Phase Inversion and Interfacial Layer Microstructure in Emulsions Stabilized by Glycosurfactant Mixtures
Abstract
1. Introduction
2. Materials and Methods
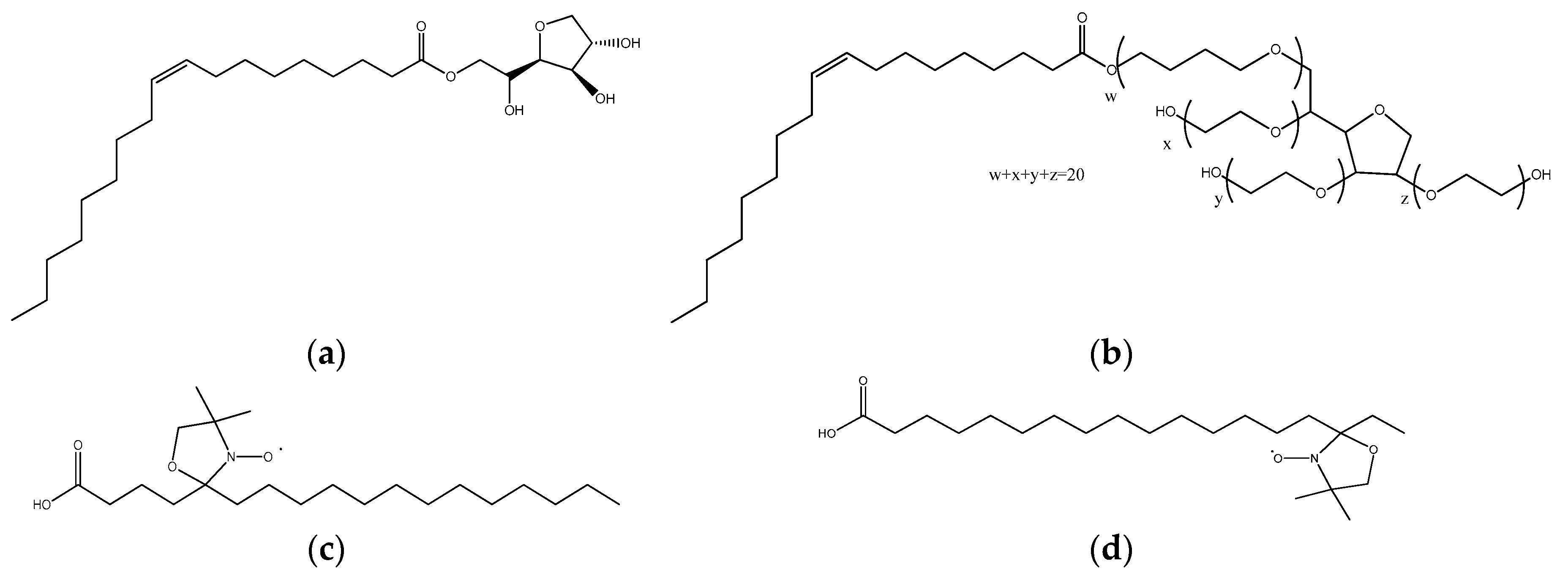
2.1. Materials
2.2. Samples Preparation
- Proper amounts of oil (either L.O. or B.O.) and Span80 were weighted in a vial and thoroughly mixed by using a bench-type vortex (ArgoLab Mix, Carpi, Italy).
- Proper amounts of water and Tween80 were weighted in another vial and thoroughly mixed by vortexing. The pH of this solution was checked to be from neutral to weakly acid (pH = 6–7).
- The contents of the two vials were combined, briefly vortexed and then sonicated using a Sonics Vibracell VCX 130 PB ultrasonic processor (Sonics&Materials, Newtown, CT, USA) equipped with a 3 mm titanium probe running at an amplitude of 40% for 20 min in an ice bath.
2.3. Pseudo-Phase Diagram Determination
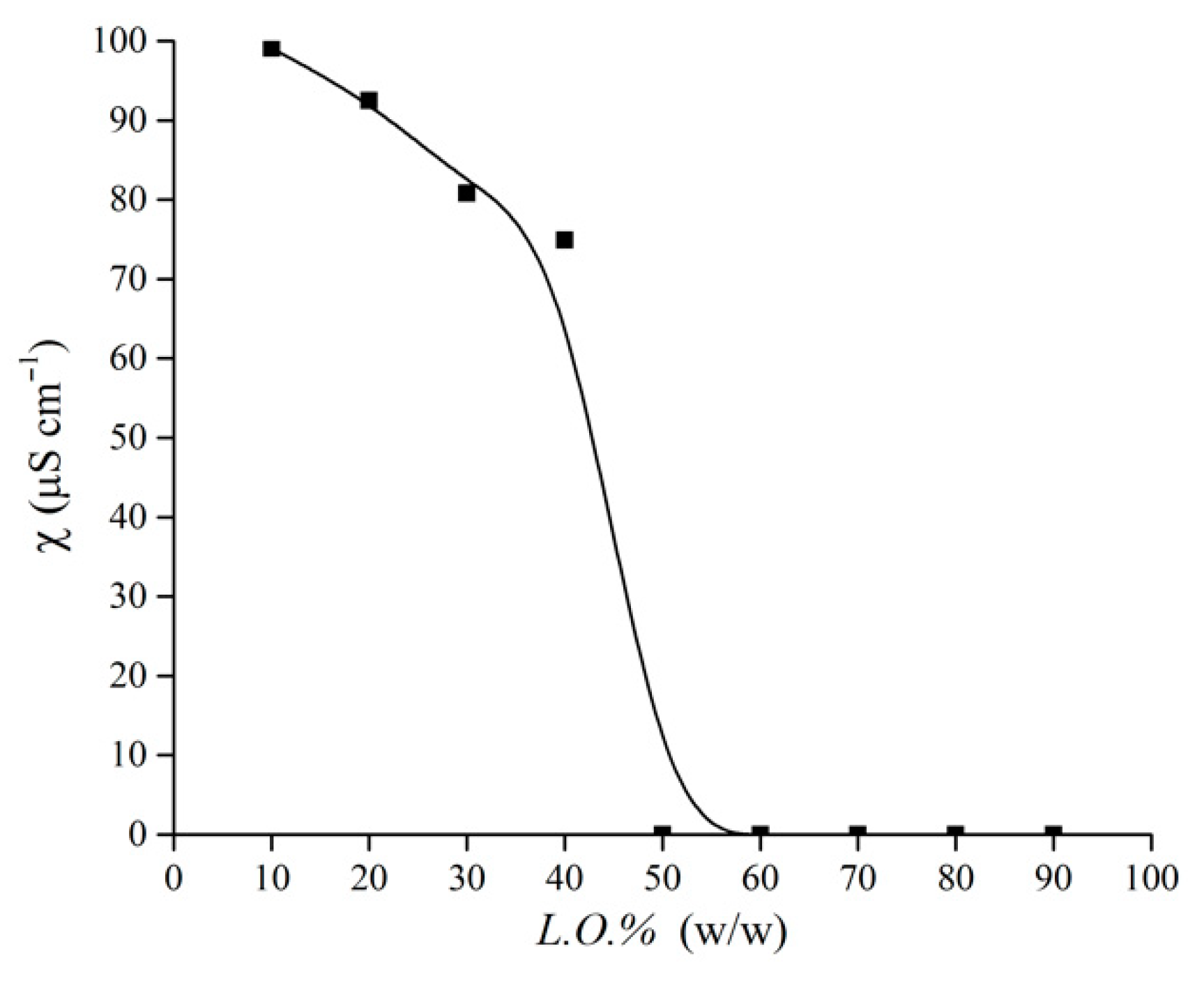
2.4. Conductometric Measurements
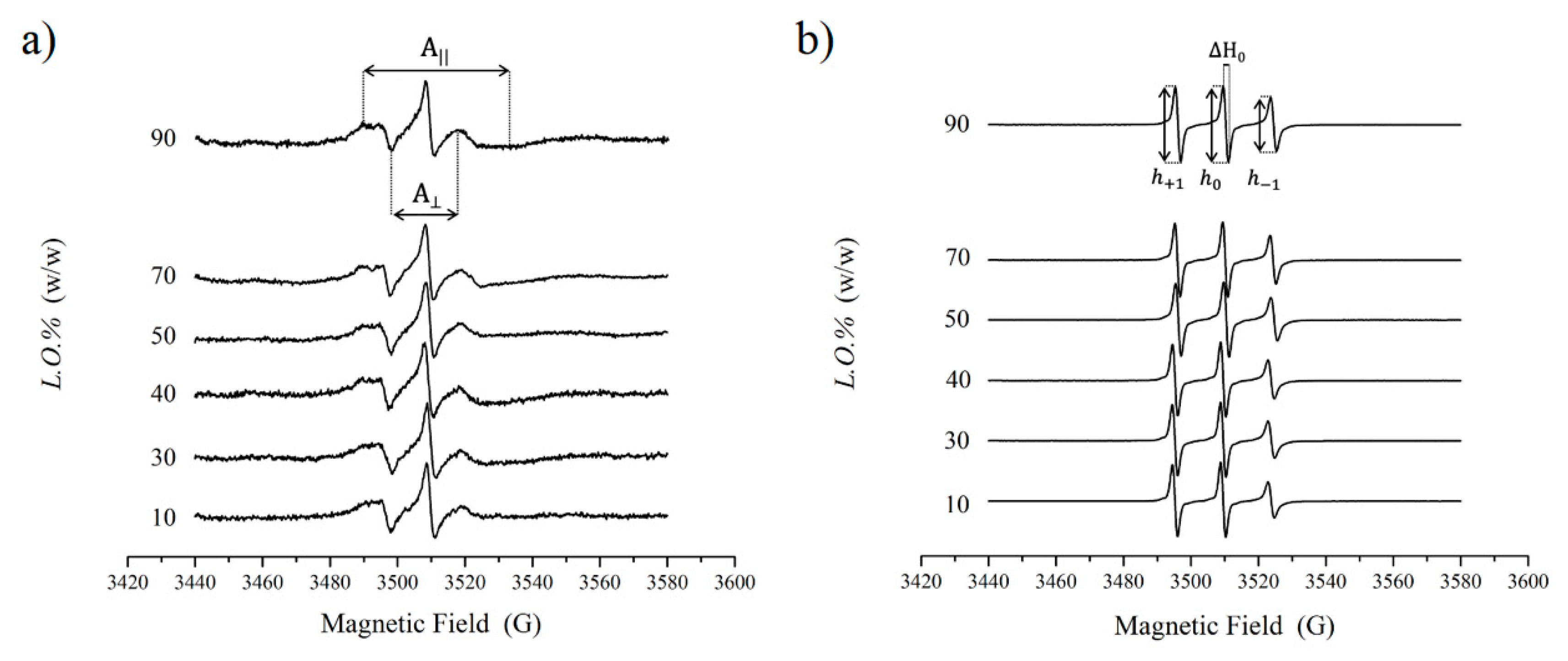
2.5. EPR Characterization
2.6. Statistical Analysis
3. Results and Discussion
3.1. Pseudo-Phase Diagrams
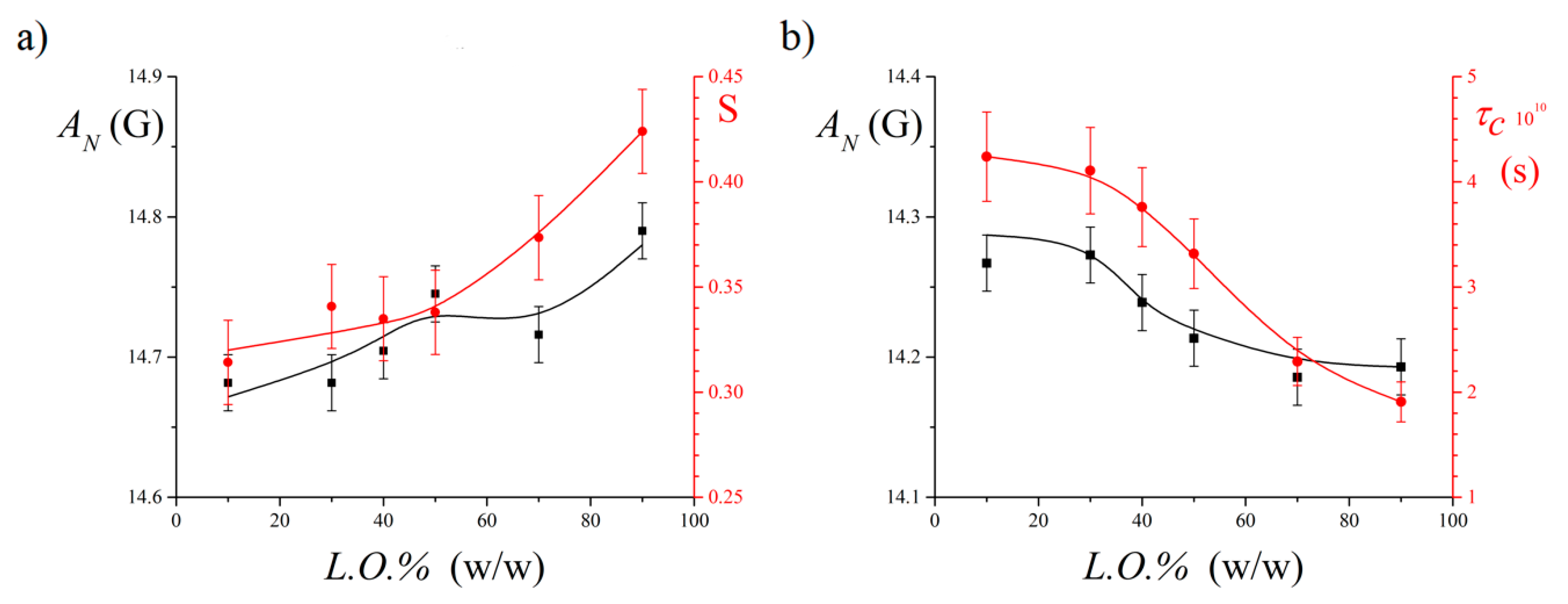
3.2. EPR Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tadros, T.F. Emulsion Formation and Stability; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: A review. Trends Food Sci. Technol. 2020, 104, 49–59. [Google Scholar] [CrossRef]
- Arab, D.; Kantzas, A.; Bryant, S.L. Nanoparticle stabilized oil in water emulsions: A critical review. J. Petrol. Sci. Eng. 2018, 163, 217–242. [Google Scholar] [CrossRef]
- Jafari, S.; McClements, D.J. Nanoemulsions: Formulation, Applications, and Characterization; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Nor Bainun, I.; Nur Hashimah, A.; Syed-Hassan, S.S.A. Nanoemulsions: Formation, characterization, properties and applications—A review. Adv. Mat. Res. 2015, 1113, 147–152. [Google Scholar] [CrossRef]
- Nikolic, I.; Mitsou, E.; Pantelic, I.; Randjelovic, D.; Markovic, B.; Papadimitriou, V.; Xenakis, A.; Lunter, D.J.; Zugic, A.; Savic, S. Microstructure and biopharmaceutical performances of curcumin-loaded low-energy nanoemulsions containing eucalyptol and pinene: Terpenes’ role overcome penetration enhancement effect? Eur. J. Pharm. Sci. 2020, 142, 105135. [Google Scholar] [CrossRef] [PubMed]
- Demisli, S.; Theochari, I.; Christodoulo, P.; Zervoua, M.; Xenaki, A.; Papadimitriou, V. Structure, activity and dynamics of extra virgin olive oil-in-water nanoemulsions loaded with vitamin D3 and calcium citrate. J. Mol. Liq. 2020, 306, 112908. [Google Scholar] [CrossRef]
- Chellapa, P.; Ariffin, F.D.; Eid, A.M.; Almahgoubi, A.A.; Mohamed, A.T.; Issa, Y.S.; Elmarzugi, N.A. Nanoemulsion for cosmetic application. Eur. J. Biomed. Pharm. Sci. 2016, 3, 8–11. [Google Scholar]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef]
- Mondal, P.K.; Mandal, B.K. A comprehensive review on the feasibility of using water emulsified diesel as a CI engine fuel. Fuel 2019, 237, 937–960. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for food, pharmaceutical and cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T.; et al. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Takamura, A.; Minowa, T.; Noro, S.; Kubo, T. Effects of Tween and Span group emulsifiers on the stability of O/W emulsions. Chem. Pharm. Bull. 1979, 27, 2921–2926. [Google Scholar] [CrossRef]
- Jhalani, A.; Sharma, D.; Soni, S.L.; Sharma, P.K.; Sharma, S. A comprehensive review on water-emulsified diesel fuel: Chemistry, engine performance and exhaust emissions. Environ. Sci. Pollut. Res. 2019, 26, 4570–4587. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, F.; Cai, W.; Li, H.; Zhang, X. Influences of addition of hydrophilic surfactants on the W/O emulsions stabilized by lipophilic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 441–448. [Google Scholar] [CrossRef]
- Hou, W.; Papadopoulos, K.D. W1/O/W2 and O1/W/O2 globules stabilized with Span80 and Tween80. Colloids Surf. A Physicochem. Eng. Asp. 1997, 125, 181–187. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Behera, B.; Sudheep, T.; Pal, K. Effect of composition on the properties of Tween80–Span80-based organogels. Des. Monomers Polym. 2012, 15, 253–273. [Google Scholar] [CrossRef]
- Peng, L.C.; Liu, C.H.; Kwan, C.C.; Huang, K.F. Optimization of water-in-oil nanoemulsions by mixed surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 136–142. [Google Scholar] [CrossRef]
- Lv, G.; Wang, F.; Cai, W.; Zhang, X. Characterization of the addition of lipophilic Span 80 to the hydrophilic Tween 80-stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 8–13. [Google Scholar] [CrossRef]
- Preetika, R.; Mehta, P.S.; Kaisare, N.S.; Basavaraj, M.G. Kinetic stability of surfactant stabilized water-in-diesel emulsion fuels. Fuel 2019, 236, 1415–1422. [Google Scholar] [CrossRef]
- Chong, W.-T.; Tan, C.-P.; Cheah, Y.-K.; Lajis, A.F.B.; Dian, N.L.H.M.; Kanagaratnam, S.; La, O.-M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Designing nanoemulsion templates for fabrication of dextrin nanoparticles via emulsion cross-linking technique. Carbohydr. Polym. 2014, 101, 650–655. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, M.; Xu, J.; Wang, Q.; Fan, Z. Stabilization of water-in-octane nano-emulsion. Part I: Stabilized by mixed surfactant systems. Fuel 2010, 89, 2838–2843. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, Y.; Gao, Z.; Ou, W.; Zhu, H.; Zhang, Q. Phase behavior and stability of nano-emulsions prepared by D phase emulsification method. J. Mol. Liq. 2019, 285, 424–429. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, Y.; Chang, S.; Zhu, H.; Zhang, Q. Influence of oil types on the formation and stability of nano-emulsions by D phase emulsification. J. Dispers. Sci. Technol. ahead of print. [CrossRef]
- Tedeschi, A.M.; D’Errico, G.; Busi, E.; Basosi, R.; Barone, V. Micellar aggregation of sulfonate surfactants studied by electron paramagnetic resonance of a cationic nitroxide: An experimental and computational approach. Phys. Chem. Chem. Phys. 2002, 4, 2180–2188. [Google Scholar] [CrossRef]
- Tedeschi, A.M.; Busi, E.; Paduano, L.; Basosi, R.; D’Errico, G. Influence of the headgroup molecular structure on the anionic surfactant-PVP interaction studied by electron paramagnetic resonance of a cationic nitroxide. Phys. Chem. Chem. Phys. 2003, 5, 5077–5083. [Google Scholar] [CrossRef]
- Vitiello, G.; Falanga, A.; Petruk, A.A.; Merlino, A.; Fragneto, G.; Paduano, L.; Galdiero, S.; D’Errico, G. Fusion of raft-like lipid bilayers operated by a membranotropic domain of the HSV-type I glycoprotein gH occurs through a cholesterol-dependent mechanism. Soft Matter 2015, 11, 3003–3016. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Mitsou, E.; Yaghmur, A.; Xenakis, A.; Papadimitriou, V. Formulation and characterization of food-grade microemulsions as carriers of natural phenolic antioxidants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 130–136. [Google Scholar] [CrossRef]
- Kogan, A.; Rozner, S.; Mehta, S.; Somasundaran, P.; Aserin, A.; Garti, N.; Ottaviani, M.F. Characterization of the nonionic microemulsions by EPR. I. Effect of solubilized drug on nanostructure. J. Phys. Chem. B 2009, 113, 691–699. [Google Scholar] [CrossRef]
- Xenakis, A.; Cazianis, C.T.; Malliaris, A. Study of the transition between different structures of some non-ionic microemulsion systems. Colloids Surf. 1992, 62, 315–319. [Google Scholar] [CrossRef]
- Baglioni, P.; Gambi, C.M.C.; Goldfarb, D. Electron spin echo modulation study of Winsor microemulsions. J. Phys. Chem. 1991, 95, 2577–2583. [Google Scholar] [CrossRef]
- Kommareddi, N.S.; John, V.T.; Waguespack, Y.Y.; McPherson, G.L. Temperature and gas pressure induced microstructural changes in AOT water-in-oil microemulsions: Characterization through electron paramagnetic resonance spectroscopy. J. Phys. Chem. 1993, 97, 5752–5761. [Google Scholar] [CrossRef]
- Avramiotis, S.; Cazianis, C.T.; Xenakis, A. Interfacial properties of lecithin microemulsions in the presence of lipase. A membrane spin-probe study. Langmuir 1999, 15, 2375–2379. [Google Scholar] [CrossRef]
- Haering, G.; Luisi, P.L.; Hauser, H. Characterization by electron spin resonance of reversed micelles consisting of the ternary system AOT-isooctane-water. J. Phys. Chem. 1988, 92, 3574–3581. [Google Scholar] [CrossRef]
- Zuev, Y.F.; Vylegzhanina, N.N.; Zakhartchenko, N.L. Effects of protein solubilization on the structure of the surfactant shell of reverse micelles. Appl. Magn. Reson. 2003, 25, 29–42. [Google Scholar] [CrossRef]
- El Kinawy, O.S.; Petersen, S.; Helmdach, L.; Ulrich, J. Parameter selection of emulsification processes: Conditions for nano- and macroemulsions. Chem. Eng. Technol. 2012, 35, 1604–1608. [Google Scholar] [CrossRef]
- Henry, J.V.L.; Fryer, P.J.; Frith, W.J.; Norton, I.T. Emulsification mechanism and storage instabilities of hydrocarbon-in-water sub-micron emulsions stabilised with Tweens (20 and 80), Brij 96v and sucrose monoesters. J. Colloid Interface Sci. 2009, 338, 201–206. [Google Scholar] [CrossRef]
- Rokach, S.; Ottaviani, M.F.; Shames, A.I.; Nir, I.; Aserin, A.; Garti, N. W/O Microemulsions as dendrimer nanocarriers: An EPR study. J. Phys. Chem. B 2012, 116, 12633–12640. [Google Scholar] [CrossRef]
- Gaffney, B.J.; McConnell, H.M. The paramagnetic resonance spectra of spin labels in phospholipid membranes. J. Magn. Reson. 1974, 16, 1–28. [Google Scholar] [CrossRef]
- Rozner, S.; Kogan, A.; Mehta, S.; Somasundaran, S.; Aserin, A.; Garti, N.; Ottaviani, M.F. Characterization of nonionic microemulsions by EPR. Part II. The effect of competitive solubilization of cholesterol and phytosterols on the nanostructure. J. Phys. Chem. B 2009, 113, 700–707. [Google Scholar] [CrossRef]
- Pradilla, D.; Barrera, A.; Saetran, M.G.; Sorland, G.; Alvarez, O. Mechanisms of physical stabilization of concentrated water-in-oil emulsions probed by pulse field gradient nuclear magnetic resonance and rheology through a multiscale approach. Langmuir 2018, 34, 9489–9499. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Feng, J.; Garcia-Celma, M.J.; Azemar, N.; Solans, C. Phase behavior and nano-emulsion formation by the phase inversion temperature method. Langmuir 2004, 20, 6594–6598. [Google Scholar] [CrossRef] [PubMed]
- Caianiello, C.; D’Avino, M.; Cavasso, D.; Paduano, L.; D’Errico, G. Bioinspired nanoemulsions stabilized by phosphoethanolamine and phosphoglycerol lipids. Nanomaterials 2020, 10, 1185. [Google Scholar] [CrossRef] [PubMed]

| System | Spin-Probe | (G) | S | (s) |
|---|---|---|---|---|
| water/L.O./(Tween80 + Span80) | ||||
| L.O.% = 10 αSpan80 = 0.0–1.0 (O/W) | 5-DSA | 14.7 ± 0.2 | 0.35 ± 0.02 | |
| 16-DSA | 14.29 ± 0.05 | 4.5 ± 0.4 | ||
| L.O.% = 90 αSpan80 = 0.2–1.0 (W/O) | 5-DSA | 14.8 ± 0.2 | 0.45 ± 0.03 | |
| 16-DSA | 14.13 ± 0.03 | 2.3 ± 0.6 | ||
| water/B.O./(Tween80 + Span80) | ||||
| B.O.% = 10 αSpan80 = 0.2–1.0 (O/W) | 5-DSA | 14.6 ± 0.2 | 0.16 ± 0.04 | |
| 16-DSA | 14.3 ± 0.1 | 2.7 ± 0.6 | ||
| B.O.% = 97 αSpan80 = 0.8–1.0 (W/O) | 5-DSA | 15.0 ± 0.1 | 0.07 ± 0.02 | |
| 16-DSA | 14.20 ± 0.02 | 0.7 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, R.; Cavasso, D.; Niccoli, M.; D’Errico, G. Phase Inversion and Interfacial Layer Microstructure in Emulsions Stabilized by Glycosurfactant Mixtures. Nanomaterials 2021, 11, 331. https://doi.org/10.3390/nano11020331
Esposito R, Cavasso D, Niccoli M, D’Errico G. Phase Inversion and Interfacial Layer Microstructure in Emulsions Stabilized by Glycosurfactant Mixtures. Nanomaterials. 2021; 11(2):331. https://doi.org/10.3390/nano11020331
Chicago/Turabian StyleEsposito, Rodolfo, Domenico Cavasso, Marcella Niccoli, and Gerardino D’Errico. 2021. "Phase Inversion and Interfacial Layer Microstructure in Emulsions Stabilized by Glycosurfactant Mixtures" Nanomaterials 11, no. 2: 331. https://doi.org/10.3390/nano11020331
APA StyleEsposito, R., Cavasso, D., Niccoli, M., & D’Errico, G. (2021). Phase Inversion and Interfacial Layer Microstructure in Emulsions Stabilized by Glycosurfactant Mixtures. Nanomaterials, 11(2), 331. https://doi.org/10.3390/nano11020331







