Degradation of Perovskite Thin Films and Solar Cells with Candle Soot C/Ag Electrode Exposed in a Control Ambient
Abstract
:1. Introduction
2. Methodology
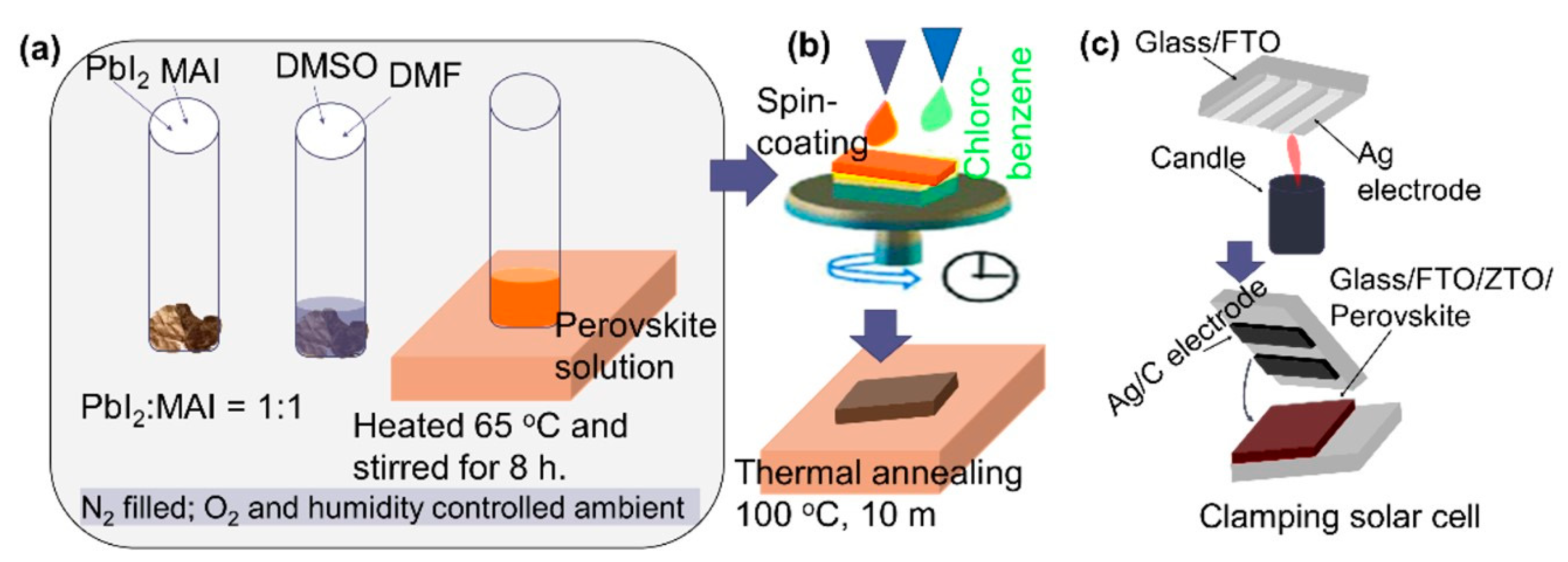
2.1. Preparation of Perovskite Films
2.2. Preparation of Perovskite Solar Cells (PSCs)
2.3. Measurement and Characterization
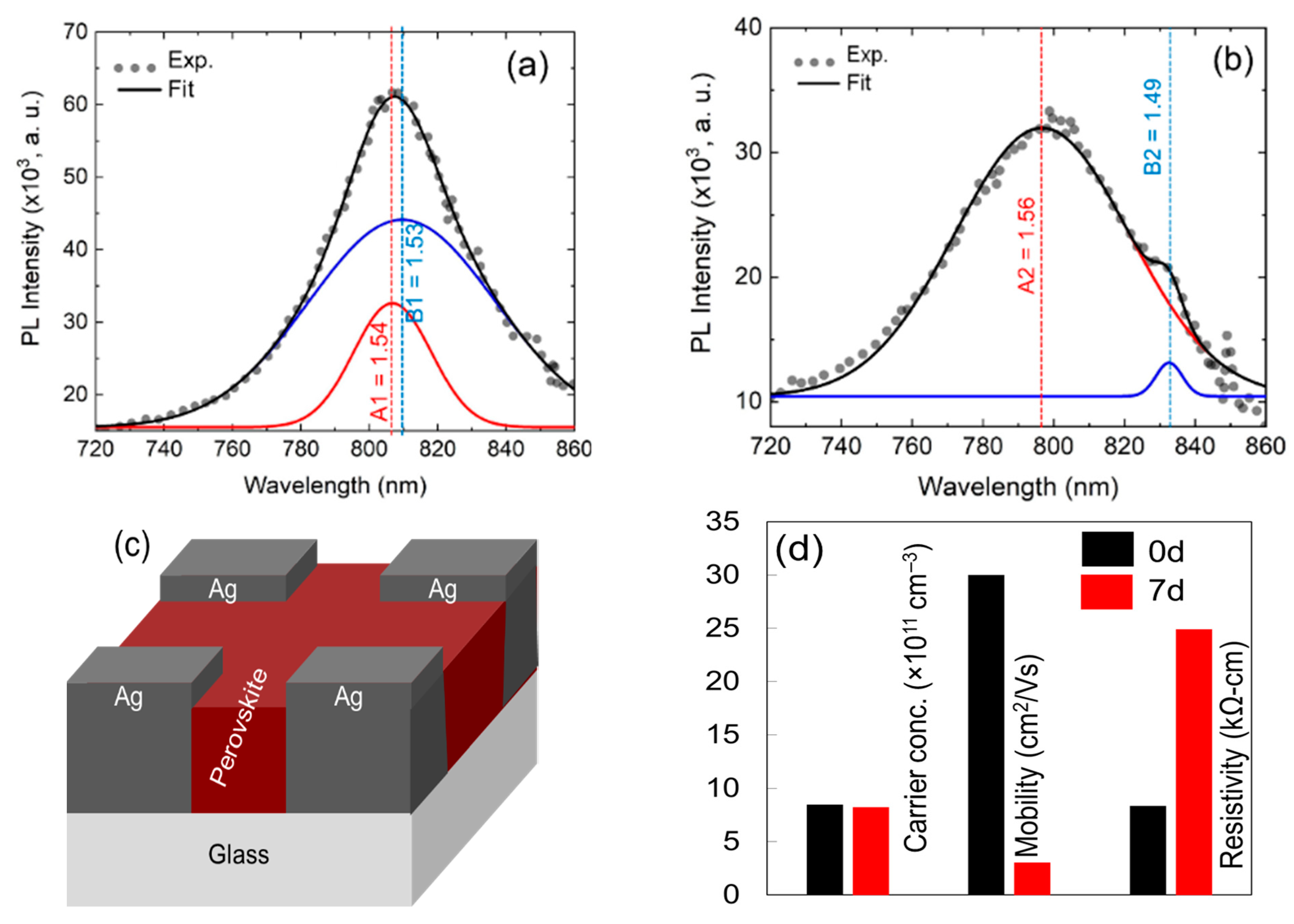
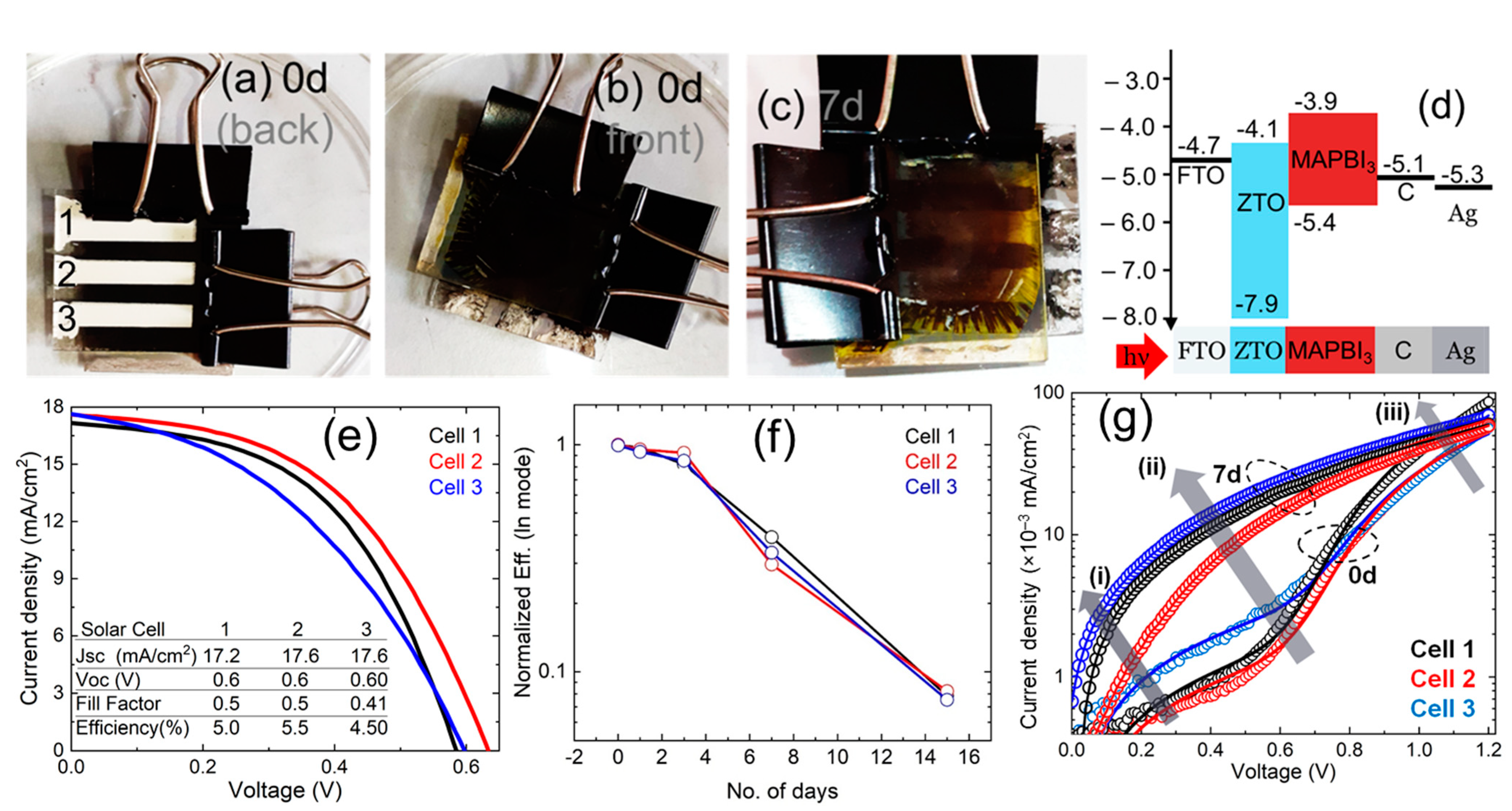
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tai, Q.; Tang, K.-C.; Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 2019, 12, 2375–2405. [Google Scholar] [CrossRef]
- Shi, Z.; Jayatissa, A.H. Perovskites-Based Solar Cells: A Review of Recent Progress, Materials and Processing Methods. Materials 2018, 11, 729. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Xue, Q.; Yao, Q.; Li, N.; Brabec, C.J.; Yip, H. Inorganic Halide Perovskite Solar Cells: Progress and Challenges. Adv. Energy Mater. 2020, 10, 2000183. [Google Scholar] [CrossRef]
- Li, D.; Shi, J.; Xu, Y.; Luo, Y.; Wu, H.; Meng, Q. Inorganic–organic halide perovskites for new photovoltaic technology. Natl. Sci. Rev. 2018, 5, 559–576. [Google Scholar] [CrossRef] [Green Version]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Il Seok, S.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Sun, J.; Jasieniak, J.J. Semi-transparent solar cells. J. Phys. D Applied Phys. 2017, 50, 093001. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Insights into Accelerated Degradation of Perovskite Solar Cells under Continuous Illumination Driven by Thermal Stress and Interfacial Junction. ACS Appl. Energy Mater. 2021, 4, 11121–11132. [Google Scholar] [CrossRef]
- Kim, M.; Alfano, A.; Perotto, G.; Serri, M.; Dengo, N.; Mezzetti, A.; Lamberti, F.; Gross, S.; Prato, M.; Salerno, M.; et al. Moisture resistance in perovskite solar cells attributed to a water-splitting layer. Commun. Mater. 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Guo, R.; Han, D.; Chen, W.; Dai, L.; Ji, K.; Xiong, Q.; Müller-Buschbaum, P.; Li, S.; Reb, L.K.; Scheel, M.A.; et al. Degradation mechanisms of perovskite solar cells under vacuum and one atmosphere of nitrogen. Nat. Energy 2021, 6, 977–986. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [Green Version]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2018, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Rahman, K.S.; Misran, H.; Asim, N.; Hossain, M.; Akhtaruzzaman, M.; Amin, N. High mobility and transparent ZTO ETM prepared by RF reactive co-sputtering for perovskite solar cell application. Results Phys. 2019, 14, 102518. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, K.; Chen, H.; Yi, Y.; Zhang, T.; Long, X.; Li, J.; Zhang, L.; Wang, J.; Yang, S. Cost-efficient clamping solar cells using candle soot for hole extraction from ambipolar perovskites. Energy Environ. Sci. 2014, 7, 3326–3333. [Google Scholar] [CrossRef]
- Liang, K.; Mitzi, D.B.; Prikas, M.T. Synthesis and Characterization of Organic−Inorganic Perovskite Thin Films Prepared Using a Versatile Two-Step Dipping Technique. Chem. Mater. 1998, 10, 403–411. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Yan, W.; Ye, S.; Peng, H.; Liu, Z.; Bian, Z.; Huang, C. High-Performance Planar Solar Cells Based On CH3NH3PbI3−xClx Perovskites with Determined Chlorine Mole Fraction. Adv. Funct. Mater. 2015, 25, 4867–4873. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Osman, M.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Aldhafiri, A.M.; Alghamdi, E.A. Effect of chlorobenzene on the optical and structural properties of CH3NH3PbI3:DMF perovskite films. J. Mater. Res. Technol. 2021, 14, 287–297. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Zheng, C.; Gao, D.; Huang, W. Advancements in the stability of perovskite solar cells: Degradation mechanisms and improvement approaches. RSC Adv. 2016, 6, 38079–38091. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Guo, B.-C.; Dai, Z.-Y.; Lin, C.-F.; Hsu, H.-P. PbI2 Single Crystal Growth and Its Optical Property Study. Crystals 2019, 9, 589. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- González-Juárez, E.; Valadez-Villalobos, K.; Garcia-Gutierrez, D.F.; Garcia-Gutierrez, D.I.; Roa, A.E.; Sanchez, E. Study on photovoltaic stability and performance by incorporating tetrabutyl phosphonium iodide into the active layer of a perovskite type photovoltaic cell. RSC Adv. 2020, 10, 31575–31585. [Google Scholar] [CrossRef]
- Zhuang, S.; Xu, D.; Xu, J.; Wu, B.; Zhang, Y.; Dong, X.; Li, G.; Zhang, B.; Du, G. Temperature-dependent photoluminescence on organic inorganic metal halide perovskite CH3NH3PbI3−xClx prepared on ZnO/FTO substrates using a two-step method. Chin. Phys. B 2017, 26, 017802. [Google Scholar] [CrossRef]
- Singh, S.; Li, C.; Panzer, F.; Narasimhan, K.L.; Graeser, A.; Gujar, T.P.; Köhler, A.; Thelakkat, M.; Huettner, S.; Kabra, D. Effect of Thermal and Structural Disorder on the Electronic Structure of Hybrid Perovskite Semiconductor CH3NH3PbI3. J. Phys. Chem. Lett. 2016, 7, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W.; Eperon, G.E.; Roe, E.T.; Warren, C.W.; Snaith, H.; Lonergan, M.C. Defect states in perovskite solar cells associated with hysteresis and performance. Appl. Phys. Lett. 2016, 109, 153902. [Google Scholar] [CrossRef]
- Duan, H.-S.; Zhou, H.; Chen, Q.; Sun, P.; Luo, S.; Song, T.-B.; Bob, B.; Yang, Y. The identification and characterization of defect states in hybrid organic–inorganic perovskite photovoltaics. Phys. Chem. Chem. Phys. 2014, 17, 112–116. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.-J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.-J.; Yum, J.-H.; Ballif, C. Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Buin, A.; Pietsch, P.; Xu, J.; Voznyy, O.; Ip, A.H.; Comin, R.; Sargent, E.H. Materials Processing Routes to Trap-Free Halide Perovskites. Nano Lett. 2014, 14, 6281–6286. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Q.D.; Xu, W.; He, Y.; Yin, W.J. Halide perovskite materials for solar cells: A theoretical review. Acta Phys. Chim. Sin. 2017, 33, 1730–1751. [Google Scholar]
- Euvrard, J.; Gunawan, O.; Mitzi, D.B. Impact of PbI2 Passivation and Grain Size Engineering in CH3NH3PbI3 Solar Absorbers as Revealed by Carrier-Resolved Photo-Hall Technique. Adv. Energy Mater. 2019, 9, 1902706. [Google Scholar] [CrossRef]
- Zuo, C.; Bolink, H.; Han, H.; Huang, J.; Cahen, D.; Ding, L. Advances in Perovskite Solar Cells. Adv. Sci. 2016, 3, 1500324. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Degradation of encapsulated perovskite solar cells driven by deep trap states and interfacial deterioration. J. Mater. Chem. C 2018, 6, 162–170. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Y.H. Catalytic role of H2O in degradation of inorganic–organic perovskite (CH3NH3PbI3) in air. Int. J. Energy Res. 2017, 41, 1063–1069. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, F.; He, J.; Lian, J.; Zeng, P.; Song, J.; Qu, J. Light-current-induced acceleration of degradation of methylammonium lead iodide perovskite solar cells. J. Power Sources 2018, 384, 303–311. [Google Scholar] [CrossRef]
- Xu, J.; Buin, A.K.; Ip, A.H.; Li, W.; Voznyy, O.; Comin, R.; Yuan, M.; Jeon, S.; Ning, Z.; McDowell, J.J.; et al. Perovskite–fullerene hybrid materials suppress hysteresis in planar diodes. Nat. Commun. 2015, 6, 7081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginting, R.T.; Jeon, M.-K.; Lee, K.-J.; Jin, W.-Y.; Kim, T.-W.; Kang, J.-W. Degradation mechanism of planar-perovskite solar cells: Correlating evolution of iodine distribution and photocurrent hysteresis. J. Mater. Chem. A 2017, 5, 4527–4534. [Google Scholar] [CrossRef]
- Islam, M.A.; Nguyen, D.C.; Ishikawa, Y. Effective minority carrier lifetime as an indicator for potential-induced degradation in p-type single-crystalline silicon photovoltaic modules. Jpn. J. Appl. Phys. 2019, 58, 106507. [Google Scholar] [CrossRef]
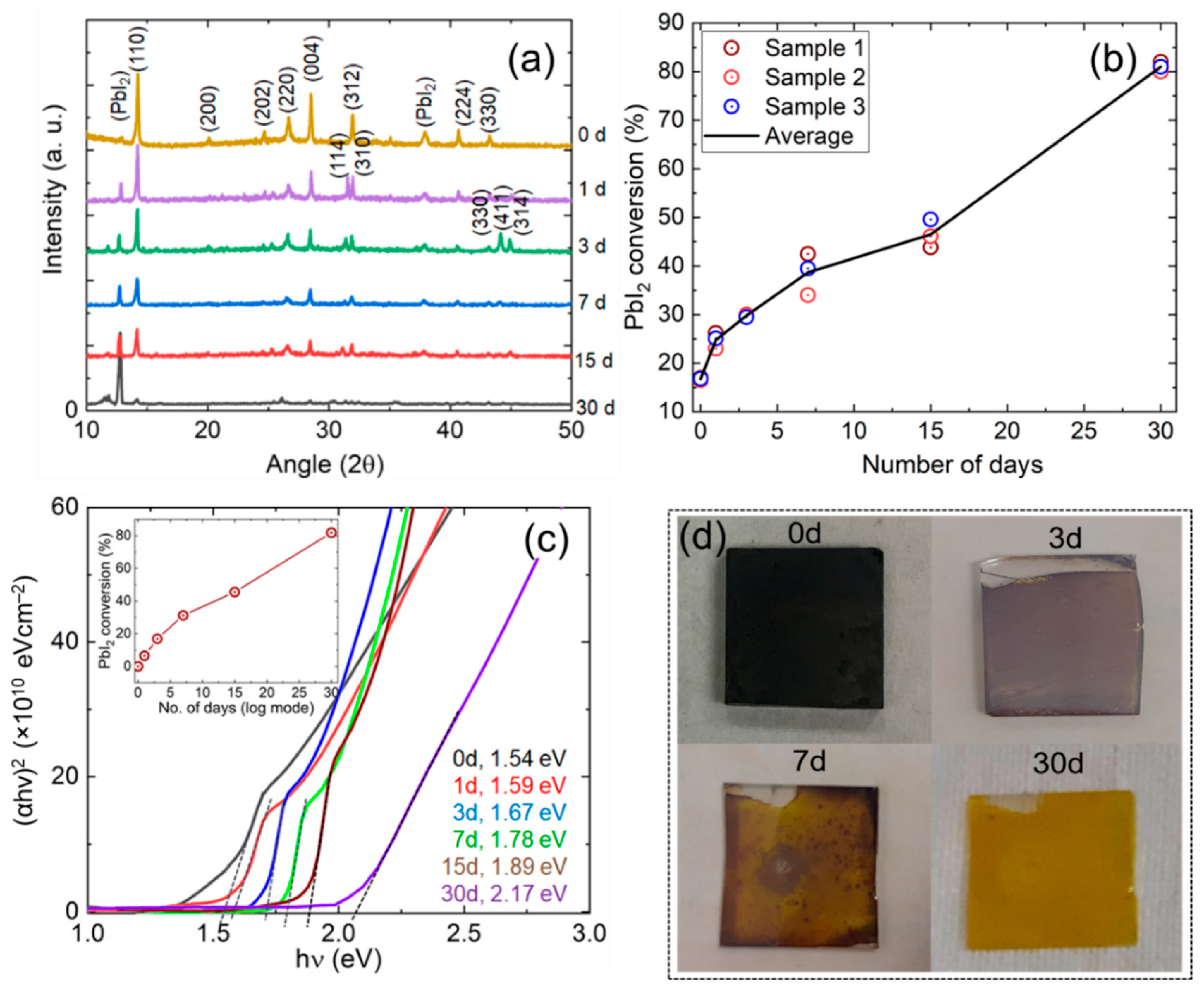
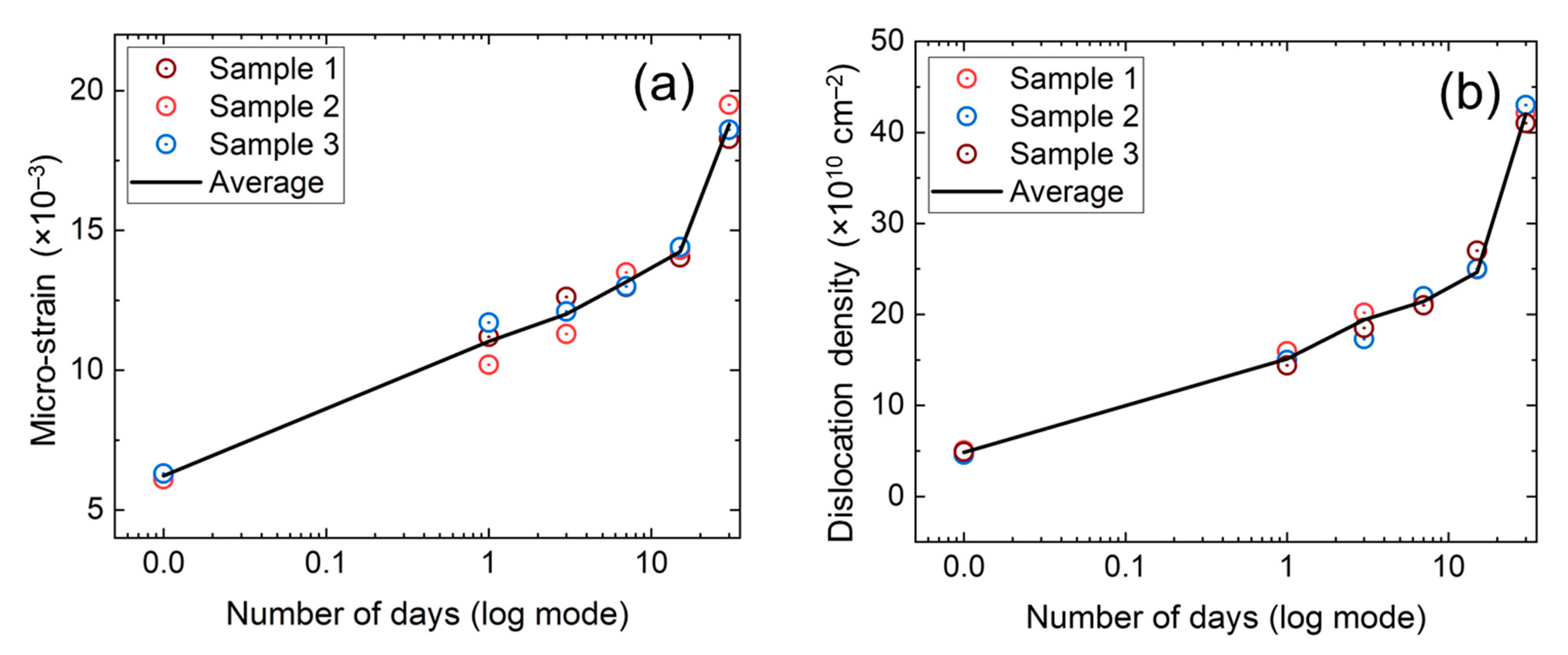



| Time | Angle, 2θ (Degree) | Average Peak Height, h (a. u.) | Average FWHM, β (×10−3 Rad.) | Average Crystallite Size, D (nm) |
|---|---|---|---|---|
| 0 days | 14.26 | 586 | 3.14 | 44.50 |
| 1 day | 14.21 | 441 | 5.58 | 25.03 |
| 3 days | 14.18 | 348 | 6.28 | 22.25 |
| 7 days | 14.18 | 228 | 6.45 | 21.65 |
| 15 days | 14.17 | 214 | 6.97 | 20.02 |
| 30 days | 14.15 | 117 | 9.07 | 15.40 |
| n | Absolute Deviation (%) | I0 (A) | Absolute Deviation (%) | Series Resistance (Rs) (Ω) | Shunt Resistance (Rsh) (Ω) | ||
|---|---|---|---|---|---|---|---|
| Fresh | Cell 1 | 2.16 | 0.025 | 5.56 × 10−8 | 2.51 × 10−3 | 68.9 | 280.5 |
| Cell 2 | 2.14 | 0.027 | 3.65 × 10−8 | 2.11 × 10−3 | 65.1 | 450.2 | |
| Cell 3 | 2.49 | 0.021 | 2.25 × 10−7 | 2.16 × 10−3 | 70.4 | 186.5 | |
| 7 days | Cell 1 | 2.74 | 0.026 | 5.45 × 10−6 | 2.88 × 10−3 | 132.5 | 118.6 |
| Cell 2 | 2.64 | 0.023 | 7.04 × 10−7 | 2.59 × 10−3 | 140.2 | 351.3 | |
| Cell 3 | 2.56 | 0.021 | 2.81 × 10−6 | 2.94 × 10−3 | 128.7 | 151.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Mohafez, H.; Sobayel, K.; Wan Muhamad Hatta, S.F.; Hasan, A.K.M.; Khandaker, M.U.; Akhtaruzzaman, M.; Muhammad, G.; Amin, N. Degradation of Perovskite Thin Films and Solar Cells with Candle Soot C/Ag Electrode Exposed in a Control Ambient. Nanomaterials 2021, 11, 3463. https://doi.org/10.3390/nano11123463
Islam MA, Mohafez H, Sobayel K, Wan Muhamad Hatta SF, Hasan AKM, Khandaker MU, Akhtaruzzaman M, Muhammad G, Amin N. Degradation of Perovskite Thin Films and Solar Cells with Candle Soot C/Ag Electrode Exposed in a Control Ambient. Nanomaterials. 2021; 11(12):3463. https://doi.org/10.3390/nano11123463
Chicago/Turabian StyleIslam, Mohammad Aminul, Hamidreza Mohafez, Khan Sobayel, Sharifah Fatmadiana Wan Muhamad Hatta, Abul Kalam Mahmud Hasan, Mayeen Uddin Khandaker, Md. Akhtaruzzaman, Ghulam Muhammad, and Nowshad Amin. 2021. "Degradation of Perovskite Thin Films and Solar Cells with Candle Soot C/Ag Electrode Exposed in a Control Ambient" Nanomaterials 11, no. 12: 3463. https://doi.org/10.3390/nano11123463









