for ectopic versus eutopic cells. Reprod. Biomed. Online 2019, 39, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Horn, I.-S.; Wichmann, G.; Mozet, C.; Dietz, A.; Dollner, R.; Tschöp, K.; Boehm, A. Heterogeneity of epithelial and stromal cells of head and neck squamous cell carcinomas in ex vivo chemoresponse. Cancer Chemother. Pharmacol. 2009, 65, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-H.; Lee, Y.-J.; Kim, J.-B.; Lee, Y.-J.; Ahn, J.-W.; Kim, S.-H.; Chae, H.-D.; Kang, B.-M. Cathepsin B in eutopic and ectopic endometrial tissues of patients with endometriosis. Dev. Reprod. 2013, 17, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of Matrix Metalloproteinases and Their Inhibitors in Endometrium: High Levels in Endometriotic Lesions. Int. J. Mol. Sci. 2020, 21, 2840. [Google Scholar] [CrossRef] [PubMed]
- Veiman, K.-L.; Künnapuu, K.; Lehto, T.; Kiisholts, K.; Pärn, K.; Langel, Ü.; Kurrikoff, K. PEG Shielded MMP Sensitive CPPs for Efficient and Tumor Specific Gene Delivery in Vivo. J. Control. Release 2015, 209, 238–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Künnapuu, K.; Veiman, K.; Porosk, L.; Rammul, E.; Kiisholts, K.; Langel, Ü.; Kurrikoff, K. Tumor Gene Therapy by Systemic Delivery of Plasmid DNA with Cell-penetrating Peptides. FASEB Bioadv 2018, 1, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; von Wahlde, M.-K.; Schäfer, S.D.; Kiesel, L.; Greve, B.; Götte, M. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brueggmann, D.; Templeman, C.; Starzinski-Powitz, A.; Rao, N.P.; Gayther, S.A.; Lawrenson, K. Novel three-dimensional in vitro models of ovarian endometriosis. J. Ovarian Res. 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumathi, V.P.; McCluggage, W.G. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J. Clin. Pathol. 2002, 55, 391–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
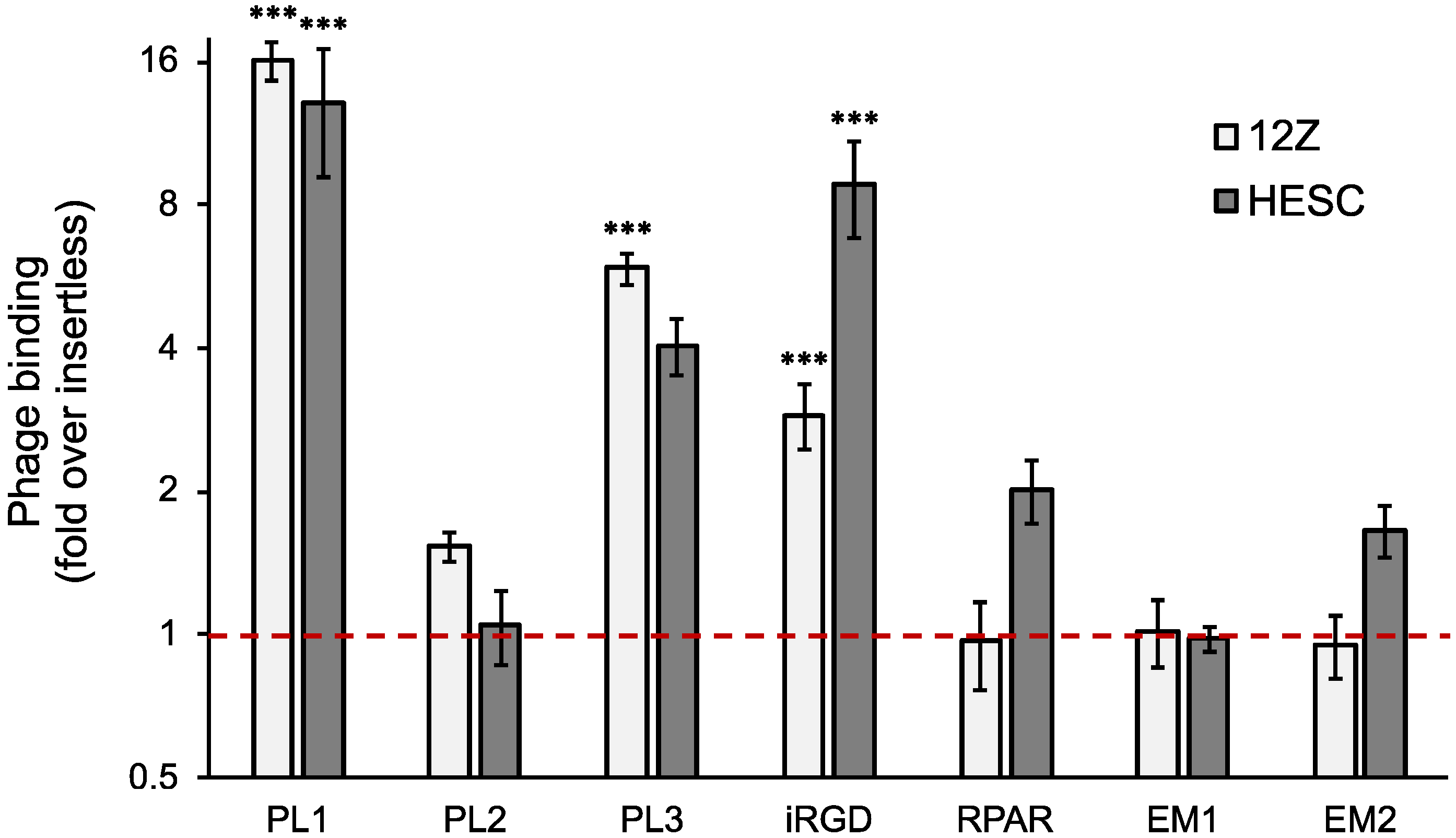

| Peptide ID | Sequence | Receptor | Reference |
|---|---|---|---|
| PL1 | PPRRGLIKLKTS | TNC-C and Fn-EDB | [16] |
| PL2 | TSKQNSR | Fn-EDB and NRP-1 | [17] |
| PL3 | AGRGRLVR | TNC-C and NRP-1 | [18] |
| RPAR | RPARPAR | NRP-1 | [20] |
| iRGD | C * RGDKGPDC * | Integrin αvβ3/5 and NRP1 after cleavage | [21] |
| EM1 | VRRADNRPG | Cyclic nucleotide-gated channel β3 (CNGB3) | [22] |
| EM2 | RTRLHTR | Unknown | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón-Gracia, L.; Kiisholts, K.; Petrikaitė, V.; Tobi, A.; Saare, M.; Lingasamy, P.; Peters, M.; Salumets, A.; Teesalu, T. Homing Peptide-Based Targeting of Tenascin-C and Fibronectin in Endometriosis. Nanomaterials 2021, 11, 3257. https://doi.org/10.3390/nano11123257
Simón-Gracia L, Kiisholts K, Petrikaitė V, Tobi A, Saare M, Lingasamy P, Peters M, Salumets A, Teesalu T. Homing Peptide-Based Targeting of Tenascin-C and Fibronectin in Endometriosis. Nanomaterials. 2021; 11(12):3257. https://doi.org/10.3390/nano11123257
Chicago/Turabian StyleSimón-Gracia, Lorena, Kristina Kiisholts, Vilma Petrikaitė, Allan Tobi, Merli Saare, Prakash Lingasamy, Maire Peters, Andres Salumets, and Tambet Teesalu. 2021. "Homing Peptide-Based Targeting of Tenascin-C and Fibronectin in Endometriosis" Nanomaterials 11, no. 12: 3257. https://doi.org/10.3390/nano11123257









