Fe3O4 Nanoparticles on 3D Porous Carbon Skeleton Derived from Rape Pollen for High-Performance Li-Ion Capacitors
Abstract
:1. Introduction
2. Materials and Methods
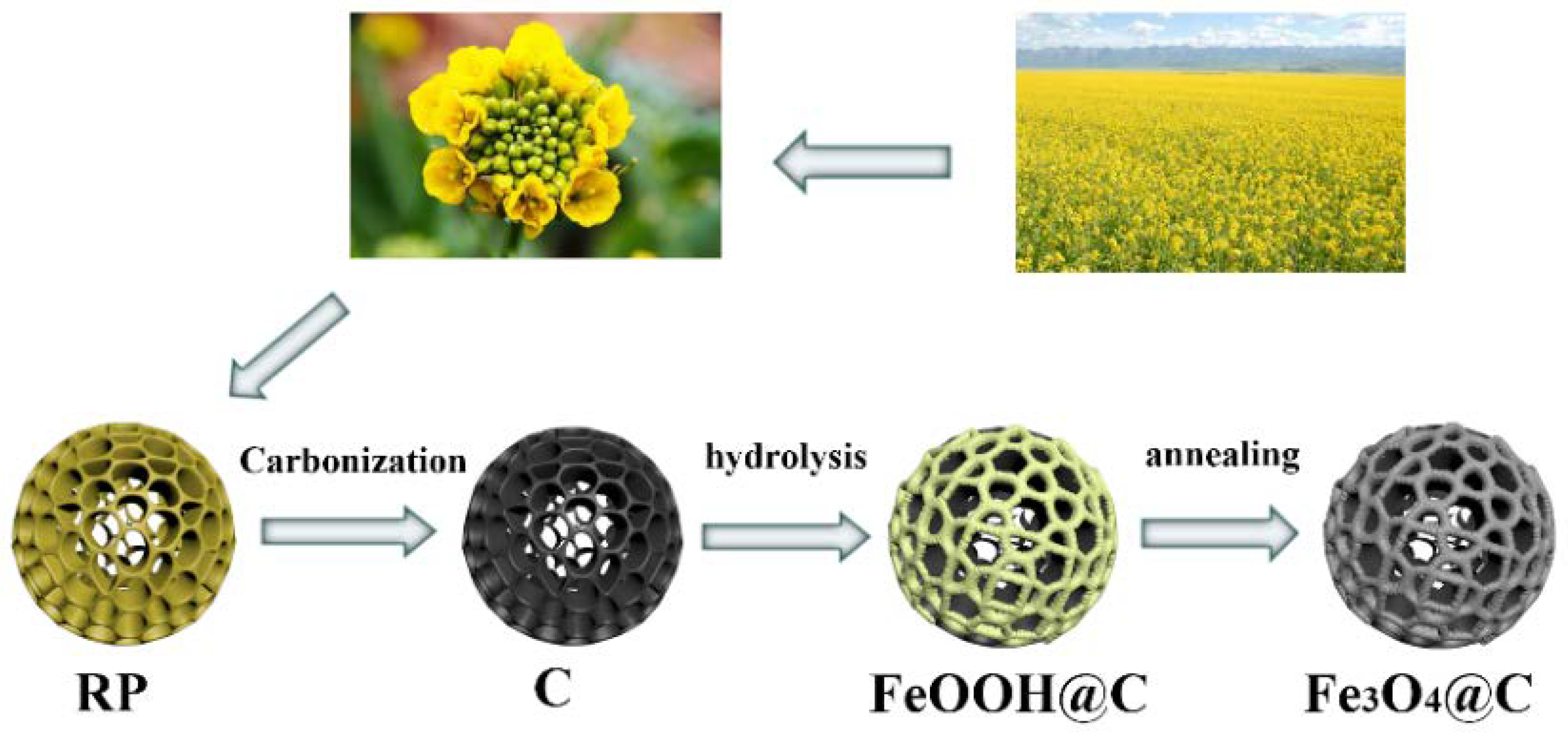
2.1. Pretreatment and Carbonization of Rape Pollen (RP)
2.2. Fabrication of Fe3O4@C Hollow Porous Composite
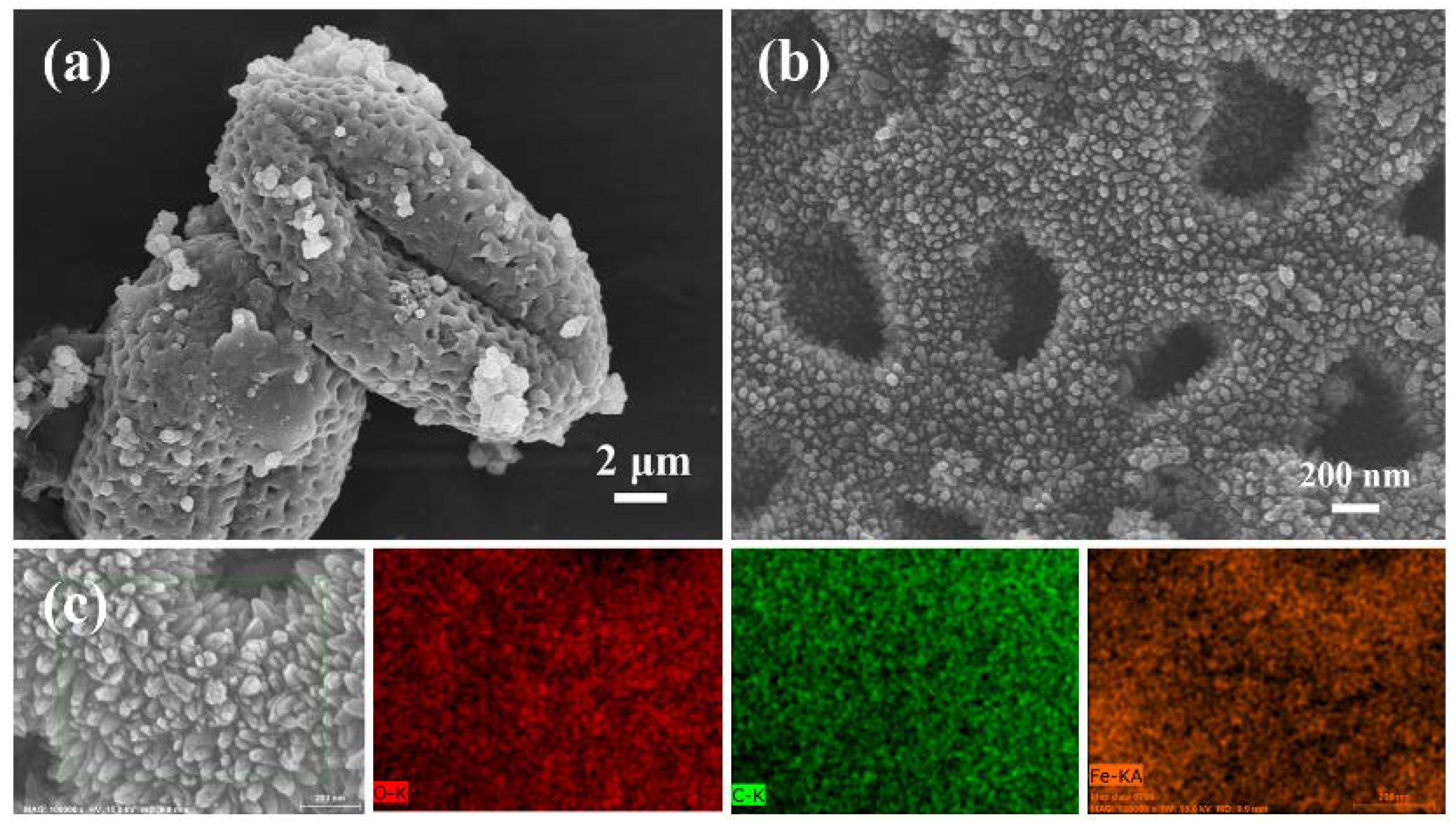
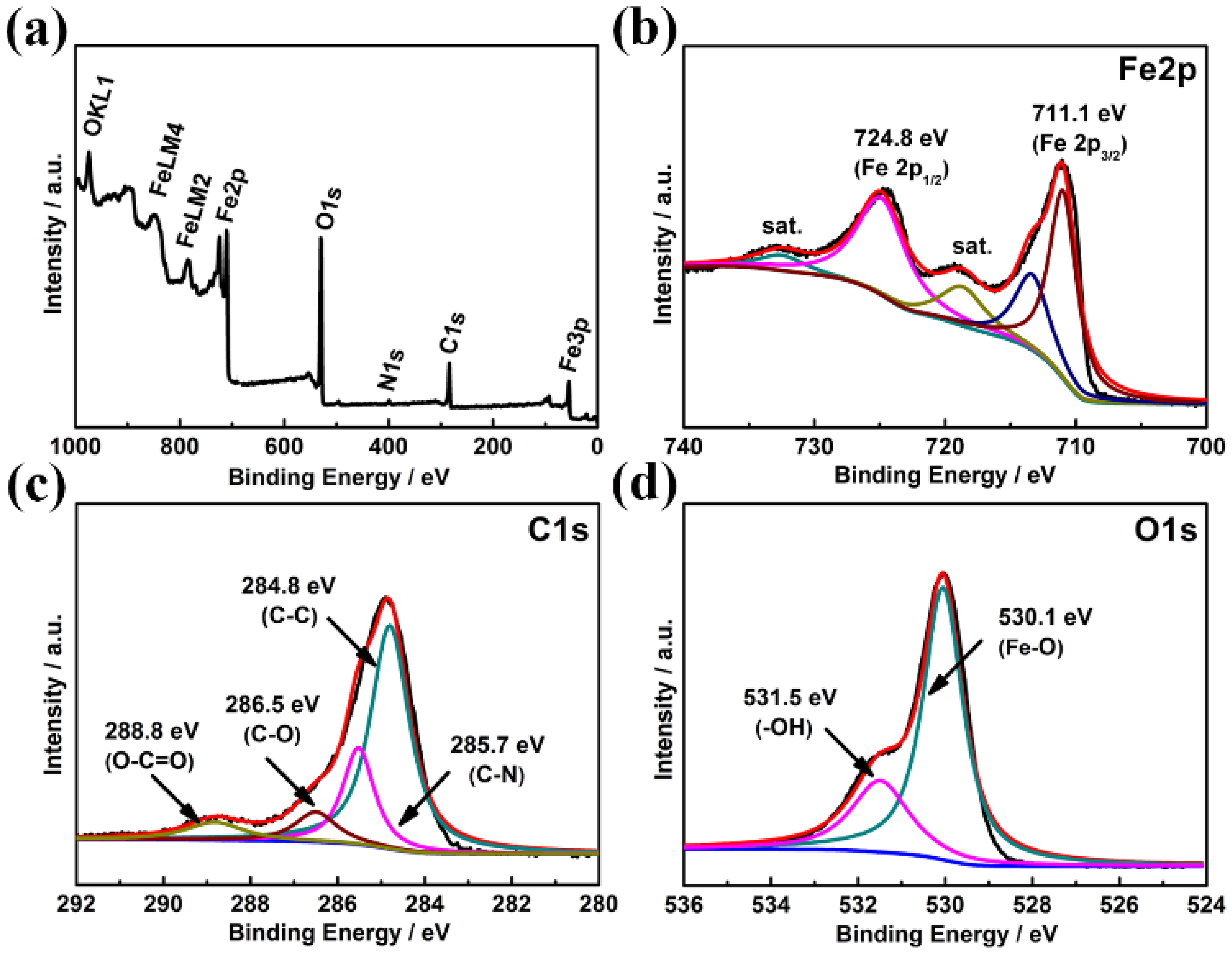
2.3. Material Characterization
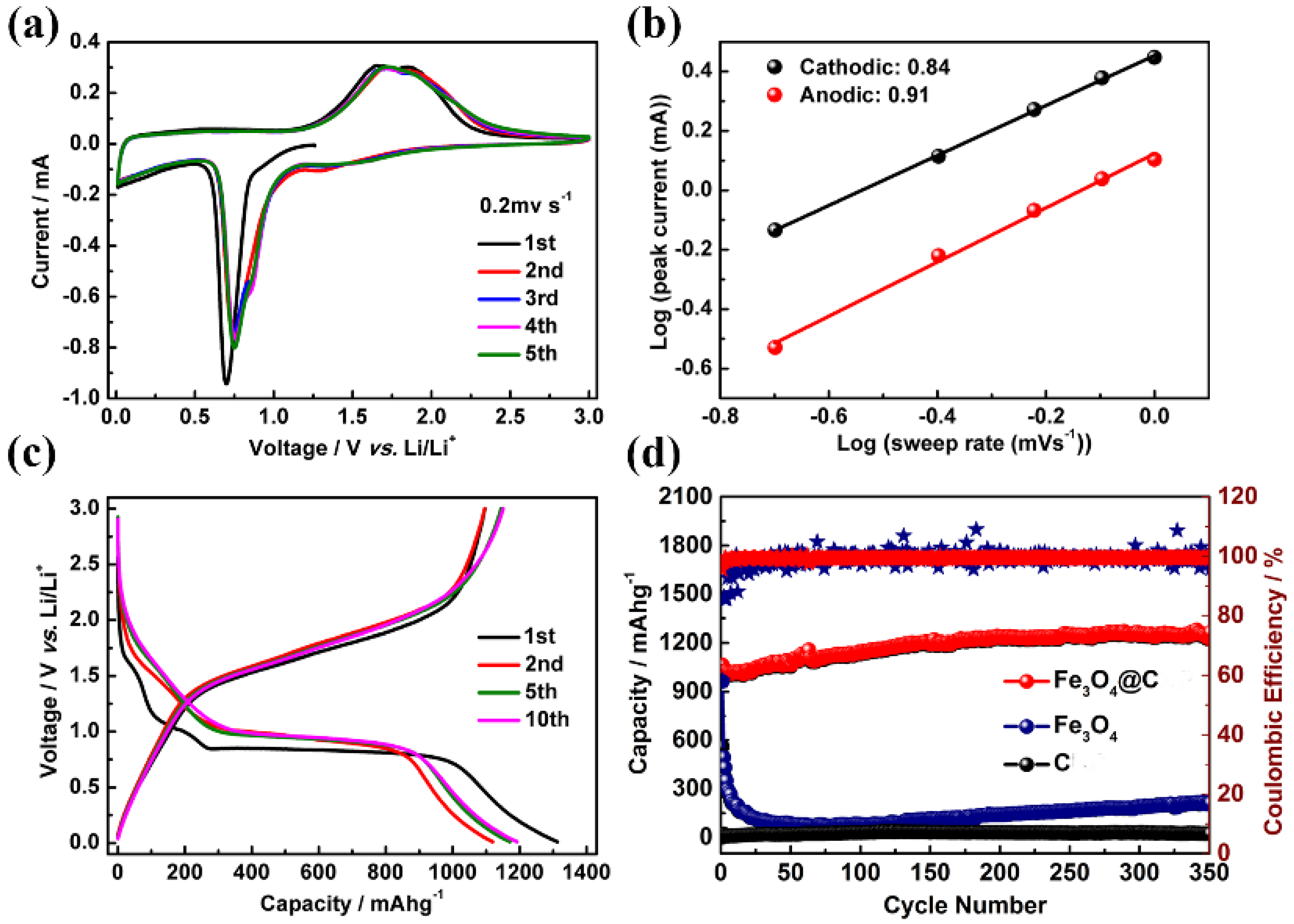
2.4. Fabrication and Evaluation of the Half Cells and LICs
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franta, B. Early oil industry knowledge of CO2 and global warming. Nat. Clim. Chang. 2018, 8, 1024–1025. [Google Scholar] [CrossRef]
- Searchinger, T.D.; Beringer, T.; Holtsmark, B.; Kammen, D.M.; Lambin, E.F.; Lucht, W.; Raven, P.; Van Ypersele, J.-P. Europe’s renewable energy directive poised to harm global forests. Nat. Commun. 2018, 9, 3741. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Xu, L.; Hu, G.; Mai, L.; Cui, Y. Nanowires for electrochemical energy storage. Chem. Rev. 2019, 119, 11042–11109. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A. LiFePO4/C nanocomposites for lithium-ion batteries. J. Power Sources 2017, 343, 395–411. [Google Scholar] [CrossRef]
- Zhu, J.; Wierzbicki, T.; Li, W. A review of safety-focused mechanical modeling of commercial lithium-ion batteries. J. Power Sources 2018, 378, 153–168. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, D.; Cheng, M.; Zhou, J.; Owusu, K.A.; Mai, L.; Yu, Y. A New view of supercapacitors: Integrated supercapacitors. Adv. Energy Mater. 2019, 9, 1901081. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy–power rivals lithium ion capacitors. Energy Environ. Sci. 2014, 8, 941–955. [Google Scholar] [CrossRef]
- Jeżowski, P.; Crosnier, O.; Deunf, E.; Poizot, P.; Beguin, F.; Brousse, T. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat. Mater. 2017, 17, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Xu, G.; Han, X.; Zhao, J.; Zhou, X.; Cui, G. Lithium ion capacitors in organic electrolyte system: Scientific problems, material development, and key technologies. Adv. Energy Mater. 2018, 8, 1801243. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode materials, electrolytes, and challenges in nonaqueous lithium-ion capacitors. Adv. Mater. 2018, 30, e1705670. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Li, C.; Sun, X.; Liu, J.; Wang, K.; Ma, Y. High-performance lithium-ion capacitors based on CoO-graphene composite anode and holey carbon nanolayer cathode. ACS Sustain. Chem. Eng. 2019, 7, 11275–11283. [Google Scholar] [CrossRef]
- Li, H.; Lang, J.; Lei, S.; Chen, J.; Wang, K.; Liu, L.; Zhang, T.; Liu, W.; Yan, X. A High-performance sodium-ion hybrid capacitor constructed by metal-organic framework-derived anode and cathode materials. Adv. Funct. Mater. 2018, 28, 1800757. [Google Scholar] [CrossRef]
- Xia, H.; Wan, Y.; Yuan, G.; Fu, Y.; Wang, X. Fe3O4/carbon core–shell nanotubes as promising anode materials for lithium-ion batteries. J. Power Sources 2013, 241, 486–493. [Google Scholar] [CrossRef]
- Jin, S.; Deng, H.; Long, D.; Liu, X.; Zhan, L.; Liang, X.; Qiao, W.; Ling, L. Facile synthesis of hierarchically structured Fe3O4/carbon micro-flowers and their application to lithium-ion battery anodes. J. Power Sources 2011, 196, 3887–3893. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Yang, Z.; Su, D.; Yang, J.; Wang, J. Fe3O4/C composite with hollow spheres in porous 3D-nanostructure as anode material for the lithium-ion batteries. J. Power Sources 2017, 363, 161–167. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, M.; Catanach, J.; Liaw, T.; Fei, L.; Deng, S.; Luo, H. Solvothermal route based in situ carbonization to Fe3O4@C as anode material for lithium ion battery. Nano Energy 2014, 8, 126–132. [Google Scholar] [CrossRef]
- Taberna, P.-L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.-M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Guo, G.; Yan, Y.; Li, T.; Wang, B.; Dong, A. Pomegranate-like, carbon-coated Fe3O4 nanoparticle superparticles for high-performance lithium storage. Energy Storage Mater. 2018, 10, 32–39. [Google Scholar] [CrossRef]
- Park, G.D.; Hong, J.H.; Jung, D.S.; Lee, J.-H.; Kang, Y.C. Unique structured microspheres with multishells comprising graphitic carbon-coated Fe3O4 hollow nanopowders as anode materials for high-performance Li-ion batteries. J. Mater. Chem. A 2019, 7, 15766–15773. [Google Scholar] [CrossRef]
- Usui, H.; Domi, Y.; Iwama, E.; Kurokawa, H.; Sakaguchi, H. α-Fe2O3 conversion anodes with improved Na-storage properties by Sb addition. Mater. Chem. Phys. 2021, 272, 125023. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.T.; Ogrodnik, A.; Gasteiger, H. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries. Mater. Today 2018, 21, 825–833. [Google Scholar] [CrossRef]
- Feng, Q.; Li, H.; Tan, Z.; Huang, Z.; Jiang, L.; Zhou, H.; Pan, H.; Zhou, Q.; Ma, S.; Kuang, Y. Design and preparation of three-dimensional MnO/N-doped carbon nanocomposites based on waste biomass for high storage and ultra-fast transfer of lithium ions. J. Mater. Chem. A 2018, 6, 19479–19487. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Cai, H.; Zhou, H.; Huang, Z.; Hou, Z.; Wu, J.; Yang, W.; Yi, H.; Fu, C.; et al. Ultra-thin Fe3C nanosheets promote the adsorption and conversion of polysulfides in lithium-sulfur batteries. Energy Storage Mater. 2018, 18, 338–348. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Tian, K.; Zhang, X.; Jin, Y.; Shi, T.; Guo, X. 3D Porous hierarchical microspheres of activated carbon from nature through nanotechnology for electrochemical double-layer capacitors. ACS Sustain. Chem. Eng. 2016, 4, 6463–6472. [Google Scholar] [CrossRef]
- Wan, L.; Wei, W.; Xie, M.; Zhang, Y.; Li, X.; Xiao, R.; Chen, J.; Du, C. Nitrogen, sulfur co-doped hierarchically porous carbon from rape pollen as high-performance supercapacitor electrode. Electrochim. Acta 2019, 311, 72–82. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D Aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. 2007, 120, 379–382. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Jia, Q.-C.; Kong, L.-B. Multi-dimensional hybrid heterostructure MoS2@C nanocomposite as a highly reversible anode for high-energy lithium-ion capacitors. Appl. Surf. Sci. 2020, 531, 147222. [Google Scholar] [CrossRef]
- Hou, W.; Zhou, Z.; Zhang, L.; Zhao, S.; Peng, B.; Hu, Z.; Ren, W.; Ye, Z.-G.; Jiang, Z.-D.; Liu, M. Low-voltage-manipulating spin dynamics of flexible Fe3O4 films through ionic gel gating for wearable devices. ACS Appl. Mater. Interfaces 2019, 11, 21727–21733. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kawaguchi, K.; Koshizaki, N.; Sohma, M.; Matsumoto, T. Fabrication and magnetoresistance of tunnel junctions using half-metallic Fe3O4. J. Appl. Phys. 2003, 93, 8032–8034. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, Z.; Li, G. Structural evolution from mesoporous α-Fe2O3 to Fe3O4@C and γ-Fe2O3 nanospheres and their lithium storage performances. CrystEngComm 2011, 13, 4709–4713. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Y.; Ren, Z.; Jian, N.; Gao, M.; Wu, Y.; Zhu, M.; Pan, F.; Liu, Y.; Pan, H. A new strategy to effectively suppress the initial capacity fading of iron oxides by reacting with LiBH4. Adv. Funct. Mater. 2017, 27, 1700342. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, T.; Wang, L.; Ma, L.; Hu, Y.; Chen, R.; Wang, Y.; Wang, C.; Yan, W.; Tie, Z.; et al. High energy density hybrid lithium-ion capacitor enabled by Co3ZnC@N-doped carbon nanopolyhedra anode and microporous carbon cathode. Energy Storage Mater. 2018, 14, 246–252. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Wang, L.; Gao, J.; Li, Q.; Ma, Y.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. High-rate capability of three-dimensionally ordered macroporous T-Nb2O5 through Li+ intercalation pseudocapacitance. J. Power Sources 2017, 361, 80–86. [Google Scholar] [CrossRef]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-dimensional graphene foam supported Fe3O4 lithium batteryanodes with long cycle life and high rate capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Han, C.; Xu, L.; Li, H.; Shi, R.; Zhang, T.; Li, J.; Wong, C.-P.; Kang, F.; Lin, Z.; Li, B. Biopolymer-assisted synthesis of 3D interconnected Fe3O4@carbon core@shell as anode for asymmetric lithium ion capacitors. Carbon 2018, 140, 296–305. [Google Scholar] [CrossRef]
- Yu, X.; Deng, J.; Zhan, C.; Lv, R.; Huang, Z.-H.; Kang, F. A high-power lithium-ion hybrid electrochemical capacitor based on citrate-derived electrodes. Electrochim. Acta 2017, 228, 76–81. [Google Scholar] [CrossRef]
- Shi, R.; Han, C.; Xu, X.; Qing, X.; Xu, L.; Li, H.; Li, J.; Wong, C.-P.; Li, B. Electrospun N-doped hierarchical porous carbon nanofiber with improved degree of graphitization for high-performance lithium ion capacitor. Chem.-A Eur. J. 2018, 24, 10460–10467. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Brandt, A.; Balducci, A. A study about the use of carbon coated iron oxide-based electrodes in lithium-ion capacitors. Electrochim. Acta 2013, 108, 219–225. [Google Scholar] [CrossRef]
- Liu, S.; Luo, Z.; Tian, G.; Zhu, M.; Cai, Z.; Pan, A.; Liang, S. TiO2 nanorods grown on carbon fiber cloth as binder-free electrode for sodium-ion batteries and flexible sodium-ion capacitors. J. Power Sources 2017, 363, 284–290. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Shi, J.; Liu, W.; Shi, Z.; Chen, S.; Wang, X.; Wang, H. Two-dimensional biomass-derived carbon nanosheets and MnO/carbon electrodes for high-performance Li-ion capacitors. J. Mater. Chem. A 2017, 5, 15243–15252. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Zhang, X.; Sun, X.; Wang, K.; Ma, Y. High performance lithium-ion hybrid capacitors employing Fe3O4–graphene composite anode and activated carbon cathode. ACS Appl. Mater. Interfaces 2017, 9, 17136–17144. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Chen, X.; Tan, S.; He, Y.; Saha, P.; Cheng, Q. Fe3O4 Nanoparticles on 3D Porous Carbon Skeleton Derived from Rape Pollen for High-Performance Li-Ion Capacitors. Nanomaterials 2021, 11, 3355. https://doi.org/10.3390/nano11123355
Sun M, Chen X, Tan S, He Y, Saha P, Cheng Q. Fe3O4 Nanoparticles on 3D Porous Carbon Skeleton Derived from Rape Pollen for High-Performance Li-Ion Capacitors. Nanomaterials. 2021; 11(12):3355. https://doi.org/10.3390/nano11123355
Chicago/Turabian StyleSun, Mingshan, Xinan Chen, Shutian Tan, Ying He, Petr Saha, and Qilin Cheng. 2021. "Fe3O4 Nanoparticles on 3D Porous Carbon Skeleton Derived from Rape Pollen for High-Performance Li-Ion Capacitors" Nanomaterials 11, no. 12: 3355. https://doi.org/10.3390/nano11123355





