Scavenger with Protonated Phosphite Ions for Incredible Nanoscale ZrO2-Abrasive Dispersant Stability Enhancement and Related Tungsten-Film Surface Chemical–Mechanical Planarization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CMP Conditions
2.3. Measurement Equipment
3. Results and Discussion
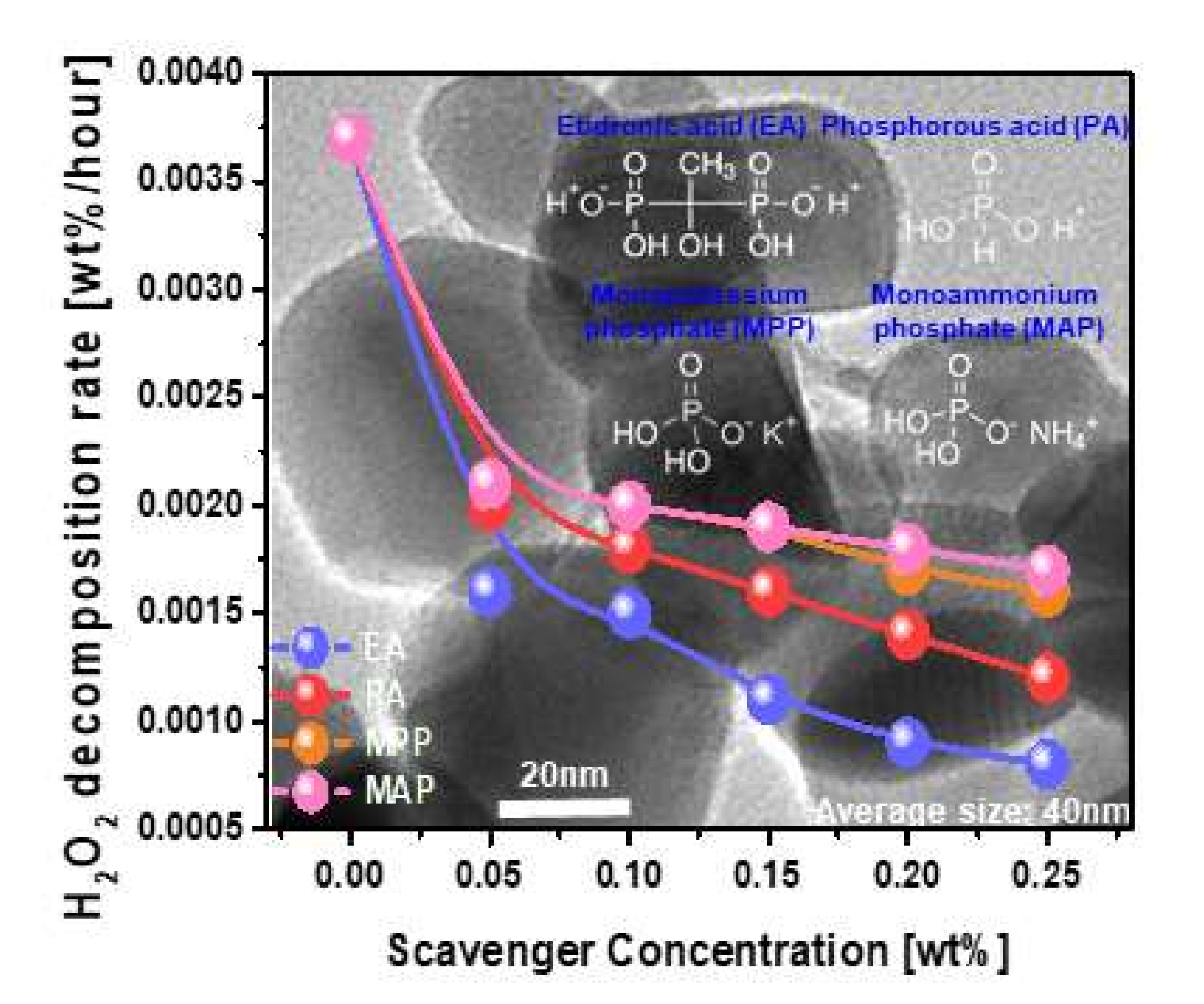
3.1. Abrasive Dispersant Stability of W-Film Surface CMP Slurry Depending on Phosphite or Phosphate-Based Scavenger Type and Concentration
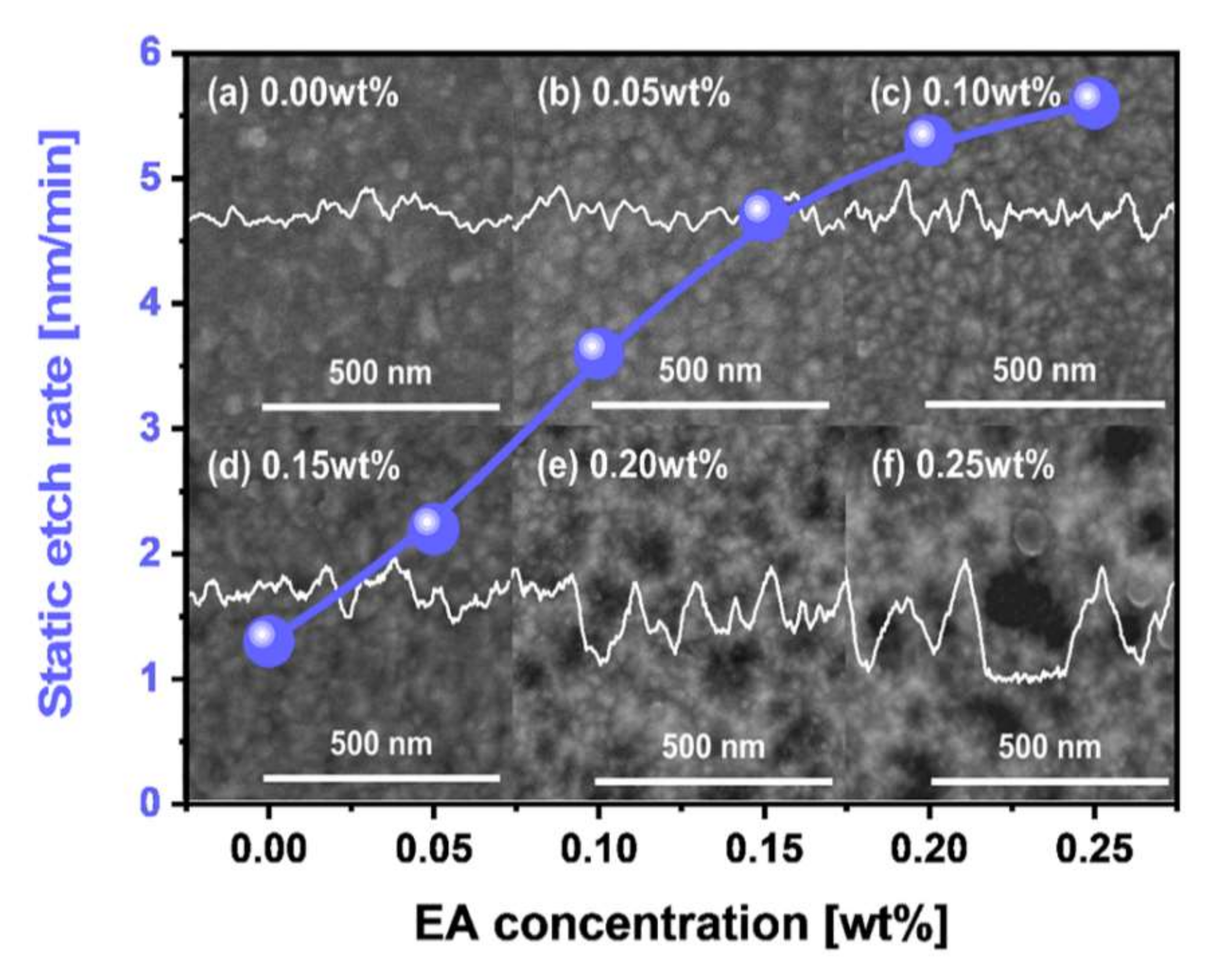
3.2. Dependency of W- and SiO2-Film Polishing Rate on Scavenger Type and Concentration
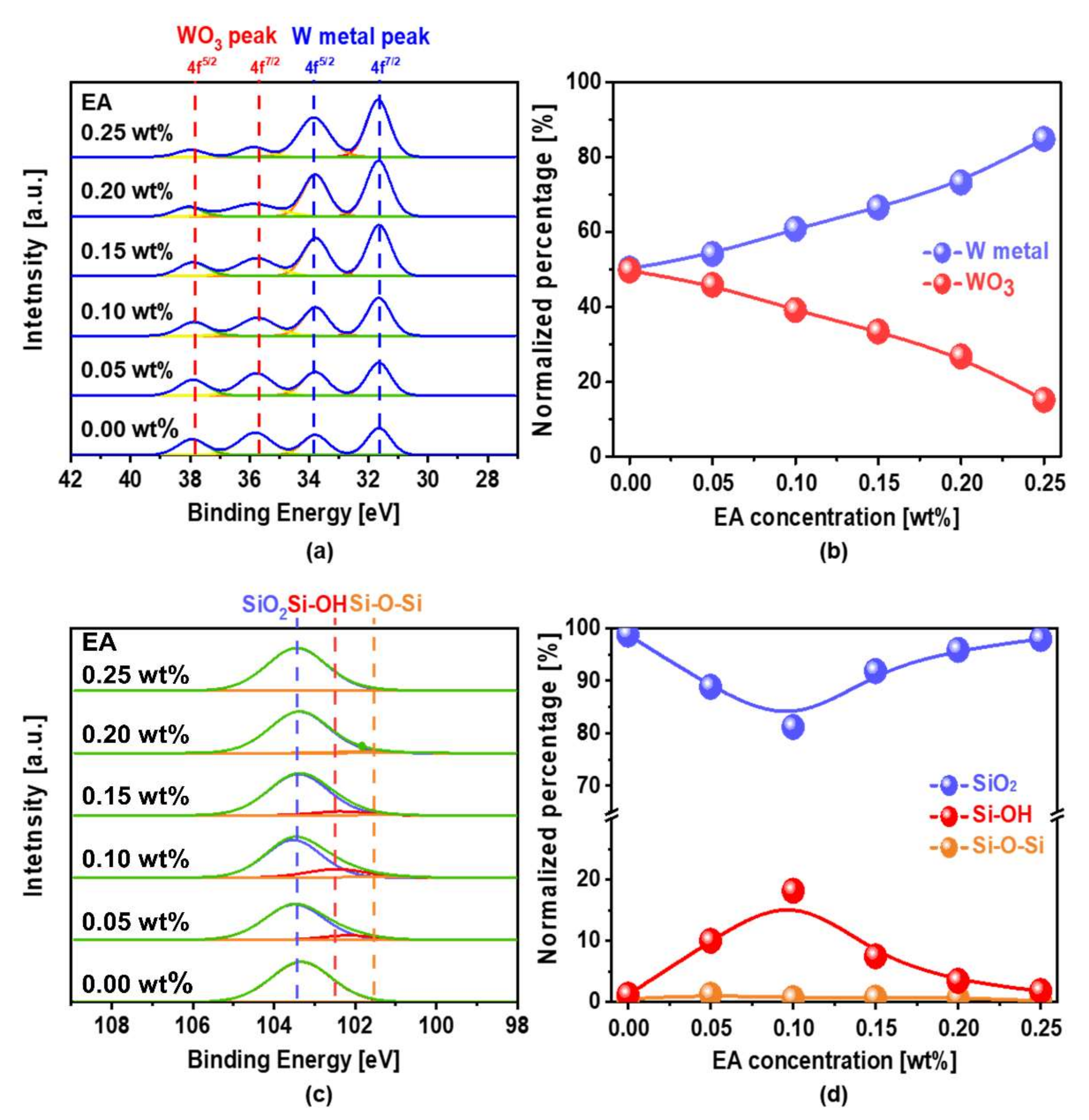
3.3. Dependencies of Chemical Properties (i.e., Corrosion, Potentiodynamic Polarization, and Chemical Composition) on Scavenger (i.e., EA) Concentration
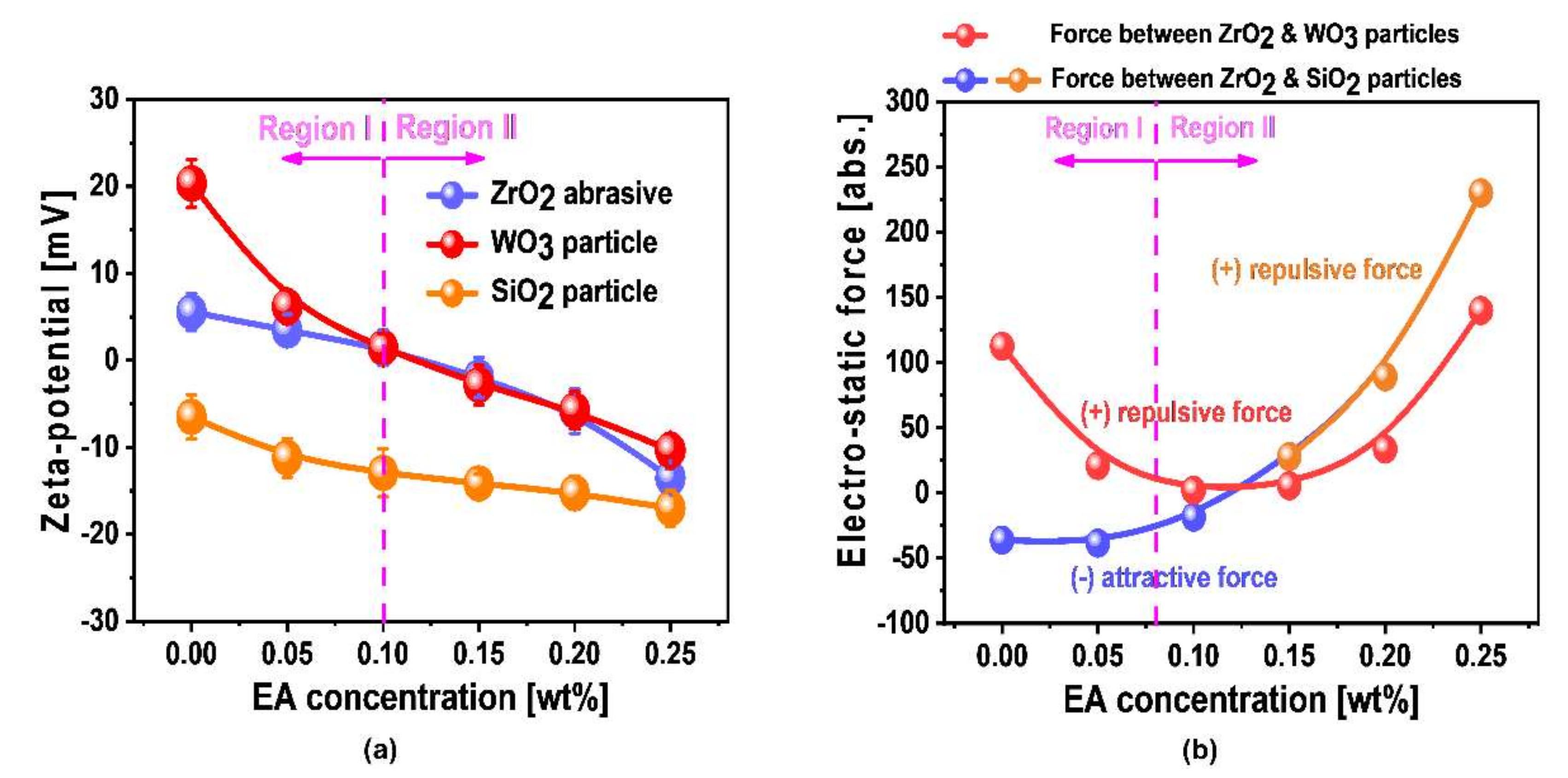
3.4. Dependency Mechanism of W- and SiO2-Film Surface Polishing Rates on Scavenger Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, G.; Bae, D.I.; Kang, M.; Hwang, S.M.; Kim, S.S.; Seo, B.; Kwon, T.Y.; Lee, T.J.; Moon, C.; Choi, Y.M.; et al. 3nm GAA Technology featuring Multi-Bridge-Channel FET for Low Power and High Performance Applications. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018; pp. 28.7.1–28.7.4. [Google Scholar] [CrossRef]
- Goda, A. 3-D NAND Technology Achievements and Future Scaling Perspectives. IEEE Trans. Electron Devices 2020, 67, 1373–1381. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Ramaswamy, N.; Mouli, C. Memory technology: Innovations needed for continued technology scaling and enabling advanced computing systems. In Proceedings of the 2020 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 12–18 December 2020; p. 10. [Google Scholar] [CrossRef]
- Kim, S.K.; Popovici, M. Future of dynamic random-access memory as main memory. MRS Bull. 2018, 43, 334–339. [Google Scholar] [CrossRef]
- Moore, S.K. Another Step Toward the End of Moore’s Law. IEEE Spectrum, 31 May 2019; pp. 9–10. Available online: https://spectrum.ieee.org/another-step-toward-the-end-of-moores-law (accessed on 31 May 2019).
- Tamboli, D.; Seal, S.; Desai, V.; Maury, A. Studies on passivation behavior of tungsten in application to chemical mechanical polishing. J. Vac. Sci. Technol. A 1999, 17, 1168–1173. [Google Scholar] [CrossRef]
- Lim, G.; Lee, J.-H.; Kim, J.; Lee, H.-W.; Hyun, S.-H. Effects of oxidants on the removal of tungsten in CMP process. Wear 2004, 257, 863–868. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Kim, N.-H.; Lee, W.-S. Chemical mechanical polishing and electrochemical characteristics of tungsten using mixed oxidizers with hydrogen peroxide and ferric nitrate. Mater. Lett. 2006, 60, 1192–1197. [Google Scholar] [CrossRef]
- Van Kranenburg, H.; Van Corbach, H.D.; Woerlee, P.H.; Lohmeier, M. W-CMP for sub-micron inverse metallisation. Microelectron. Eng. 1997, 33, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.-B.; Bae, J.-Y.; Kim, S.-I.; Choi, H.-E.; Kim, P.; Lee, J.-C.; Son, Y.-H.; Yun, S.-S.; Park, J.-H.; Park, J.-G. Influence of Scavenger on Abrasive Stability Enhancement and Chemical and Mechanical Properties for Tungsten-Film Chemical-Mechanical-Planarization. ECS J. Solid State Sci. Technol. 2020, 9, 065001. [Google Scholar] [CrossRef]
- Seo, E.-B.; Bae, J.-Y.; Kim, S.-I.; Choi, H.-E.; Son, Y.-H.; Yun, S.-S.; Park, J.-H.; Park, J.-G. Interfacial Chemical and Mechanical Reactions between Tungsten-Film and Nano-Scale Colloidal Zirconia Abrasives for Chemical-Mechanical-Planarization. ECS J. Solid State Sci. Technol. 2020, 9, 054001. [Google Scholar] [CrossRef]
- Perez-Benito, J.F. Iron(III)-hydrogen peroxide reaction: Kinetic evidence of a hydroxyl-mediated chain mechanism. J. Phys. Chem. A. 2004, 108, 4853–4858. [Google Scholar] [CrossRef]
- Dong, H.; Sans, C.; Li, W.; Qiang, Z. Promoted discoloration of methyl orange in H2O2/Fe(III) Fenton system: Effects of gallic acid on iron cycling. Sep. Purif. Technol. 2016, 171, 144–150. [Google Scholar] [CrossRef]
- Poddar, M.K.; Ryu, H.-Y.; Yerriboina, N.P.; Jeong, Y.-A.; Lee, J.-H.; Kim, T.-G.; Kim, J.-H.; Park, J.-D.; Lee, M.-G.; Park, C.-Y.; et al. Nanocatalyst-induced hydroxyl radical (˙OH) slurry for tungsten CMP for next-generation semiconductor processing. J. Mater. Sci. 2020, 55, 3450–3461. [Google Scholar] [CrossRef]
- Seo, E.-B.; Park, J.-G.; Bae, J.-Y.; Park, J.-H. Highly Selective Polishing Rate Between a Tungsten Film and a Silicon-Dioxide Film by Using a Malic-Acid Selectivity Agent in Tungsten-Film Chemical-Mechanical Planarization. J. Korean Phys. Soc. 2020, 76, 1127–1132. [Google Scholar] [CrossRef]
- Ulanski, P.; Bothe, E.; Hildenbrand, K.; Rosiak, J.M.; von Sonntag, C. Hydroxyl-radical-induced reactions of poly(acrylic acid); a pulse radiolysis, EPR and product study. Part I. Deoxygenated aqueous solutions. J. Chem. Soc. Perkin Trans. 1996, 2, 13–22. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nishiyama, T.; Sato, E.; Horibe, H. Degradation of Poly(acrylic acid) in aqueous solution by using O3 microbubble. J. Photopolym. Sci. Technol. 2018, 31, 409–412. [Google Scholar] [CrossRef]
- Stein, D.J.; Hetherington, D.L.; Cecchia, J.L. Investigation of the Kinetics of Tungsten Chemical Mechanical Polishing in Potassium Iodate-Based Slurries: II. Roles of Colloid Species and Slurry Chemistry. J. Electrochem. Soc. 1999, 146, 1934–1938. [Google Scholar] [CrossRef]
- Lim, J.-H.; Park, J.-H.; Park, J.-G. Effect of iron(III) nitrate concentration on tungsten chemical-mechanical-planarization performance. Appl. Surf. Sci. 2013, 282, 512–517. [Google Scholar] [CrossRef]
- Spindler, M.; Herold, S.; Acker, J.; Brachmann, E.; Oswald, S.; Menzel, S.; Rane, G. Chemical etching of Tungsten thin films for high-temperature surface acoustic wave-based sensor devices. Thin Solid Films 2016, 612, 322–326. [Google Scholar] [CrossRef]
- Ru, L.; Hu, Y.; Gu, Y.; Zhang, F.; Cheng, B.; Zhou, Y.; Deng, S. Reinforcement of Silicon-Containing Arylacetylene/Quarts Fiber composites by poly(imide-co-siloxane) macromolecular coupling agent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 397, 012018. [Google Scholar] [CrossRef] [Green Version]
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A.P. Characterization and applications of nanoparticles modified in-flight with silica or silica-organic coatings. Nanomaterials 2018, 8, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-I.; Jeong, G.-P.; Lee, S.-J.; Lee, J.-C.; Lee, J.-M.; Park, J.-H.; Bae, J.-Y.; Park, J.-G. Scavenger with Protonated Phosphite Ions for Incredible Nanoscale ZrO2-Abrasive Dispersant Stability Enhancement and Related Tungsten-Film Surface Chemical–Mechanical Planarization. Nanomaterials 2021, 11, 3296. https://doi.org/10.3390/nano11123296
Kim S-I, Jeong G-P, Lee S-J, Lee J-C, Lee J-M, Park J-H, Bae J-Y, Park J-G. Scavenger with Protonated Phosphite Ions for Incredible Nanoscale ZrO2-Abrasive Dispersant Stability Enhancement and Related Tungsten-Film Surface Chemical–Mechanical Planarization. Nanomaterials. 2021; 11(12):3296. https://doi.org/10.3390/nano11123296
Chicago/Turabian StyleKim, Seong-In, Gi-Ppeum Jeong, Seung-Jae Lee, Jong-Chan Lee, Jun-Myeong Lee, Jin-Hyung Park, Jae-Young Bae, and Jea-Gun Park. 2021. "Scavenger with Protonated Phosphite Ions for Incredible Nanoscale ZrO2-Abrasive Dispersant Stability Enhancement and Related Tungsten-Film Surface Chemical–Mechanical Planarization" Nanomaterials 11, no. 12: 3296. https://doi.org/10.3390/nano11123296
APA StyleKim, S.-I., Jeong, G.-P., Lee, S.-J., Lee, J.-C., Lee, J.-M., Park, J.-H., Bae, J.-Y., & Park, J.-G. (2021). Scavenger with Protonated Phosphite Ions for Incredible Nanoscale ZrO2-Abrasive Dispersant Stability Enhancement and Related Tungsten-Film Surface Chemical–Mechanical Planarization. Nanomaterials, 11(12), 3296. https://doi.org/10.3390/nano11123296






