Room Temperature Processed Double Electron Transport Layers for Efficient Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Deposition of Nb2O5/ZnO Films
2.3. Device Fabrication
2.4. Characterization
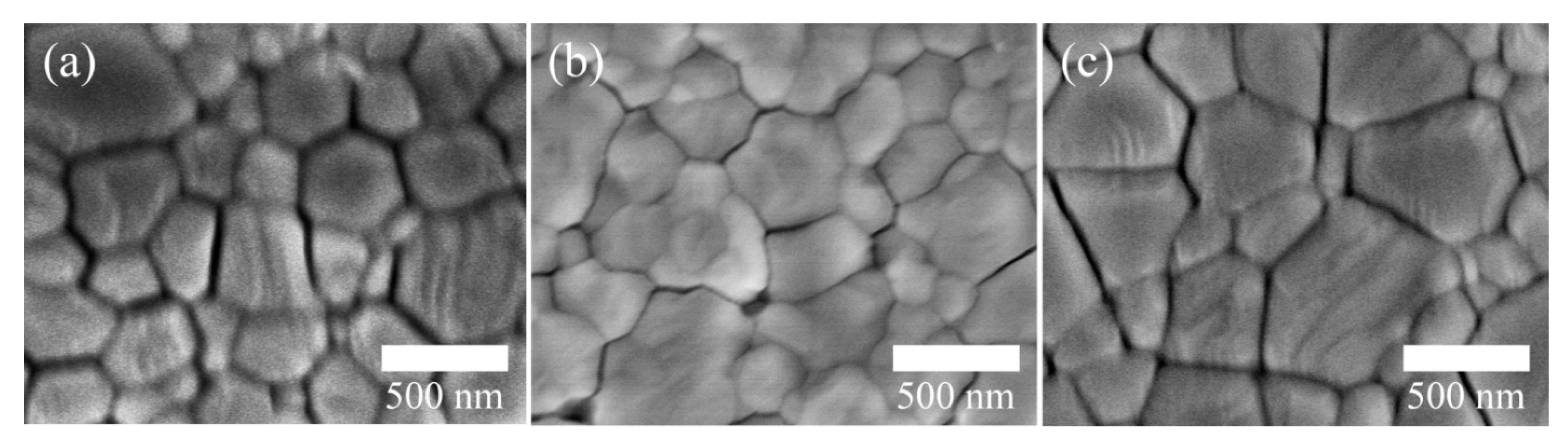
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Shi, T.; Yan, Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv. Mater. 2014, 26, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.J.; Xie, J.S.; Li, G.; Wang, Y.; Fang, D.S.; Yao, Y.X.; Xie, D.Y.; Cui, C.; Yan, K.Y.; Xu, J.B.; et al. An Interlayer with Strong Pb-Cl Bond Delivers Ultraviolet-Filter-Free, Efficient, and Photostable Perovskite Solar Cells. iScience 2019, 21, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Chu, L.; Hu, R.; Zhang, R.; Ma, Y.; Pu, Y.; Zhang, J.; Yang, J.; Li, X.; Huang, W. Diameter engineering on TiO2 nanorod arrays for improved hole-conductor-free perovskite solar cells. Sol. Energy 2018, 166, 42–49. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, W.; Hu, R.; Ma, Y.; Sun, Y.; Zhang, J.; Pu, Y.; Yang, J.; Chu, L.; Li, X. Enhancing perovskite quality and energy level alignment of TiO2 nanorod arrays-based solar cells via interfacial modification. Sol. Energy Mater. Sol. Cells 2019, 191, 183–189. [Google Scholar] [CrossRef]
- Manser, S.J.; Saidaminov, M.I.; Christians, J.A.; Bakr, O.M.; Kamat, P.V. Making and breaking of lead halide perovskites. Acc. Chem. Res. 2016, 49, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-freesolid-state organic-inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wu, C.; Luo, W.; Guo, X.; Qu, B.; Xiao, L.; Chen, Z. ZnO/SnO2 double electron ransport layer guides improved open circuit voltage for highly efficient CH3NH3PbI3-based planar perovskite solar cell. ACS Appl. Energy Mater. 2018, 1, 2215–2221. [Google Scholar] [CrossRef]
- Ran, C.; Xi, J.; Gao, W.; Yuan, F.; Lei, T.; Jiao, B.; Hou, X.; Wu, Z. Bilateral Interface Engineering toward Efficient 2D-3D Bulk Heterojunction Tin Halide Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 713–721. [Google Scholar] [CrossRef]
- Li, M.H.; Yeh, H.H.; Chiang, Y.H.; Jeng, U.S.; Su, C.J.; Shiu, H.W.; Hsu, Y.J.; Kosugi, N.; Ohigashi, T.; Chen, Y.A.; et al. Highly Efficient 2D/3D Hybrid Perovskite Solar Cells via Low-Pressure Vapor-Assisted Solution Process. Adv. Mater. 2018, 30, 1801401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Liu, P.F.; He, T.W.; Zhao, L.L.; Zhang, X.L.; Yang, J.; Yang, H.G.; Liu, H.R.; Qin, R.P.; Yuan, M.J. Tuning Surface Wettability of Buffer Layers by Incorporating Polyethylene Glycols for Enhanced Performance of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 26670–26679. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Galatopoulos, F.; Papadas, I.T.; Ioakeimidis, A.; Eleftheriou, P.; Choulis, S.A. Surface Treatment of Cu:NiOx Hole-Transporting Layer Using β-Alanine for Hysteresis-Free and Thermally Stable Inverted Perovskite Solar Cells. Nanomaterials 2020, 10, 1961. [Google Scholar] [CrossRef]
- Chandrasekhar, P.S.; Komarala, V.K. Graphene/ZnO nanocomposite as an electron transport layer for perovskite solar cells: The effect of graphene concentration on photovoltaic performance. RSC Adv. 2017, 7, 28610–28615. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Fukui, R.; Fukazawa, M.; Suzukiac, Y. SrTiO3/TiO2 composite electron transport layer for perovskite solar cells. Mater. Lett. 2017, 187, 111–113. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Huang, Z.; He, Y.; Luo, W.; Ting, H.; Li, T.; Sun, W.; Zhang, Q.; Chen, Z.; Xiao, L. TiO2/SnOxCly double layer for highly efficient planar perovskite solar cells. Org. Electron. 2017, 50, 485–490. [Google Scholar] [CrossRef]
- Zhao, E.; Gao, L.; Yang, S.; Wang, L.; Cao, J.; Ma, T. In situ fabrication of 2D SnS2 nanosheets as a new electron transport layer for perovskite solar cells. Nano Res. 2018, 11, 5913–5923. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Chan, K.H.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Low temperature processed ZnO thin film as electron transport layer for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 251–264. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; Angelis, F.D.; Kelly, T.L. Origin of the thermal instability in CH3NH3PbI3 thin films deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Azmi, R.; Hadmojo, W.T.; Sinaga, S.; Lee, C.L.; Yoon, S.C.; Jung, I.H.; Jang, S.Y. High-efficiency low-temperature ZnO based perovskite solar cells based on highly polar nonwetting self-assembled molecular layers. Adv. Energy Mater. 2017, 8, 1701683. [Google Scholar] [CrossRef]
- Stubhan, T.; Salinas, M.; Ebel, A.; Krebs, F.C.; Hirsch, A.; Halik, M.; Brabec, C.J. Increasing the Fill Factor of Inverted P3HT:PCBM Solar Cells Through Surface Modification of Al-Doped ZnO via Phosphonic Acid-Anchored C60 SAMs. Adv. Energy Mater. 2012, 2, 32–535. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Chen, R.; Wu, Y.; Hui, Y.; Mao, B.W.; Zheng, N. Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells with ZnO as Electron-Transport Layer: Effect of Surface Passivation. Adv. Mater. 2018, 30, 1705596. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Gu, Z.; Ye, T.; Fu, W.; Wu, G.; Li, H.; Chen, H. Enhanced photovoltaic performance of CH3NH3PbI3 perovskite solar cells through interfacial engineering using self-assembling monolayer. J. Am. Chem. Soc. 2015, 137, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Kang, M.S.; Lee, J.K.; Shin, B.C.; Lee, J.W. Enhanced electron diffusion length of mesoporous TiO2 film by using Nb2O5 energy barrier for dye-sensitized solar cells. Appl. Phys. Lett. 2006, 89, 013103. [Google Scholar] [CrossRef]
- Ling, X.; Yuan, J.; Liu, D.; Wang, Y.; Zhang, Y.; Chen, S.; Wu, H.; Jin, F.; Wu, F.; Shi, G.; et al. Room-temperature processed Nb2O5 as the electron-transporting layer for efficient planar perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 23181–23188. [Google Scholar] [CrossRef]
- Cui, J.; Meng, F.; Zhang, H.; Cao, K.; Yuan, H.; Cheng, Y.; Huang, F.; Wang, M. CH3NH3PbI3-Based Planar Solar Cells with Magnetron-Sputtered Nickel Oxide. ACS Appl. Mater. Interfaces 2014, 6, 22862–22870. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, W.; Li, Y.; Abate, A.; Wei, M. Facile Deposition of Nb2O5 Thin Film as an Electron-Transporting Layer for Highly Efficient Perovskite Solar Cells. ACS Appl. Nano Mater. 2018, 1, 4101–4109. [Google Scholar] [CrossRef]
- Salim, E.T.; Admon, S.J.; Abood, M.K.; Fakhri, M.A. Some physical properties of Nb2O5 thin films prepared using nobic acid based colloidal suspension at room temperature. Mater. Res. Express 2017, 4, 106407. [Google Scholar] [CrossRef]
- Valerio, T.L.; Maia, G.A.R.; Gonçalves, L.F.; Viomar, A.; Banczek, E.P.; Rodrigues, P.R.P. Study of the Nb2O5 Insertion in ZnO to Dye-sensitized Solar Cell. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; Yang, D.; Ren, X.; Zhu, X.; Jin, Z.; Zi, W.; Wei, Q.; Liu, S. E-beam evaporated Nb2O5 as an effective electron transport layer for large flexible perovskite solar cells. Nano Energy 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, S.; Jia, X.; Fang, X.; Zhang, S.; Zhang, J.; Liu, W.; Ding, J.; Yuan, N. Totally room-temperature solution-processing method for fabricating flexible perovskite solar cells using an Nb2O5-TiO2 electron transport layer. RSC Adv. 2018, 8, 12823–12831. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Wang, Y.; Zhang, Q. Progress in perovskite solar cells based on ZnO nanostructures. Sol. Energy 2018, 163, 289–306. [Google Scholar] [CrossRef]
- Lim, K.G.; Ahn, S.; Lee, T.W. Energy level alignment of dipolar interface layer in organic and hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2915–2924. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Wang, X.; Tao, J.; Jiang, J.; Hua, Z.; Chu, J. Enhanced performance of carbon-based planar CsPbBr3 perovskite solar cells with room-temperature sputtered Nb2O5 electron transport layer. Sol. Energy 2019, 191, 263–271. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Jia, Z.; Qin, Z.; Chu, L.; Yang, J.; Zhang, J.; Huang, W.; Li, X. High Stable, Transparent and Conductive ZnO/Ag/ZnO Nanofilm Electrodes on Rigid/Flexible Substrates. Energies 2016, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, D.G.; Jung, H.S.; Lee, H.H.; Kim, H. ITO and electron-transport layer-free planar perovskite solar cells on transparent Nb-doped anatase TiO2−x electrodes. J. Alloys Compd. 2020, 845, 155531. [Google Scholar] [CrossRef]
- Firdaus, C.M.; Rizamb, M.S.B.S.; Rusopa, M.; Hidayahc, S.R. Characterization of ZnO and ZnO: TiO2 Thin Films Prepared by Sol-Gel Spray-Spin Coating Technique. Procedia Eng. 2012, 41, 1367–1373. [Google Scholar] [CrossRef]
- Wei, H.; Xiao, J.; Yang, Y.; Lv, S.; Shi, J.; Xu, X.; Dong, J.; Luo, Y.; Li, D.; Meng, Q. Free-standing flexible carbon electrode for highly efficient hole-conductor-free perovskite solar cells. Carbon 2015, 93, 861–868. [Google Scholar] [CrossRef]
- Merdasa1, A.; Tsarev, S.; Akbulatov, A.F.; Troshin, P.; Unger, E.L. Microscopic insight into the reversibility of photodegradation in MAPbI3 thin films. J. Lumin. 2020, 219, 116916. [Google Scholar] [CrossRef]
- Kosyachenko, L.A. Solar Cells Silicon Wafer-Based Technologies; IntechOpen: London, UK, 2011. [Google Scholar]
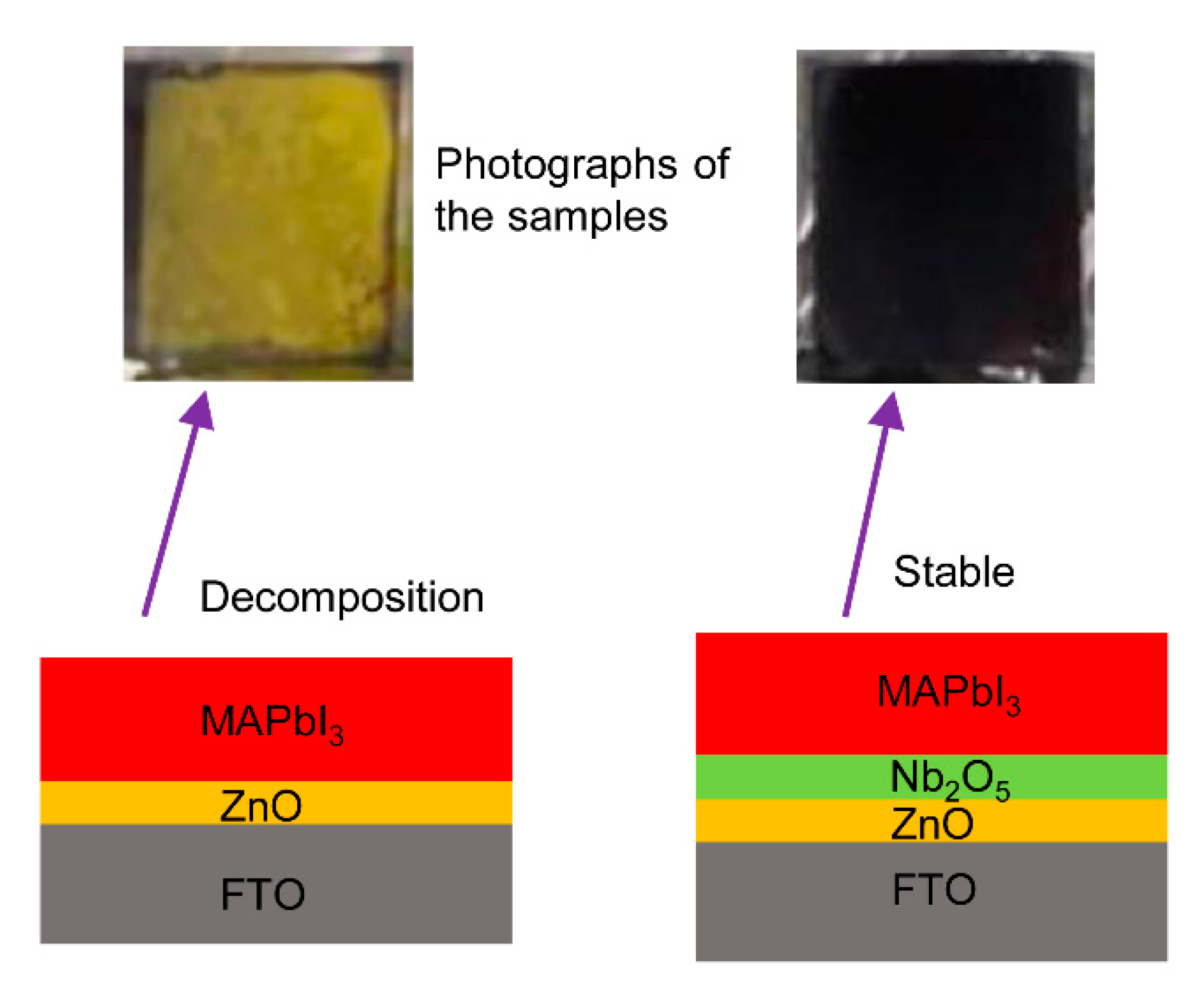

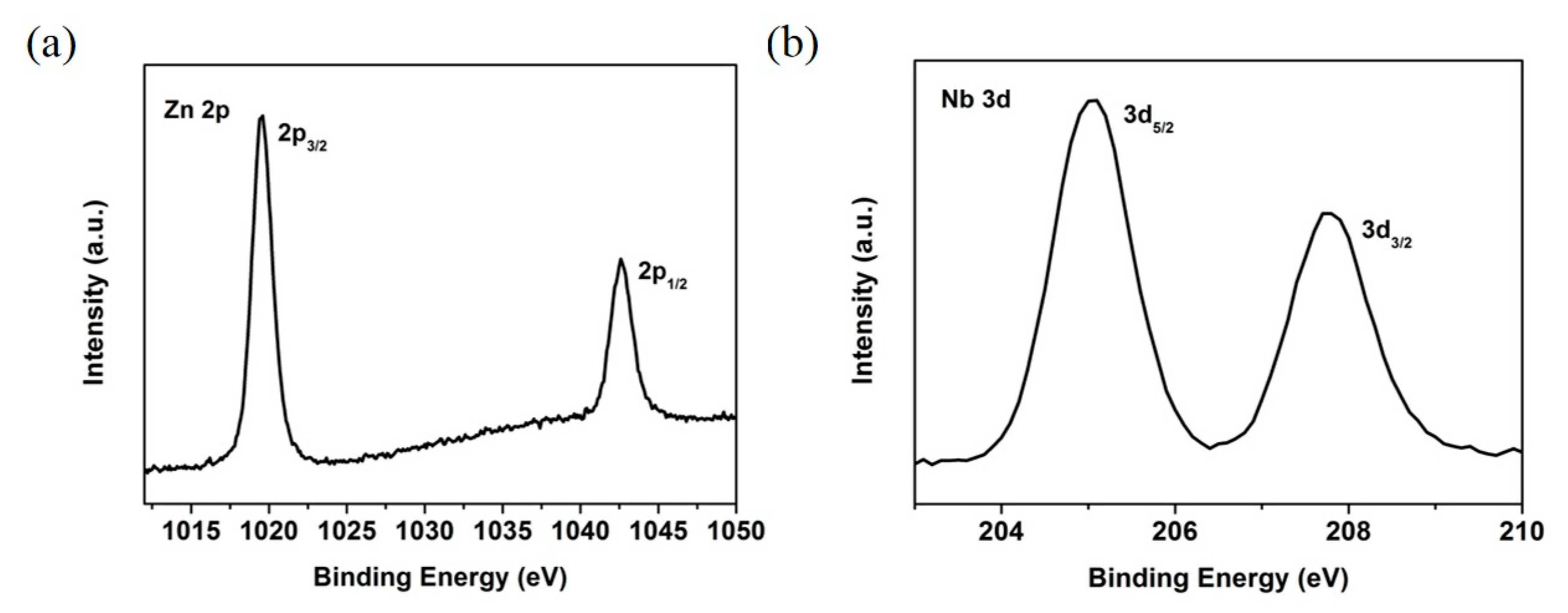
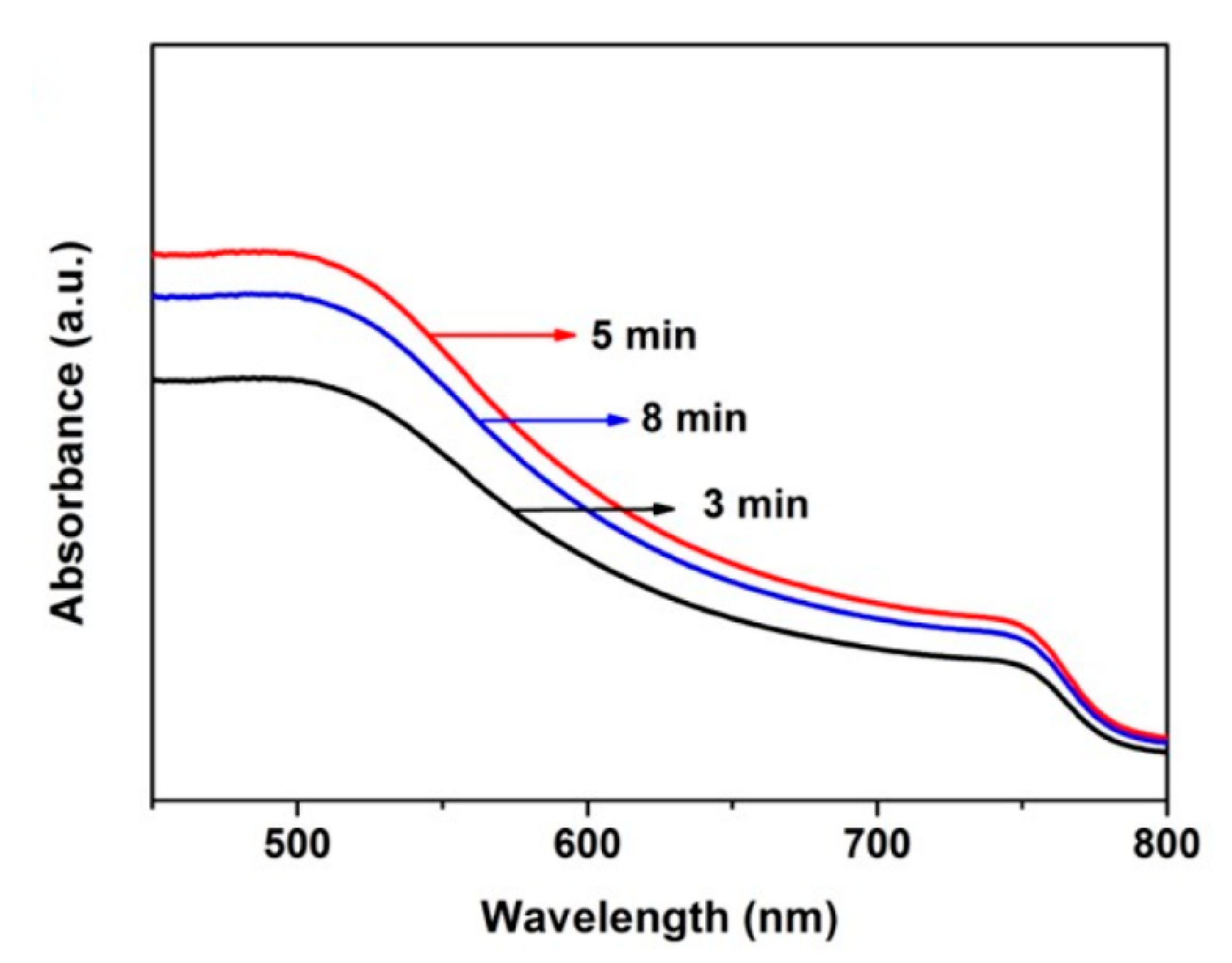
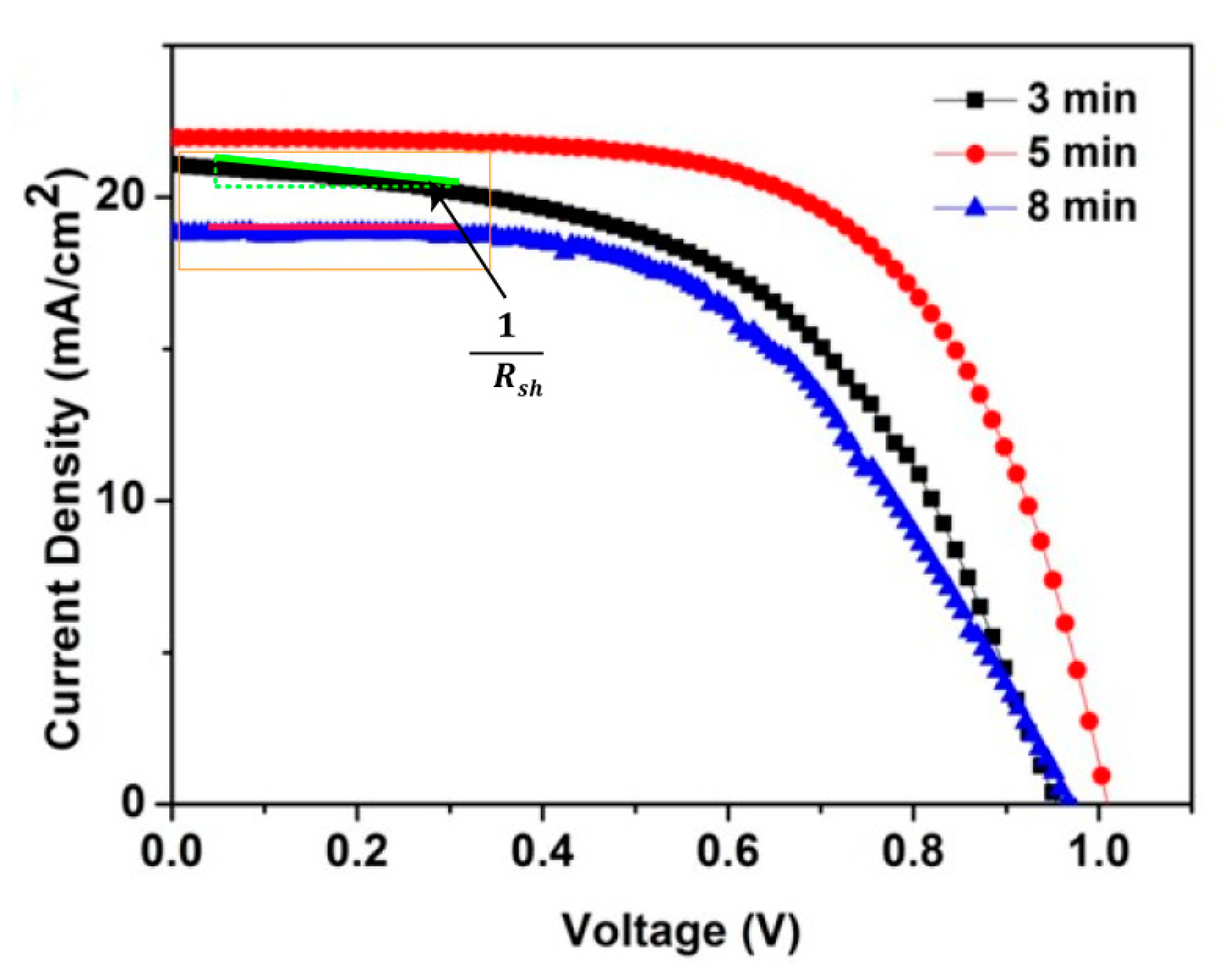
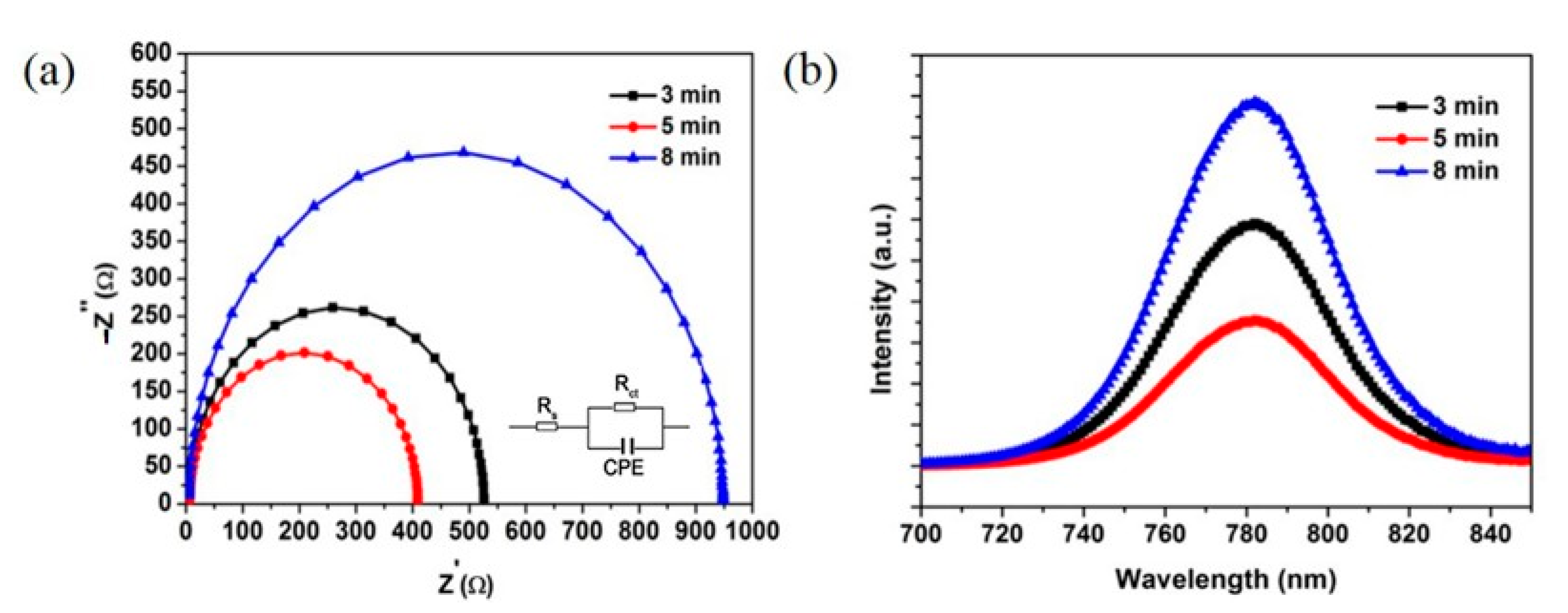
| Sputter ZnO Time (min) | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 3 | 0.94 | 21.0 | 52.4 | 10.5 |
| 5 | 1.0 | 21.9 | 62.7 | 13.8 |
| 8 | 0.97 | 18.9 | 53 | 9.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Zhang, R.; Xia, X.; Steichen, P.; Liu, N.; Yang, J.; Chu, L.; Li, X. Room Temperature Processed Double Electron Transport Layers for Efficient Perovskite Solar Cells. Nanomaterials 2021, 11, 329. https://doi.org/10.3390/nano11020329
Huang W, Zhang R, Xia X, Steichen P, Liu N, Yang J, Chu L, Li X. Room Temperature Processed Double Electron Transport Layers for Efficient Perovskite Solar Cells. Nanomaterials. 2021; 11(2):329. https://doi.org/10.3390/nano11020329
Chicago/Turabian StyleHuang, Wen, Rui Zhang, Xuwen Xia, Parker Steichen, Nanjing Liu, Jianping Yang, Liang Chu, and Xing’ao Li. 2021. "Room Temperature Processed Double Electron Transport Layers for Efficient Perovskite Solar Cells" Nanomaterials 11, no. 2: 329. https://doi.org/10.3390/nano11020329





