Pt3Ni@C Composite Material Designed and Prepared Based on Volcanic Catalytic Curve and Its High-Performance Static Lithium Polysulfide Semiliquid Battery
Abstract
:1. Introduction
2. Experimental Section
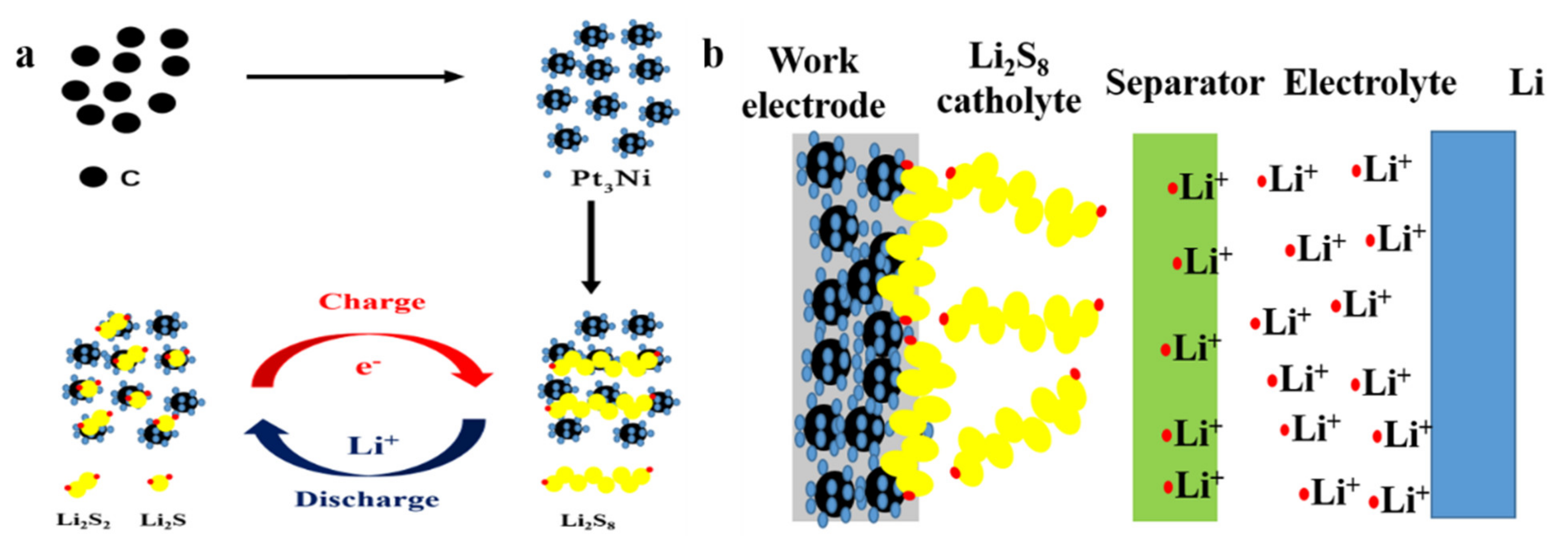
2.1. Preparation of Pt3Ni@C Composite Materials
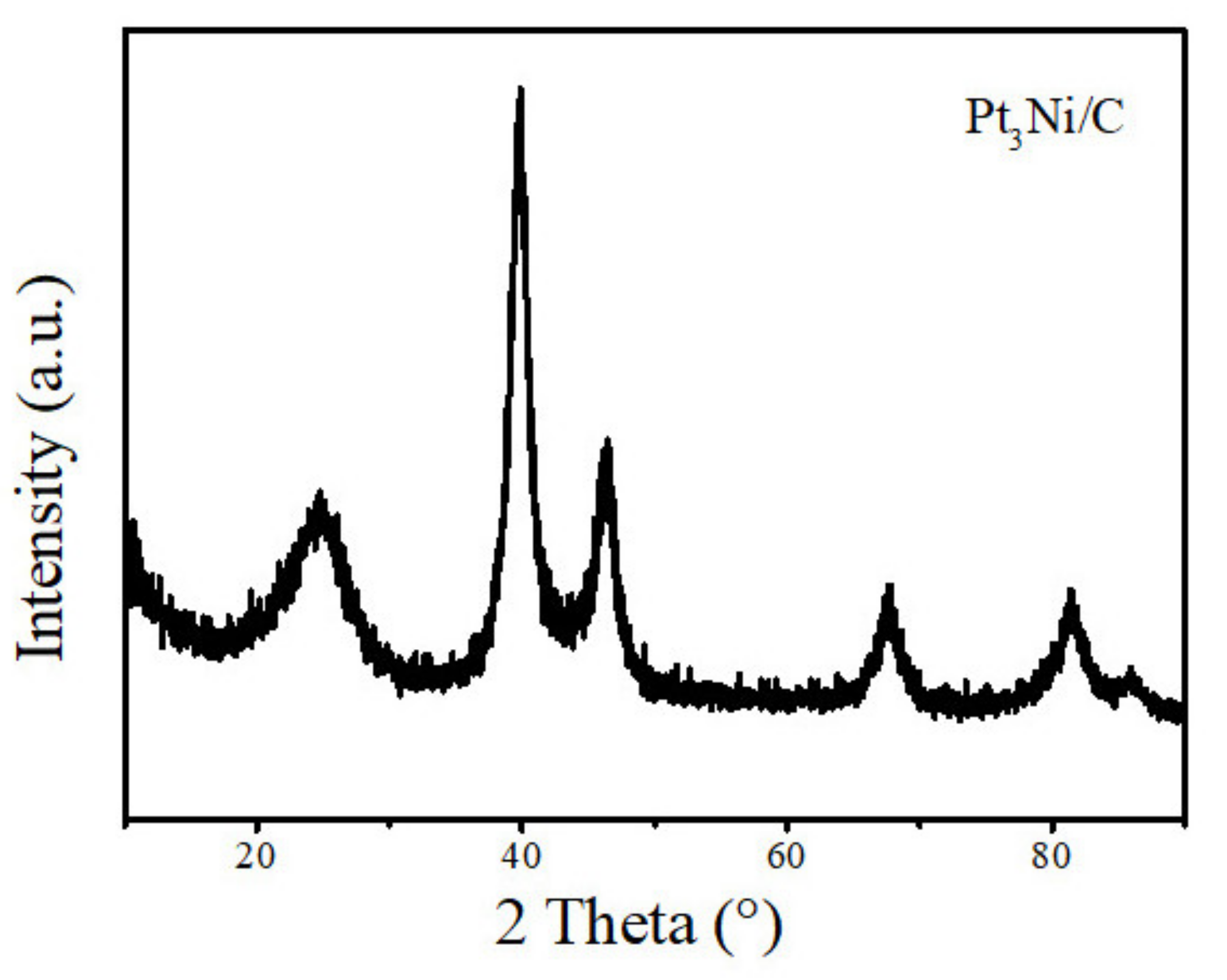
2.2. Material Characterization
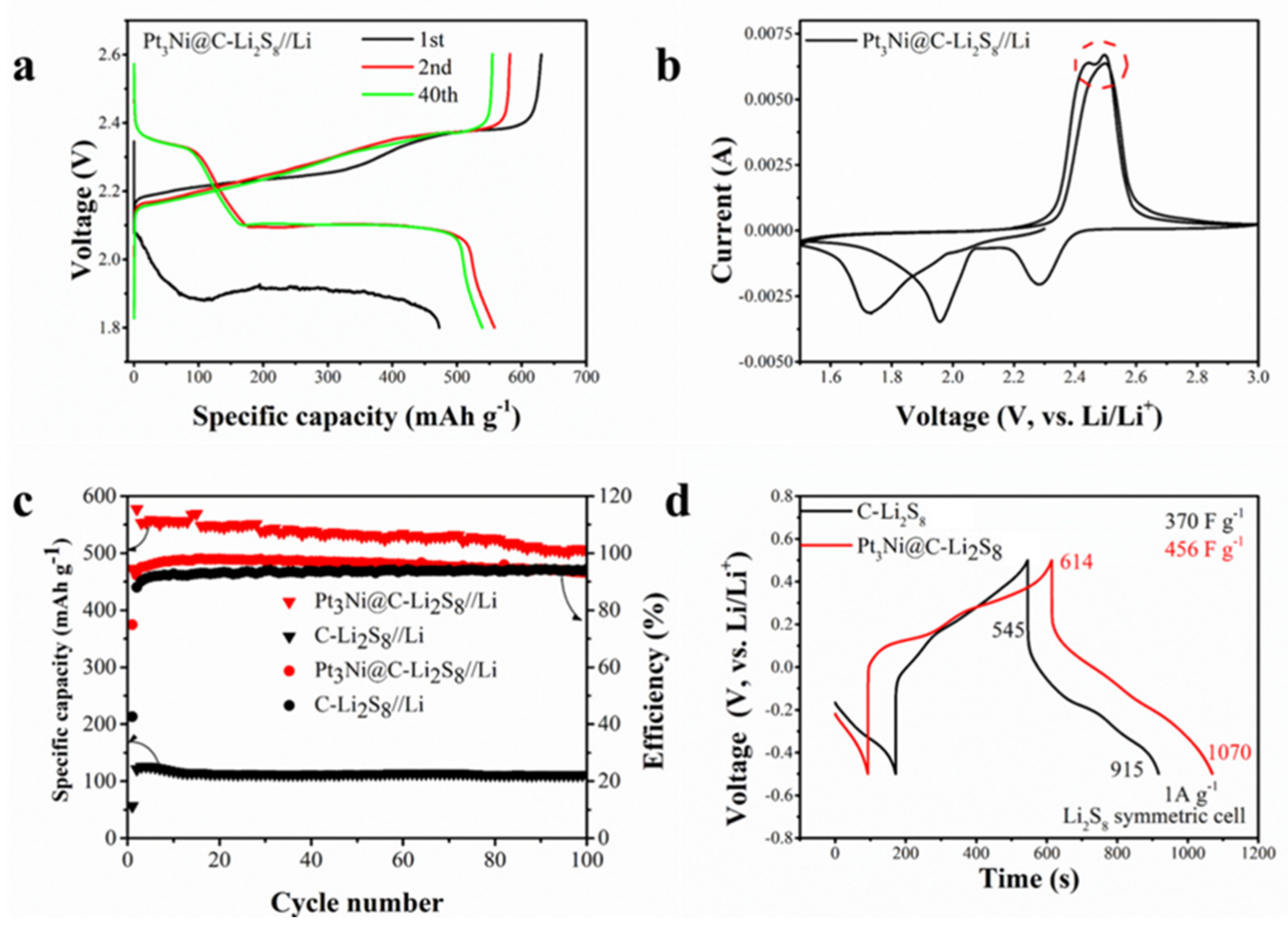
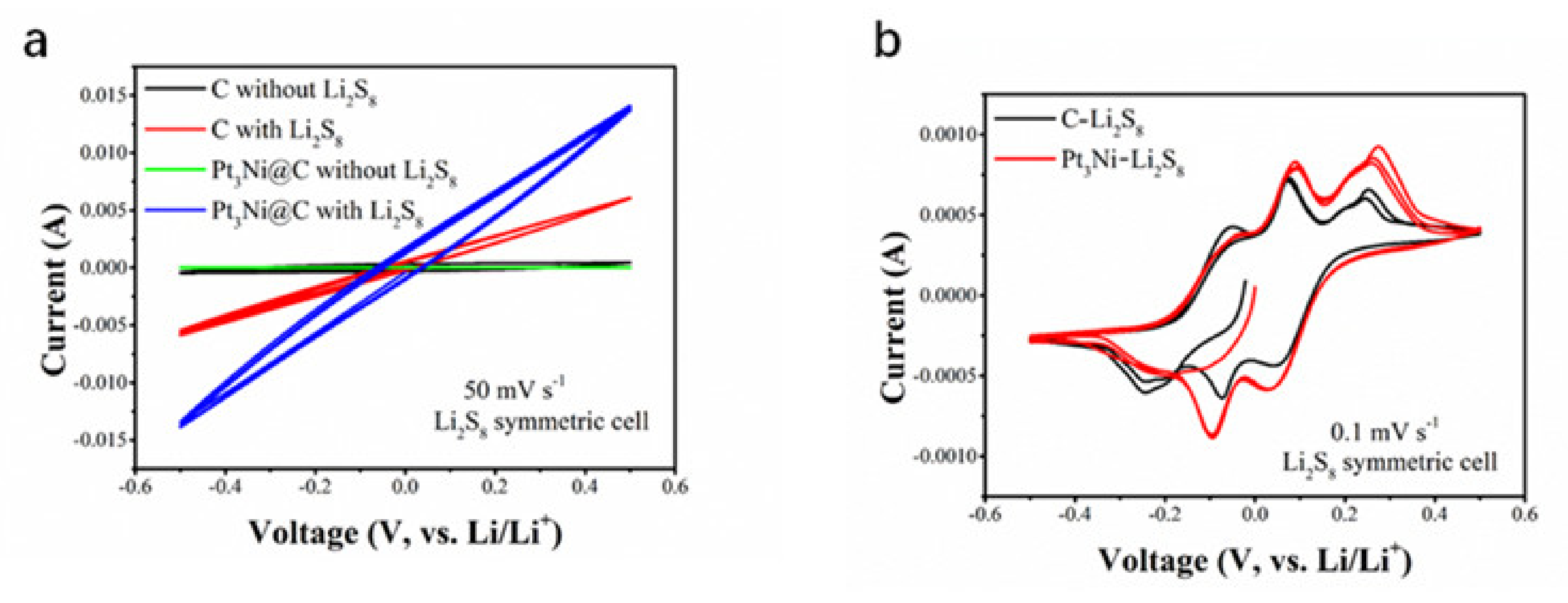
2.3. Cell Assembly and Electrochemical Measurements
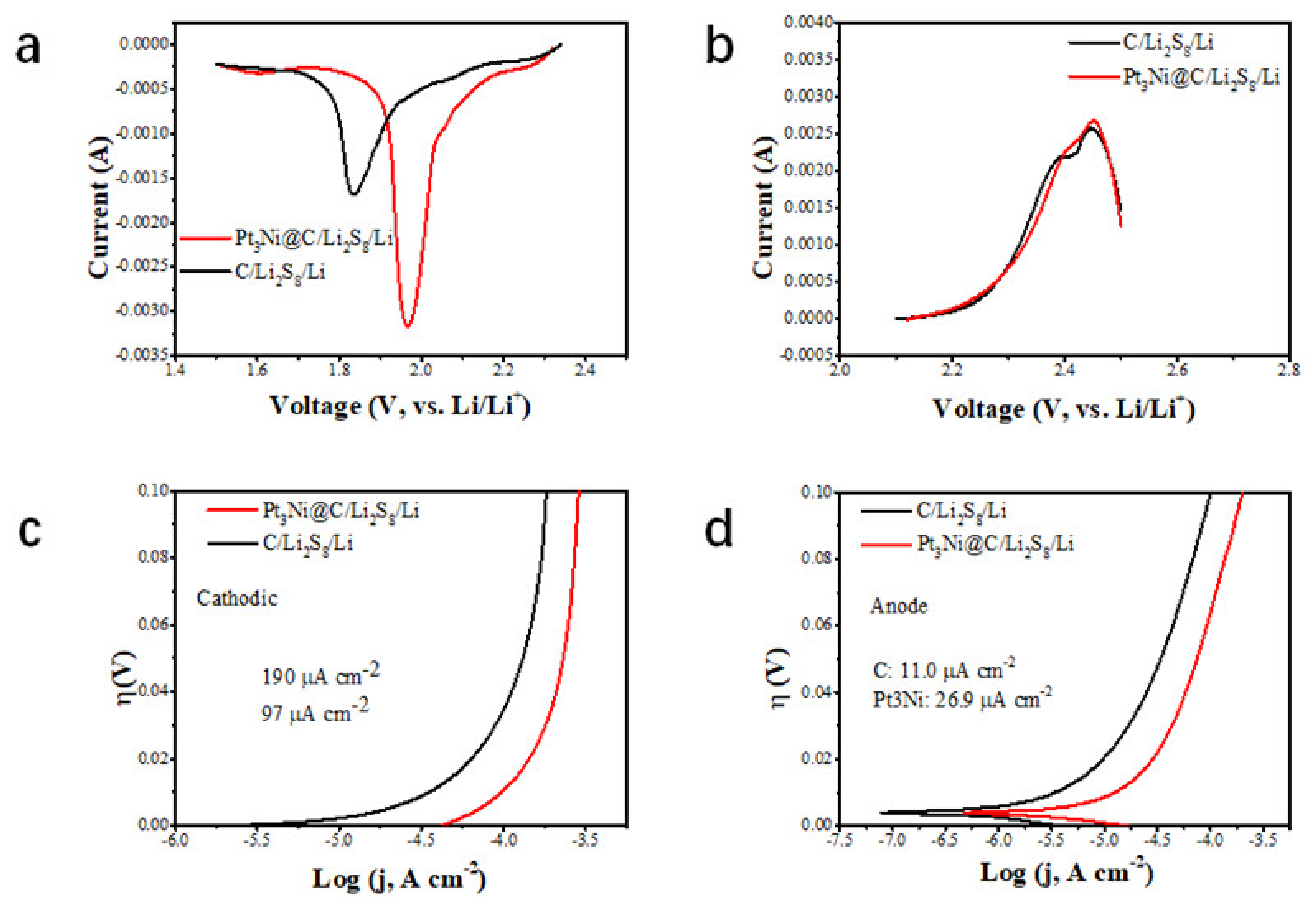
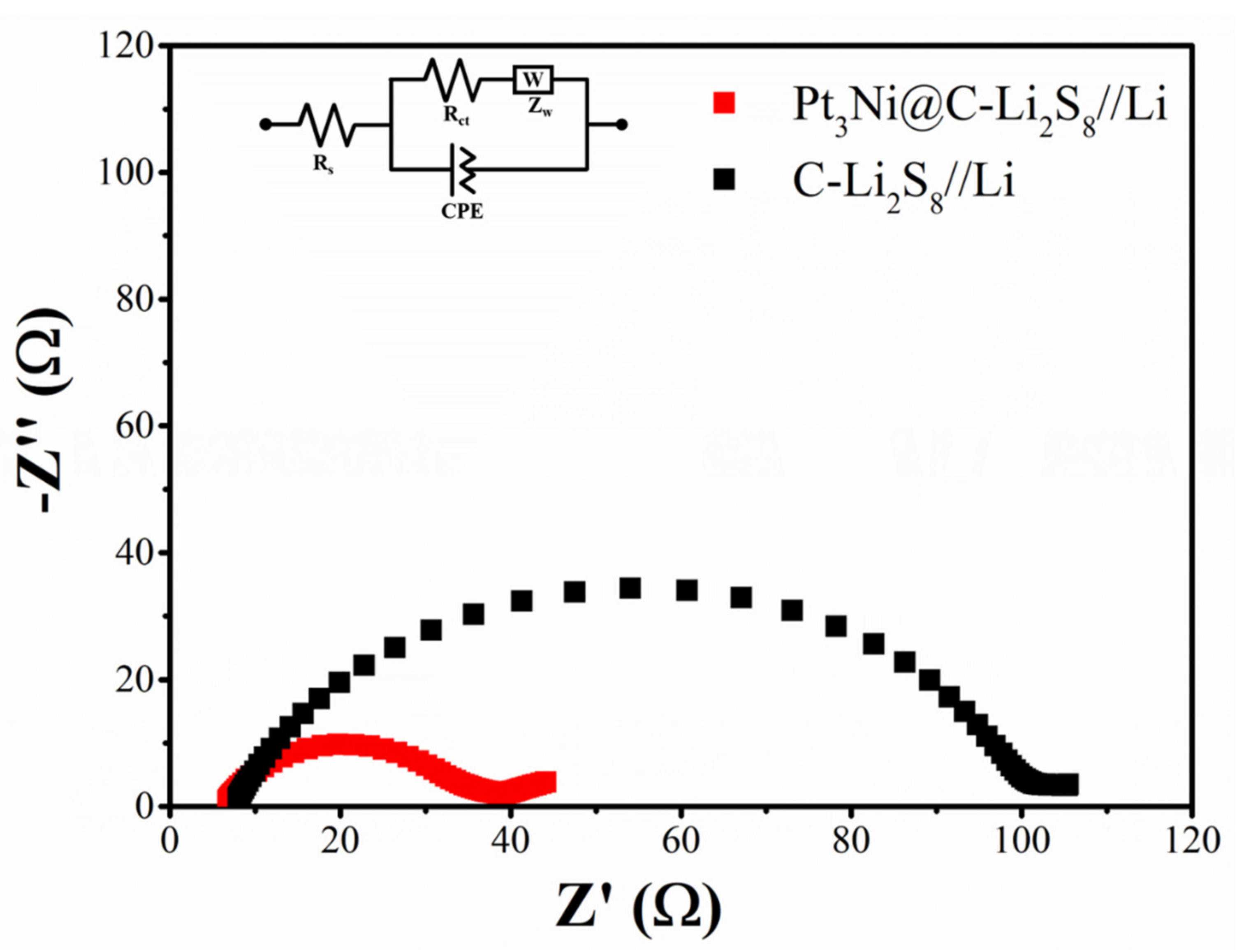
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zheng, G.; Cui, Y. A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy Environ. Sci. 2013, 6, 1552–1558. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium-sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium–sulphur batteries. J. Power Sources 2014, 255, 204–218. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Cheng, X.-B.; Chen, W.-J.; Titirici, M.-M.; Huang, J.-Q.; Yuan, T.-Q.; Zhang, Q. A review of naturally derived nanostructured materials for safe lithium metal batteries. Mater. Today Nano 2019, 8, 100049. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Siadati, M.H.; De La Rosa, M.P.; Berhault, G.; Wilcoxon, J.P.; Bearden, R.; Abrams, B.L. Catalytic Properties of Single Layers of Transition Metal Sulfide Catalytic Materials. Catal. Rev. 2006, 48, 1–41. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-Impregnated Disordered Carbon Nanotubes Cathode for Lithium–Sulfur Batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tran, D.T.; Zhang, Z. Poly(acrylic acid) gel as a polysulphide blocking layer for high-performance lithium/sulphur battery. J. Mater. Chem. A 2014, 2, 18288–18292. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef] [Green Version]
- de León, C.P.; Frías-Ferrer, A.; González-García, J.; Szánto, D.A.; Walsh, F.C. Redox flow cells for energy conversion. J. Power Sources 2006, 160, 716–732. [Google Scholar] [CrossRef] [Green Version]
- Skyllaskazacos, M.; Chakrabarti, H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55–R79. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q. Next-Generation, High-Energy-Density Redox Flow Batteries. ChemPlusChem 2015, 80, 312–322. [Google Scholar] [CrossRef]
- Li, W.; Liang, Z.; Lu, Z.; Tao, X.; Liu, K.; Yao, H.; Cui, Y. Magnetic Field-Controlled Lithium Polysulfide Semiliquid Battery with Ferrofluidic Properties. Nano Lett. 2015, 15, 7394–7399. [Google Scholar] [CrossRef]
- Darling, R.M.; Gallagher, K.G.; Kowalski, J.A.; Ha, S.; Brushett, F.R. Pathways to low-cost electrochemical energy storage: A comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 2014, 7, 3459–3477. [Google Scholar] [CrossRef] [Green Version]
- Brushett, F.R.; Vaughey, J.T.; Jansen, A.N. An All-Organic Non-aqueous Lithium-Ion Redox Flow Battery. Adv. Energy Mater. 2012, 2, 1390–1396. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.-M. Semi-Solid Lithium Rechargeable Flow Battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Hamelet, S.; Tzedakis, T.; Leriche, J.B.; Sailler, S.; Larcher, D.; Taberna, P.L.; Simon, P.; Tarascon, J.M. Non-Aqueous Li-Based Redox Flow Batteries. J. Electrochem. Soc. 2012, 159, A1360–A1367. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Smith, K.C.; Dong, Y.; Baram, N.; Fan, F.Y.; Xie, J.; Limthongkul, P.; Carter, W.C.; Chiang, Y.-M. Aqueous semi-solid flow cell: Demonstration and analysis. Phys. Chem. Chem. Phys. 2013, 15, 15833–15839. [Google Scholar] [CrossRef]
- Wei, T.-S.; Fan, F.; Helal, A.; Smith, K.C.; McKinley, G.H.; Chiang, Y.-M.; Lewis, J.A. Biphasic Electrode Suspensions for Li-Ion Semi-solid Flow Cells with High Energy Density, Fast Charge Transport, and Low-Dissipation Flow. Adv. Energy Mater. 2015, 15, 1500535–1500588. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.Y.; Woodford, W.H.; Li, Z.; Baram, N.; Smith, K.C.; Helal, A.; McKinley, G.H.; Carter, W.C.; Chiang, Y.-M. Polysulfide Flow Batteries Enabled by Percolating Nanoscale Conductor Networks. Nano Lett. 2014, 3, 2210–2218. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Morcrette, M.; Gangulibabu, A. Gueguen, Rémi Dedryvère, Jean-Marie Tarascon, Li-S batteries: Simple ap-proaches for superior performance. Energy Environ. Sci. 2013, 6, 176–182. [Google Scholar] [CrossRef]
- Lu, Y.; Goodenough, J.B.; Kim, Y. Aqueous Cathode for Next-Generation Alkali-Ion Batteries. J. Am. Chem. Soc. 2011, 133, 5756–5759. [Google Scholar] [CrossRef]
- Lu, Y.; Goodenough, J.B. Rechargeable alkali-ion cathode-flow battery. J. Mater. Chem. 2011, 21, 10113–10117. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Q.; Liang, Z.; Liu, H.; Li, Q.; Lu, Y.-C. Sulphur-impregnated flow cathode to enable high-energy-density lithium flow batteries. Nat. Commun. 2015, 6, 5877–5885. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2012, 46, 1125–1134. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New Approaches for High Energy Density Lithium–Sulfur Battery Cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystal-lites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 51, 12798–12801. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, J.; Ying, Z.; Cai, Q.; Zheng, G.; Gan, Y.; Huang, H.; Xia, Y.; Lian, C.; Zhang, W.; et al. Strong Sulfur Binding with Conducting Magnéli-Phase TinO2n-1 Nanomaterials for Improving Lithium-Sulfur Batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zheng, G.; Hsu, P.-C.; Kong, D.; Cha, J.J.; Li, W.; Seh, Z.W.; McDowell, M.T.; Yan, K.; Liang, Z.; et al. Improving lithium–sulphur batteries through spatial control of sulphur species deposition on a hybrid electrode surface. Nat. Commun. 2014, 5, 3943. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-D.; Zhuang, Q.-C.; Tian, L.-L.; Qin, Y.-P.; Fang, L.; Sun, S.-G. Impedance Spectra of Nonhomogeneous, Multilayered Po-rous Composite Graphite Electrodes for Li-Ion Batteries: Experimental and Theoretical Studies. J. Phys. Chem. C 2011, 115, 9210–9219. [Google Scholar] [CrossRef]
- Qian, Y.; Lyu, Z.; Kim, D.-H.; Kang, D.J. Enhancing the output power density of polydimethylsiloxane-based flexible triboelectric nanogenerators with ultrathin nickel telluride nanobelts as a co-triboelectric layer. Nano Energy 2021, 90, 106536. [Google Scholar] [CrossRef]
- Qian, Y.; Du, J.; Kang, D.J. Enhanced electrochemical performance of porous Co-doped TiO2 nanomaterials prepared by a solvothermal method. Microporous Mesoporous Mater. 2019, 273, 148–155. [Google Scholar] [CrossRef]
- Sikha, G.; White, R.E. Analytical Expression for the Impedance Response for a Lithium-Ion Cell. J. Electrochem. Soc. 2008, 155, A893. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, H.; Tian, C.; Geng, D.; Wang, D.; Bai, S. One-Pot Synthesis of Highly Effcient Carbon-Supported Polyhedral Pt3Ni Alloy Nanoparticles for Oxygen Reduction Reaction. Electrocatalysis 2019, 10, 613–620. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yao, Y.; Chen, Y.; Hou, J.; Ni, Z.; Wang, Y.; Hu, X.; Sun, Y.; Ai, R.; Xian, Y.; et al. Pt3Ni@C Composite Material Designed and Prepared Based on Volcanic Catalytic Curve and Its High-Performance Static Lithium Polysulfide Semiliquid Battery. Nanomaterials 2021, 11, 3416. https://doi.org/10.3390/nano11123416
Wang Y, Yao Y, Chen Y, Hou J, Ni Z, Wang Y, Hu X, Sun Y, Ai R, Xian Y, et al. Pt3Ni@C Composite Material Designed and Prepared Based on Volcanic Catalytic Curve and Its High-Performance Static Lithium Polysulfide Semiliquid Battery. Nanomaterials. 2021; 11(12):3416. https://doi.org/10.3390/nano11123416
Chicago/Turabian StyleWang, Ying, Yao Yao, Yu Chen, Jiyue Hou, Zhicong Ni, Yanjie Wang, Xiuqiong Hu, Yanzhong Sun, Rui Ai, Yulin Xian, and et al. 2021. "Pt3Ni@C Composite Material Designed and Prepared Based on Volcanic Catalytic Curve and Its High-Performance Static Lithium Polysulfide Semiliquid Battery" Nanomaterials 11, no. 12: 3416. https://doi.org/10.3390/nano11123416



