Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Co@xPC Catalyst
2.3. Catalyst Characterization
2.4. Measurement Method of Hydrogen Production
2.5. DFT Calculations
3. Results and Discussion
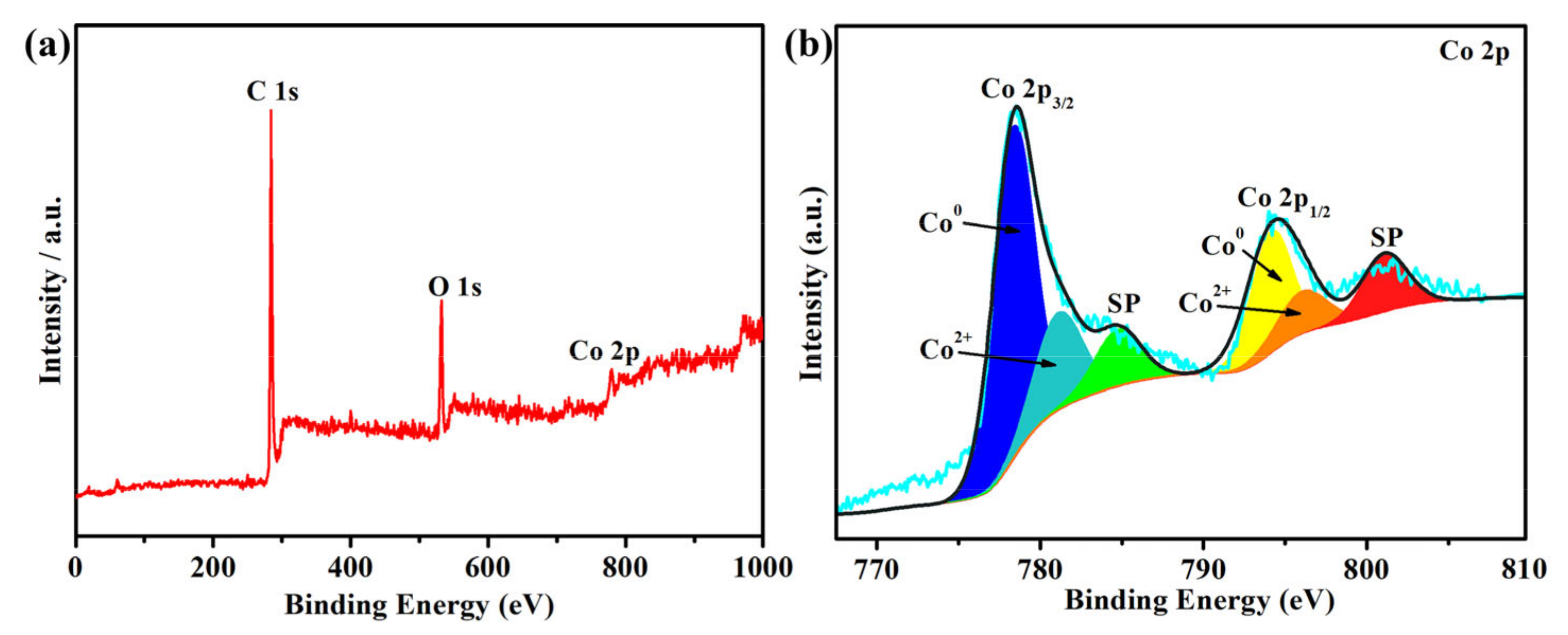
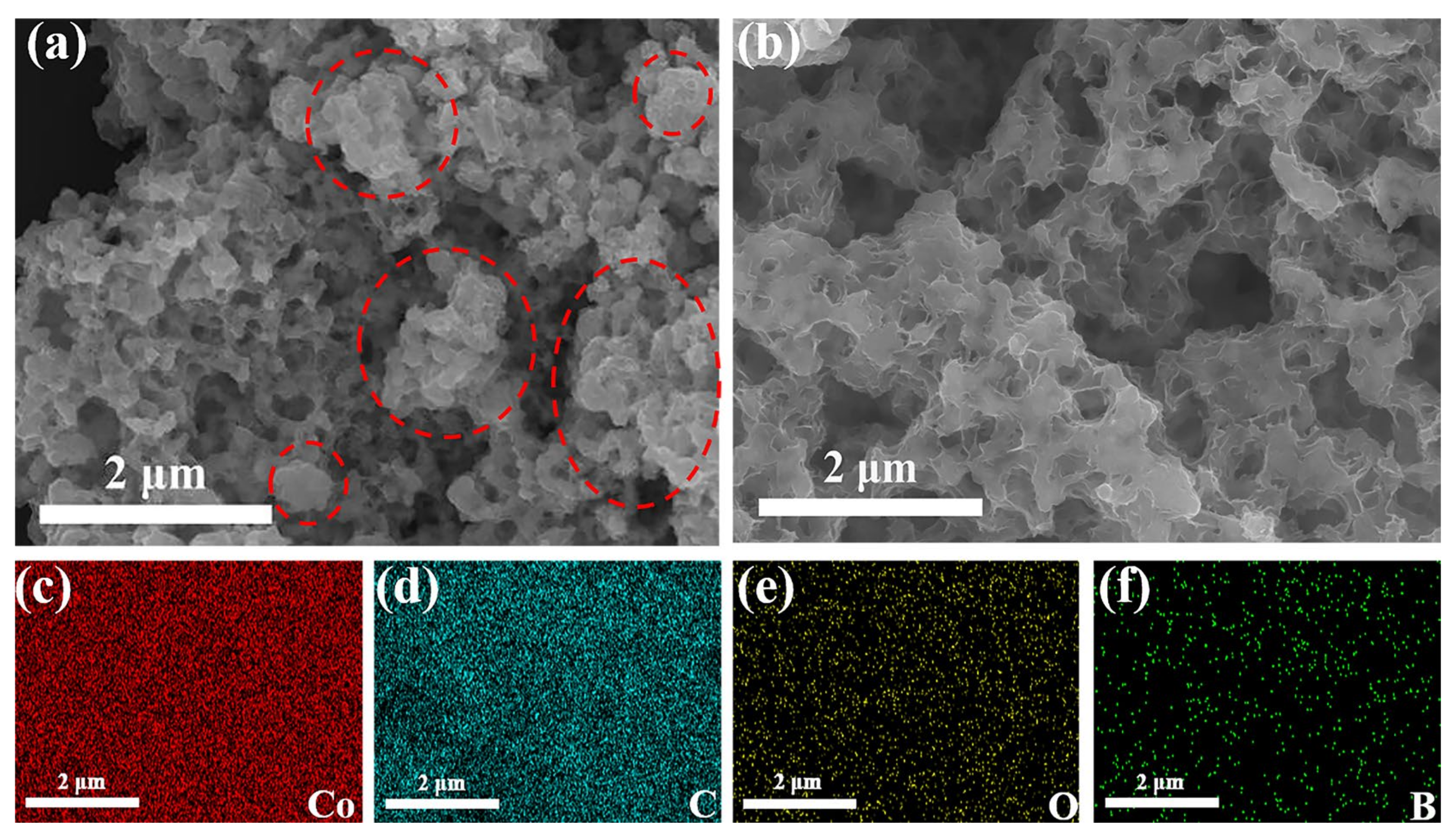
3.1. Characterization of the as-Prepared Catalysts
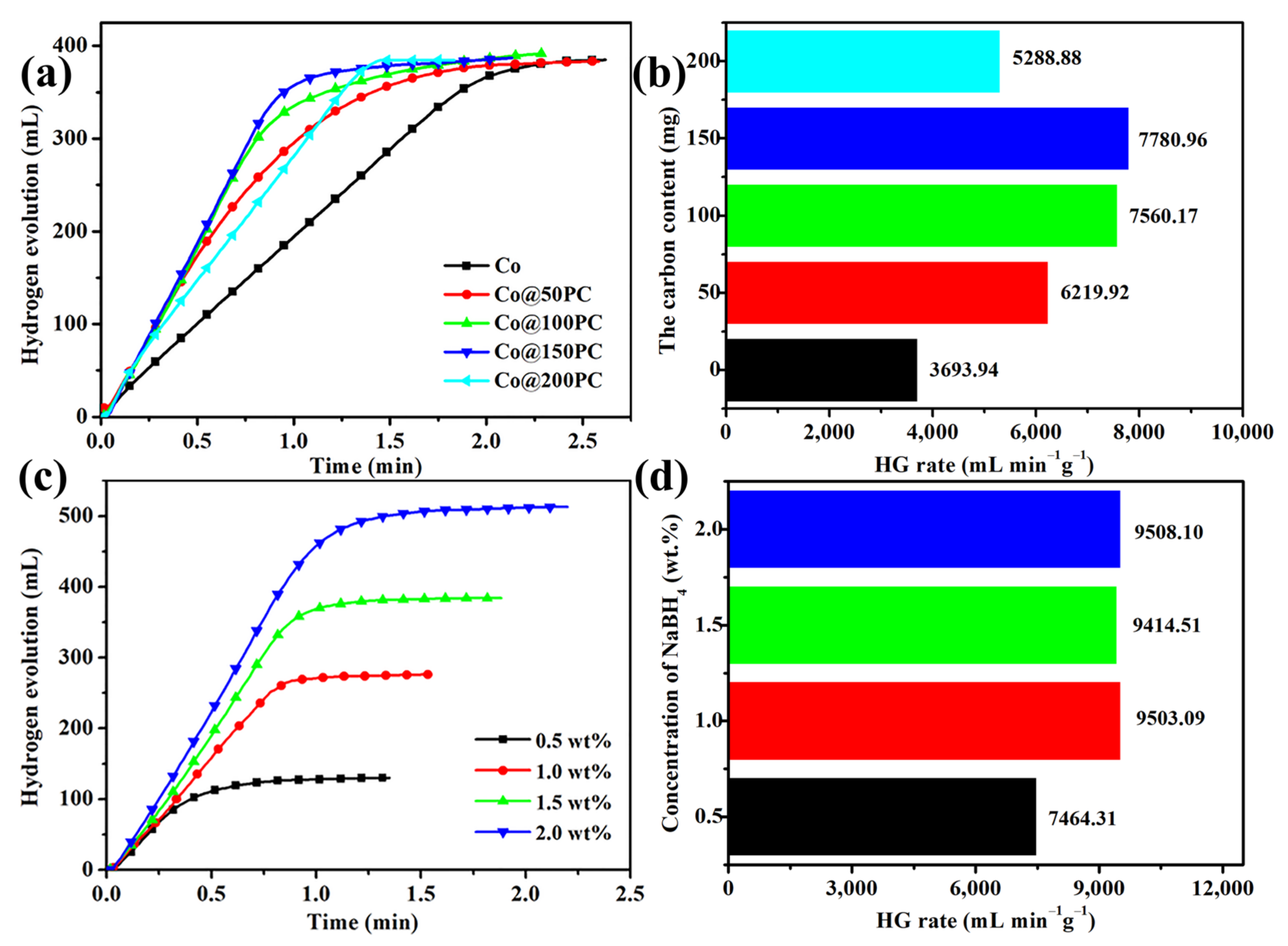
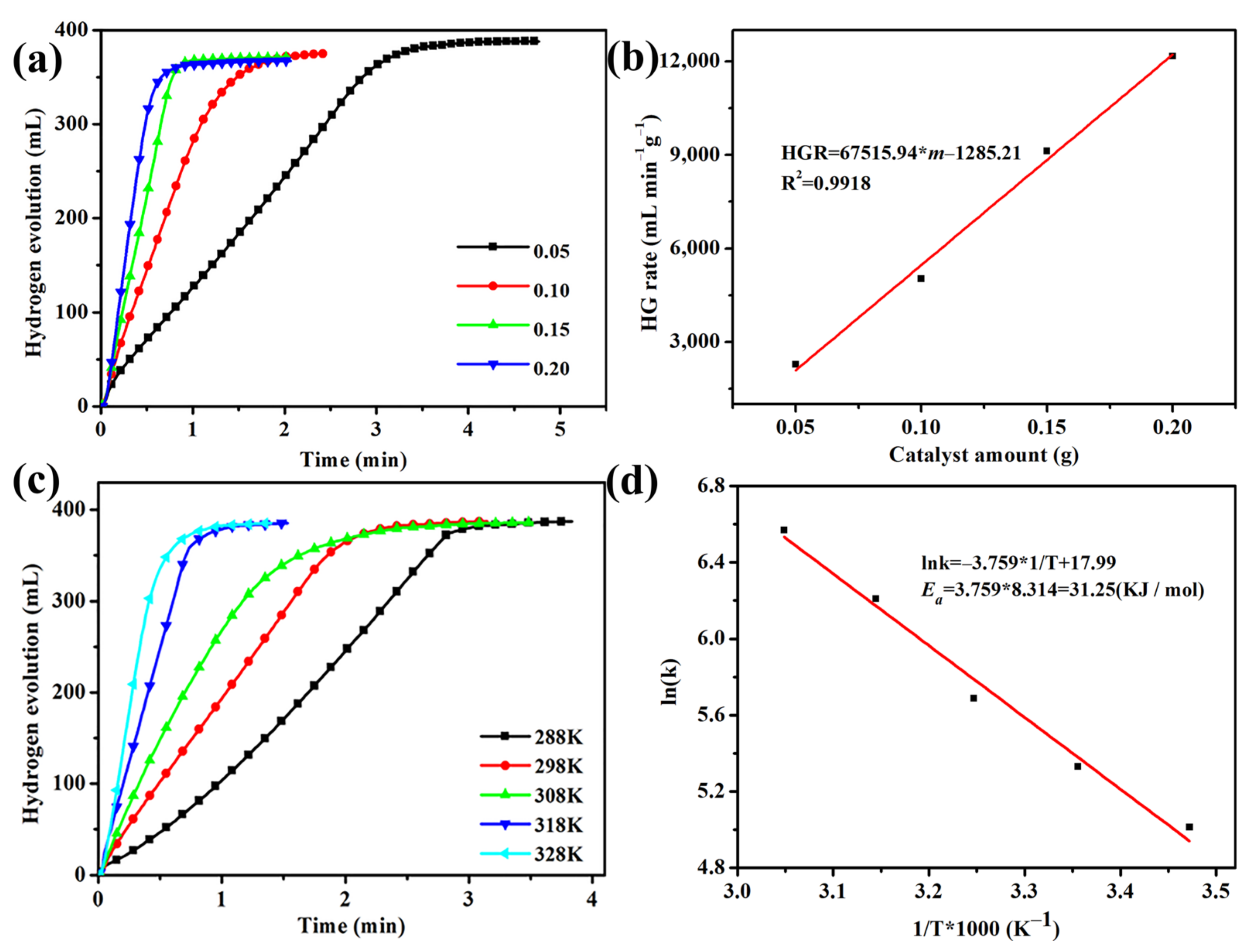
3.2. Catalytic Activity Tests of Co@xPC for Hydrolysis of NaBH4
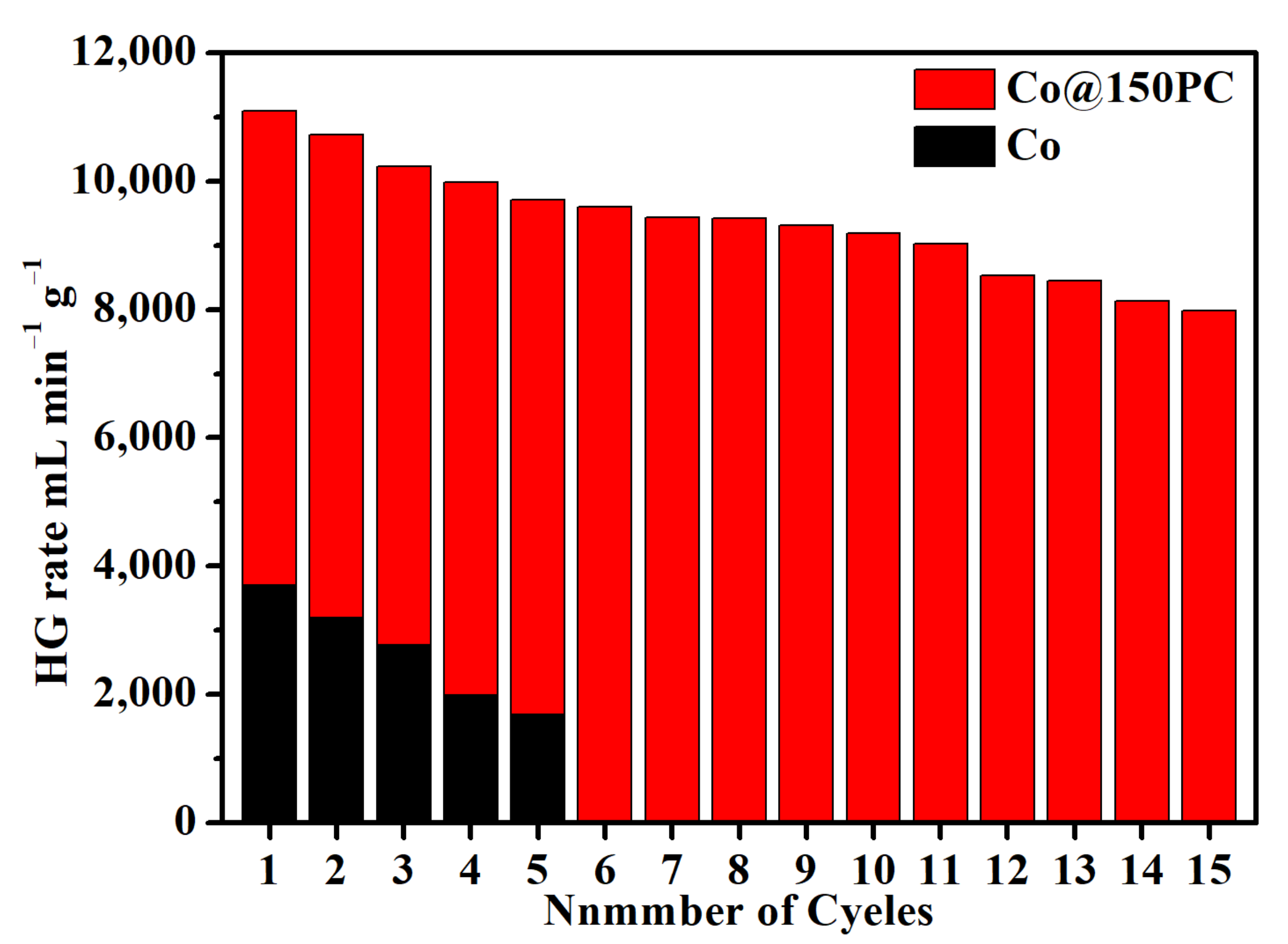
3.3. Catalytic Stability Tests of Co@150PC for Hydrolysis of NaBH4
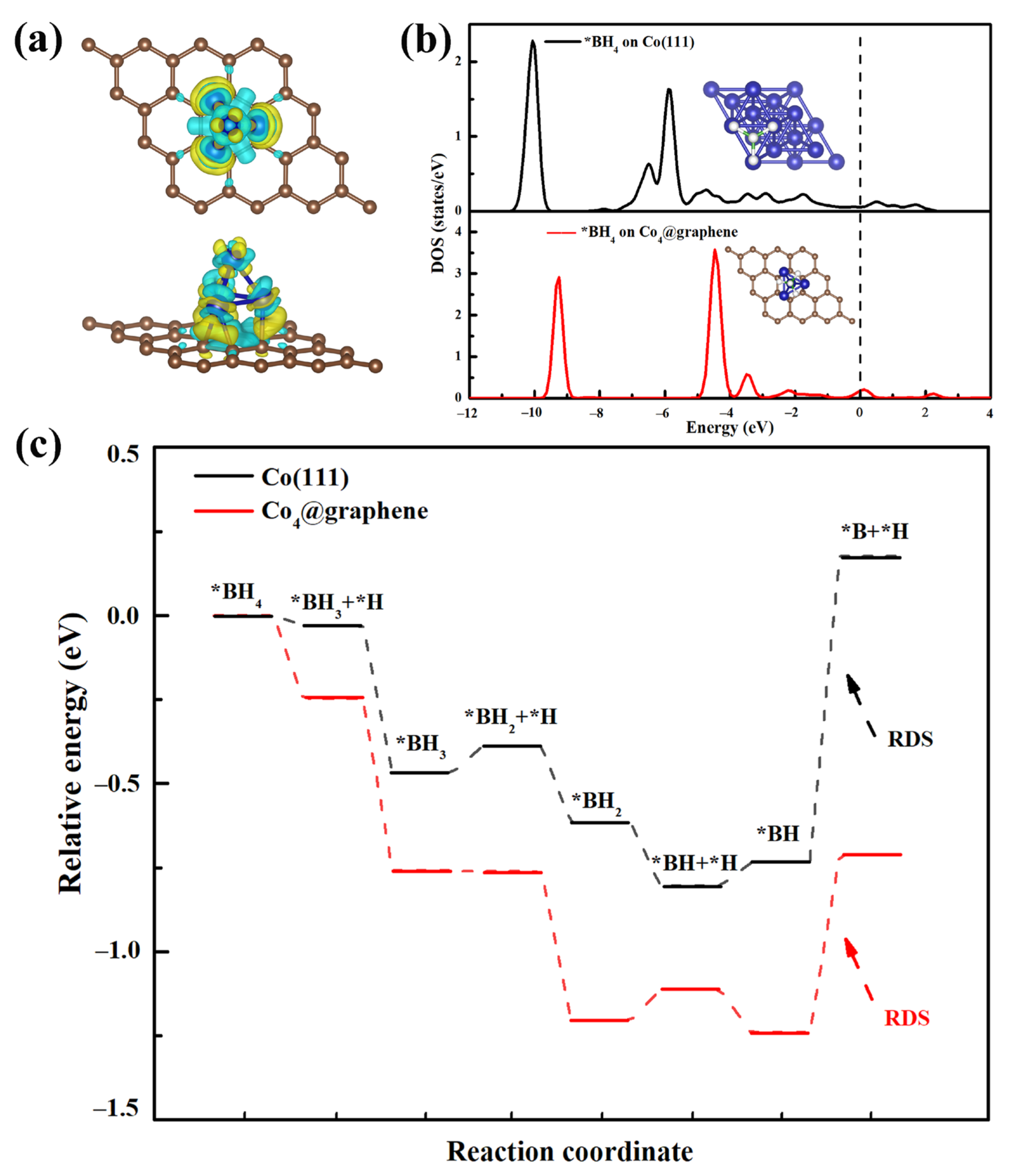
3.4. DFT Calculations of Co@150PC
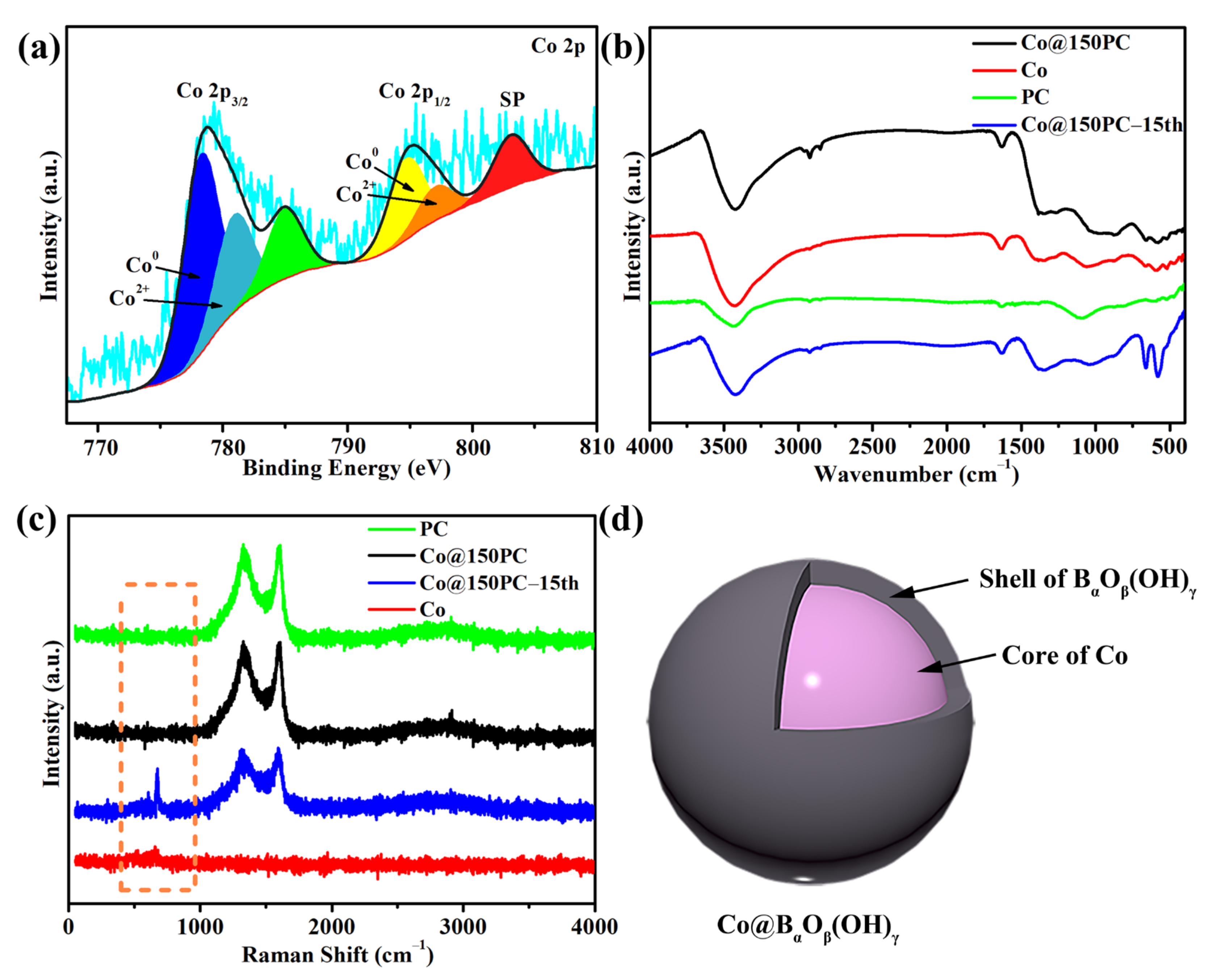
3.5. Mechanism Analysis on Performance Decrease of Co@150PC during Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef]
- Das, P.; Wu, Z.S.; Li, F.; Liang, J.; Cheng, H.M. Two-dimensional energy materials: Opportunities and perspectives. Energy Storage Mater. 2019, 22, 15–17. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.Z.; Wang, X.Z.; Yao, Z.D.; Wang, C.T.; Fan, X.L.; Chen, L.X. Highly synergetic catalytic mechanism of Ni@g-C3N4 on the superior hydrogen storage performance of Li-Mg-B-H system. Energy Storage Mater. 2018, 13, 199–206. [Google Scholar] [CrossRef]
- Cui, B.Y.; Wu, G.M.; Qiu, S.J.; Zou, Y.J.; Yan, E.H.; Xu, F.; Sun, L.X.; Chu, H.L. Ruthenium Supported on Cobalt-Embedded Porous Carbon with Hollow Structure as Efficient Catalysts toward Ammonia-Borane Hydrolysis for Hydrogen Production. Adv. Sustain. Syst. 2021, 5, 2100209. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Fu, Q.; Wu, J.; Fan, G.Y. Ultrafine Pd nanoparticles supported on soft nitriding porous carbon for hydrogen production from hydrolytic dehydrogenation of dimethyl amine-borane. Nanomaterials 2020, 10, 1612. [Google Scholar] [CrossRef]
- Qiu, S.J.; Chu, H.L.; Zou, Y.L.; Xiang, C.L.; Xu, F.; Sun, L.X. Light metal borohydrides/amides combined hydrogen storage systems: Composition, structure and properties. J. Mater. Chem. A 2017, 5, 25112–25130. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Zhong, H.; Ouyang, L.Z.; Ye, J.S.; Liu, J.W.; Wang, H.; Yao, X.D.; Zhu, M. An one-step approach towards hydrogen production and storage through regeneration of NaBH4. Energy Storage Mater. 2017, 7, 222–228. [Google Scholar] [CrossRef]
- Fangaj, E.; Ceyhan, A.A. Apricot Kernel shell waste treated with phosphoric acid used as a green, metal-free catalyst for hydrogen generation from hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2020, 45, 17104–17117. [Google Scholar] [CrossRef]
- Guella, G.; Zanchetta, C.; Patton, B.; Miotello, A. New insights on the mechanism of palladium-catalyzed hydrolysis of sodium borohydride from B-11 NMR measurements. J. Phys. Chem. B 2006, 110, 17024–17033. [Google Scholar] [CrossRef]
- Muir, S.S.; Yao, X.D. Progress in sodium borohydride as a hydrogen storage material: Development of hydrolysis catalysts and reaction systems. Int. J. Hydrog. Energy 2011, 36, 5983–5997. [Google Scholar] [CrossRef]
- Zhang, H.M.; Xu, G.C.; Zhang, L.; Wang, W.F.; Miao, W.K.; Chen, K.L.; Cheng, L.N.; Li, Y.; Han, S.M. Ultrafine cobalt nanoparticles supported on carbon nanospheres for hydrolysis of sodium borohydride. Renew. Energy 2020, 162, 345–354. [Google Scholar] [CrossRef]
- Kılınç, D.; Şahin, O. Synthesis of polymer supported Ni(II)-Schiff Base complex and its usage as a catalyst in sodium borohydride hydrolysis. Int. J. Hydrog. Energy 2018, 43, 10717–10727. [Google Scholar] [CrossRef]
- Kılınç, D.; Saka, C.; Şahin, O. Hydrogen generation from catalytic hydrolysis of sodium borohydride by a novel Co(II)–Cu(II) based complex catalyst. J. Power Sources 2012, 217, 256–261. [Google Scholar] [CrossRef]
- Ocon, J.D.; Tuan, T.N.; Yi, Y.M.; de Leon, R.L.; Lee, J.K.; Lee, J. Ultrafast and stable hydrogen generation from sodium borohydride in methanol and water over Fe–B nanoparticles. J. Power Sources 2013, 243, 444–450. [Google Scholar] [CrossRef]
- Shi, L.M.; Xie, W.; Jian, Z.Y.; Liao, X.M.; Wang, Y.J. Graphene modified Co–B catalysts for rapid hydrogen production from NaBH4 hydrolysis. Int. J. Hydrog. Energy 2019, 44, 17954–17962. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Y.J.; Li, B.; Liu, Y.L. Novel Ni–Co–B hollow nanospheres promote hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2018, 43, 15245–15254. [Google Scholar] [CrossRef]
- Marchionni, A.; Bevilacqua, M.; Filippi, J.; Folliero, M.G.; Innocenti, M.; Lavacchi, A.; Miller, H.A.; Pagliaro, M.V.; Vizza, F. High volume hydrogen production from the hydrolysis of sodium borohydride using a cobalt catalyst supported on a honeycomb matrix. J. Power Sources 2015, 299, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Ai, L.H.; Gao, X.Y.; Jiang, J. In situ synthesis of cobalt stabilized on macroscopic biopolymer hydrogel as economical and recyclable catalyst for hydrogen generation from sodium borohydride hydrolysis. J. Power Sources 2014, 257, 213–220. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, M.S.; Chen, Y.W. Hydrogen generation by hydrolysis of sodium borohydride on CoB/SiO2 catalyst. Int. J. Hydrog. Energy 2011, 36, 1418–1423. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, H.M.; Xu, D.Y.; Ma, L.; Yi, B.L. Hydrogen generation utilizing alkaline sodium borohydride solution and supported cobalt catalyst. J. Power Sources 2007, 164, 544–548. [Google Scholar] [CrossRef]
- Rakap, M.; Ozkar, S. Hydroxyapatite-supported cobalt(0) nanoclusters as efficient and cost-effective catalyst for hydrogen generation from the hydrolysis of both sodium borohydride and ammonia-borane. Cataly. Today 2012, 183, 17–25. [Google Scholar] [CrossRef]
- Chu, H.L.; Li, N.P.; Qiu, X.Y.; Wang, Y.; Qiu, S.J.; Zeng, J.L.; Zou, Y.J.; Xu, F.; Sun, L.X. Poly(N-vinyl-2-pyrrolidone)-stabilized ruthenium supported on bamboo leaf-derived porous carbon for NH3BH3 hydrolysis. Int. J. Hydrog. Energy 2019, 44, 29255–29262. [Google Scholar] [CrossRef]
- Lu, Y.H.; Zhang, S.L.; Yin, J.M.; Bai, C.C.; Zhang, J.H.; Li, Y.X.; Yang, Y.; Ge, Z.; Zhang, M.; Wei, L.; et al. Mesoporous activated carbon materials with ultrahigh mesopore volume and effective specific surface area for high performance supercapacitors. Carbon 2017, 124, 64–71. [Google Scholar] [CrossRef]
- Wei, S.; Xue, S.S.; Huang, S.S.; Huang, C.S.; Chen, B.Y.; Zhang, H.Z.; Sun, L.X.; Xu, F.; Xia, Y.P.; Cheng, R.G.; et al. Multielement synergetic effect of NiFe2O4 and h-BN for improving the dehydrogenation properties of LiAlH4. Inorg. Chem. Front. 2021, 8, 3111–3126. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Chaugule, A.A.; Chung, W.J.; Kim, H. Fe2O3 hollow nanorods/CNT composites as an efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2016, 222, 1316–1325. [Google Scholar] [CrossRef]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. J. Cheminform. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Alaf, M.; Gultekin, D.; Akbulut, H. Electrochemical properties of free-standing Sn/SnO2/multi-walled carbon nano tube anode papers for Li-ion batteries. Appl. Surf. Sci. 2013, 275, 244–251. [Google Scholar] [CrossRef]
- Heena, H.; Lombardo, L.; Luo, W.; Kin, W.; Zuttel, A. Hydrogen storage properties of various carbon supported NaBH4 prepared via metathesis. Int. J. Hydrog. Energy 2018, 43, 7108–7116. [Google Scholar]
- Li, J.H.; Hong, X.Y.; Wang, Y.L.; Luo, Y.M.; Huang, P.R.; Li, B.; Zhang, K.X.; Zou, Y.J.; Sun, L.X.; Xu, F.; et al. Encapsulated cobalt nanoparticles as a recoverable catalyst for the hydrolysis of sodium borohydride. Energy Storage Mater. 2020, 27, 187–197. [Google Scholar] [CrossRef]
- ElSheikh, A.M.A.; Backovic, G.; Oliveira, R.C.P.; Sequeira, C.A.C.; McGregor, J.; Sljukic, B.; Santos, D.M.F. Carbon-Supported Trimetallic Catalysts (PdAuNi/C) for Borohydride Oxidation Reaction. Nanomaterials 2021, 14, 1441. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chen, Z.L.; Wu, D.Y.; Liu, H.; Dong, C.K.; Song, Y.; Sun, D.L. Subunit volume control mechanism for dehydrogenation performance of AB3-type superlattice intermetallics. J. Power Sources 2019, 427, 145–153. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B. 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.L.; Ju, S.L.; Shao, Y.F.; Chen, W.; Zhang, T.F.; Huang, Y.Q.; Zhang, H.Y.; Xia, G.L.; Yu, X.B. Magnesium hydride nanoparticles anchored on MXene sheets as high capacity anode for lithium-ion batteries. J. Energy Chem. 2021, 62, 431–439. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Mat. 2009, 21, 084204. [Google Scholar] [CrossRef]
- Akça, A.; Genç, A.E.; Kutlu, B. BH4 dissociation on various metal (111) surfaces: A DFT study. Appl. Surf. Sci. 2019, 473, 681–692. [Google Scholar] [CrossRef]
- Zou, Y.J.; Yin, Y.; Gao, Y.B.; Xiang, C.L.; Chu, H.L.; Qiu, S.J.; Yan, E.H.; Xu, F.; Sun, L.X. Chitosan-mediated Co–Ce–B nanoparticles for catalyzing the hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2018, 43, 4912–4921. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.J.; Bandal, H.A.; Appiah-Ntiamoah, R.; Jadhav, A.R.; Kim, H.; Appiah-Ntiamoah, A.R.; Jadhav, H.K. Cobalt nanoparticles supported on magnetic core-shell structured carbon as a highly efficient catalyst for hydrogen generation from NaBH4 hydrolysis. Int. J. Hydrog. Energy 2018, 43, 9296–9306. [Google Scholar] [CrossRef]
- Liu, W.L.; Ju, S.L.; Yu, X.B. Phosphorus-Amine-Based Synthesis of Nanoscale Red Phosphorus for Application to Sodium-Ion Batteries. ACS Nano 2020, 14, 974–984. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.L.; Xie, Z.J.; An, C.H.; Jiang, G.X.; Wang, Y.J. 3D graphene-encapsulated nearly monodisperse Fe3O4 nanoparticles as high-performance lithium-ion battery anodes. J. Alloy. Compd. 2020, 815, 152337. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Y.; Wu, M.; Wang, K.; Zhang, W.K.; Gan, Y.P.; Huang, H.; Chen, J.; Xia, Y.; Zhang, J.; et al. Green synthesis of graphite from CO2 without graphitization process of amorphous carbon. Nat. Commun. 2021, 12, 119. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, L.; Rodriguez-Perez, I.A.; Miao, W.K.; Chen, K.L.; Wang, W.F.; Li, Y.; Han, S.M. Carbon nanospheres supported bimetallic Pt-Co as an efficient catalyst for NaBH4 hydrolysis. Appl. Surf. Sci. 2021, 540, 148296. [Google Scholar] [CrossRef]
- Baydaroglu, F.; Ozdemir, E.; Hasimoglu, A. An effective synthesis route for improving the catalytic activity of carbon-supported Co-B catalyst for hydrogen generation through hydrolysis of NaBH4. Int. J. Hydrog. Energy 2014, 39, 1516–1522. [Google Scholar] [CrossRef]
- Peng, C.L.; Li, T.S.; Zou, Y.J.; Xiang, C.L.; Xu, F.; Zhang, J.; Sun, L.X. Bacterial cellulose derived carbon as a support for catalytically active Co-B alloy for hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2021, 46, 666–675. [Google Scholar] [CrossRef]
- Wei, Y.S.; Wang, R.; Meng, L.Y.; Wang, Y.; Li, G.D.; Xin, S.G.; Zhao, X.S.; Zhang, K. Hydrogen generation from alkaline NaBH4 solution using a dandelion-like Co-Mo-B catalyst supported on carbon cloth. Int. J. Hydrog. Energy 2016, 42, 9945–9951. [Google Scholar] [CrossRef]
- Wang, F.H.; Wang, Y.A.; Zhang, Y.J.; Luo, Y.M.; Zhu, H. Highly dispersed RuCo bimetallic nanoparticles supported on carbon black: Enhanced catalytic activity for hydrogen generation from NaBH4 methanolysis. J. Mater. Sci. 2018, 53, 6831–6841. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Wang, Y.; Zhao, R.X.; Shen, P.K.; Wei, Z.D. Accurately measuring the hydrogen generation rate for hydrolysis of sodium borohydride on multiwalled carbon nanotubes/Co-B catalysts. Int. J. Hydrog. Energy 2008, 33, 7110–7115. [Google Scholar] [CrossRef]
- Xu, J.N.; Du, X.X.; Wei, Q.L.; Huang, Y.M. Efficient Hydrolysis of Sodium Borohydride by Co-B Supported on Nitrogen-doped Carbon. Chem. Sel. 2020, 5, 6683–6690. [Google Scholar] [CrossRef]
- Zhang, H.M.; Feng, X.L.; Cheng, L.N.; Hou, X.W.; Li, Y.; Han, S.M. Non-noble Co anchored on nanoporous graphene oxide, as an efficient and long-life catalyst for hydrogen generation from sodium borohydride. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 112–119. [Google Scholar] [CrossRef]
- Makiabadi, M.; Shamspur, T.; Mostafavi, A. Performance improvement of oxygen on the carbon substrate surface for dispersion of cobalt nanoparticles and its effect on hydrogen generation rate via NaBH4 hydrolysis. Int. J. Hydrog. Energy 2020, 45, 1706–1718. [Google Scholar] [CrossRef]
- Zhu, J.; Li, R.; Niu, W.L.; Wu, Y.J.; Gou, X.L. Fast hydrogen generation from NaBH4 hydrolysis catalyzed by carbon aerogels supported cobalt nanoparticles. Int. J. Hydrog. Energy 2013, 38, 10864–10870. [Google Scholar] [CrossRef]
- Xu, D.Y.; Dai, P.; Liu, X.M.; Cao, C.Q.; Guo, Q.J. Carbon-supported cobalt catalyst for hydrogen generation from alkaline sodium borohydride solution. J. Power Sources 2008, 182, 616–620. [Google Scholar] [CrossRef]
- Netskina, O.V.; Kochubey, D.I.; Prosvirin, I.P.; Malykhin, S.E.; Komova, O.V.; Kanazhevskiy, V.V.; Chukalkin, Y.G.; Bobrovskii, V.I.; Kellerman, D.G.; Ishchenko, A.V.; et al. Cobalt-boron catalyst for NaBH4 hydrolysis: The state of the active component forming from cobalt chloride in a reaction medium. Mol. Catal. 2017, 441, 100–108. [Google Scholar] [CrossRef]
- Retnamma, R.; Novais, A.Q.; Rangel, C.M. Kinetics of hydrolysis of sodium borohydride for hydrogen production in fuel cell applications: A review. Int. J. Hydrog. Energy 2011, 36, 9772–9790. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Reaction mechanisms of the hydrolysis of sodium borohydride: A discussion focusing on cobalt-based catalysts. CR Chim. 2014, 17, 707–716. [Google Scholar] [CrossRef]
- Jia, Y.Z.; Gao, S.Y.; Xia, S.P.; Li, J. FT-IR spectroscopy of supersaturated aqueous solutions of magnesium borate. Spectrochim. Acta Part A 2000, 56, 1291–1297. [Google Scholar]
- Sasaki, K.; Toshiyuki, K.; Guo, B.L.; Ideta, K.; Hayashi, Y.; Hirajima, T.; Miyawaki, J. Calcination effect of borate-bearing hydroxyapatite on the mobility of borate. J. Hazard. Mater. 2018, 344, 90–97. [Google Scholar] [CrossRef]
- Akdim, O.; Demirci, U.B.; Miele, P. Deactivation and reactivation of cobalt in hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2012, 36, 13669–13675. [Google Scholar] [CrossRef]

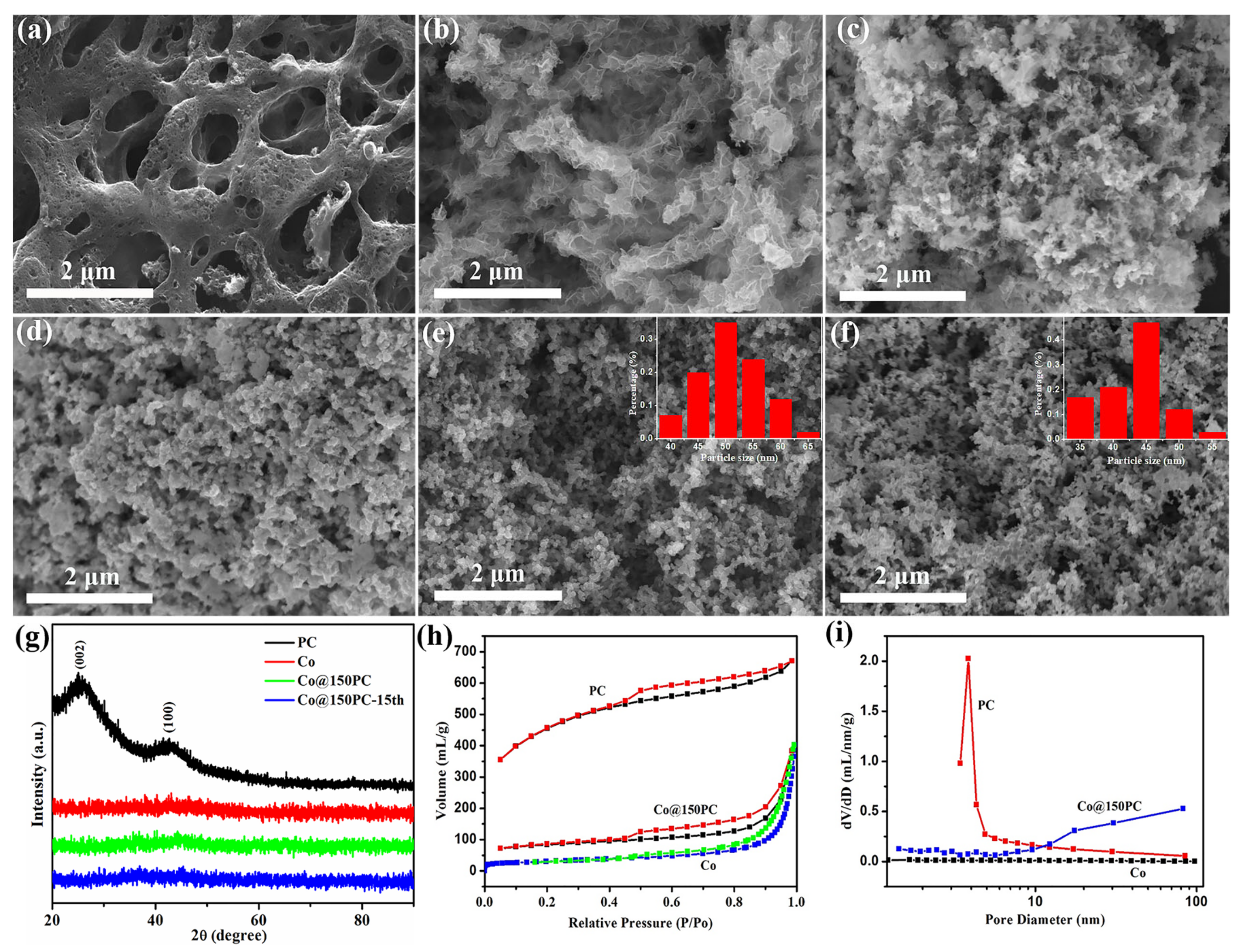
| Catalyst | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| PC | 1527.499 | 0.530 | 3.837 |
| Co | 87.098 | 0.528 | 0.478 |
| Co@150PC | 274.101 | 0.348 | 1.429 |
| Catalyst Sample | Maximum Hydrogen Production Rate (mLH2∙min−1∙gM−1) | Ea (kJ mol−1) | Durability | References |
|---|---|---|---|---|
| Co-Fe3O4@C | 1403 | 49.2 | 59.3% after 5 cycles | [42] |
| CNSs@Pt0.1Co0.9 | 8943 | 38.0 | 85.12% after 5 cycles | [46] |
| Co-B/C | 8033.89 | 56.72 | - | [47] |
| Co–B/C | 3887.1 | 56.37 | 25% after 6 cycles | [48] |
| Co–Mo–B/CC | 1280.8 | 51.0 | 75.1% after 3 cycles | [49] |
| Ru–Co/C | 9360 | 36.83 | 70% after 8 cycles | [50] |
| Co–B/MWCNT | 5100 | 40.40 | - | [51] |
| Co-B/N-C-700 | 2649 | 37.57 | - | [52] |
| Co/PGO | 5955 | 55.2 | 73% after 5 cycles | [53] |
| Co@NMGC | 3575 | 35.2 | 82.5% after 20 cycles | [31] |
| Modified CCS/Co | 11,600 | 33.4 | - | [54] |
| CAs/Co | 11,220 | 38.4 | 96.4% after 5 cycles | [55] |
| Co/C | 530 | 44.1 | - | [56] |
| Co@150PC | 11,086.4 | 31.25 | 72% after 15 cycles | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Liu, J.; Chu, H.; Wei, S.; Yin, Q.; Kang, L.; Luo, X.; Sun, L.; Xu, F.; Huang, P.; et al. Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon. Nanomaterials 2021, 11, 3259. https://doi.org/10.3390/nano11123259
Bu Y, Liu J, Chu H, Wei S, Yin Q, Kang L, Luo X, Sun L, Xu F, Huang P, et al. Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon. Nanomaterials. 2021; 11(12):3259. https://doi.org/10.3390/nano11123259
Chicago/Turabian StyleBu, Yiting, Jiaxi Liu, Hailiang Chu, Sheng Wei, Qingqing Yin, Li Kang, Xiaoshuang Luo, Lixian Sun, Fen Xu, Pengru Huang, and et al. 2021. "Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon" Nanomaterials 11, no. 12: 3259. https://doi.org/10.3390/nano11123259
APA StyleBu, Y., Liu, J., Chu, H., Wei, S., Yin, Q., Kang, L., Luo, X., Sun, L., Xu, F., Huang, P., Rosei, F., Pimerzin, A. A., Seifert, H. J., Du, Y., & Wang, J. (2021). Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon. Nanomaterials, 11(12), 3259. https://doi.org/10.3390/nano11123259








