Inactivation of Bacteria Using Bioactive Nanoparticles and Alternating Magnetic Fields
Abstract
:1. Introduction
2. Materials and Methods
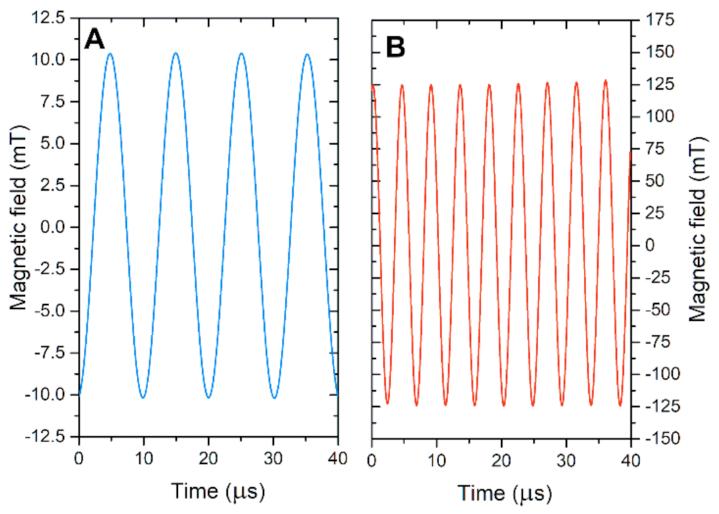
2.1. Alternating Magnetic Fields
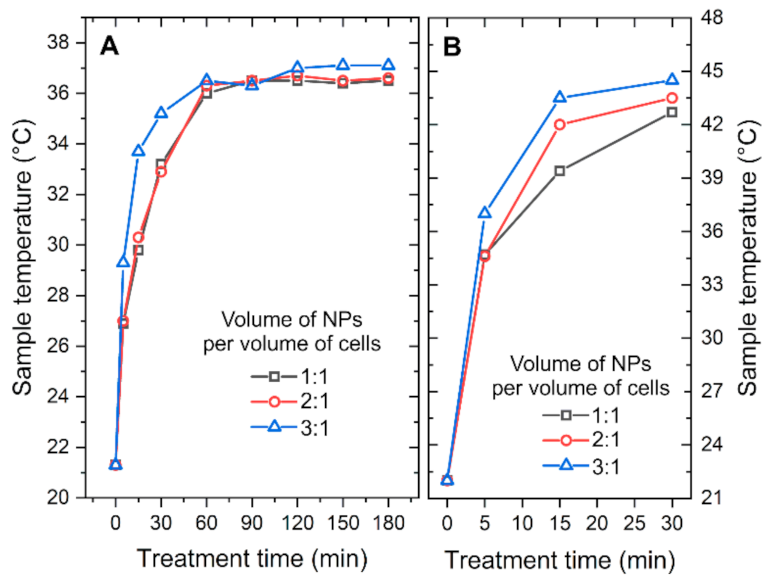
2.2. Thermal Influence
2.3. Nisin-Loaded Magnetic Nanoparticles
2.4. Bacterial Cells
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
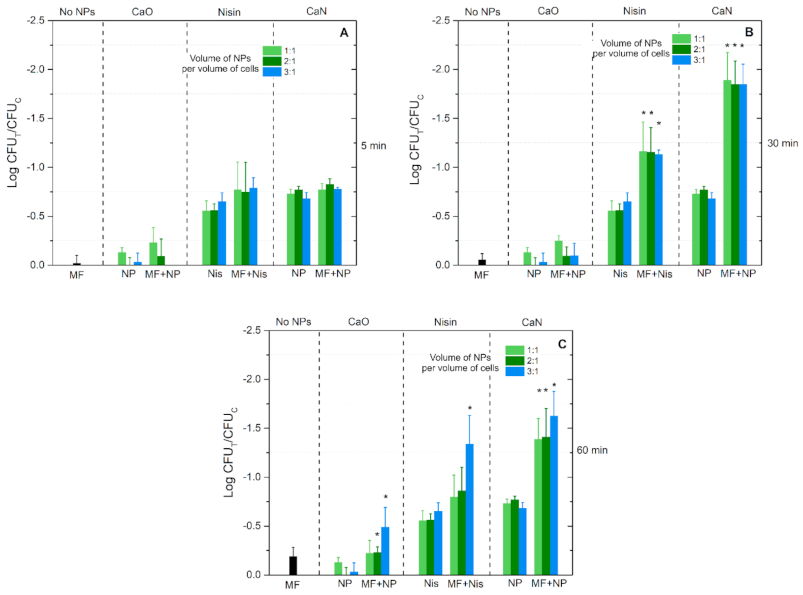
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.; Materón, E.M.; Ibáñez-Redín, G.; Faria, R.C.; Correa, D.S.; Oliveira, O.N. Electrical detection of pathogenic bacteria in food samples using information visualization methods with a sensor based on magnetic nanoparticles functionalized with antimicrobial peptides. Talanta 2019, 194, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, A.; Jovanovic, J.; Monteiro, S.; Decleer, M.; Andjelkovic, M.; Foubert, A.; Beloglazova, N.; Tsilla, V.; Sas, B.; Madder, A.; et al. Detection of toxins involved in foodborne diseases caused by Gram-positive bacteria. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1605–1657. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vertedor, D.; Rodrigues, N.; Ítala, M.G.; Veloso, A.C.A.; Peres, A.M.; Pereira, J.A. Impact of thermal sterilization on the physicochemical-sensory characteristics of Californian-style black olives and its assessment using an electronic tongue. Food Control. 2020, 117, 107369. [Google Scholar] [CrossRef]
- Auksornsri, T.; Bornhorst, E.R.; Tang, J.; Tang, Z.; Songsermpong, S. Developing model food systems with rice based products for microwave assisted thermal sterilization. LWT 2018, 96, 551–559. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, Y.-l.; Zhu, Y.; Yang, L.; Yu, N.; Wei, Y.; Zhang, H.; Sun, A.-D. Design of a treatment chamber for low-voltage pulsed electric field sterilization. Innov. Food Sci. Emerg. Technol. 2017, 42, 180–189. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Jensen, J.L.; Keener, K.M.; Campanella, O.H. Modeling the inactivation of Bacillus subtilis spores during cold plasma sterilization. Innov. Food Sci. Emerg. Technol. 2019, 52, 334–342. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Wang, W.; Pei, J.; Zhang, J.; Yue, T.; Youravong, W.; Li, Z. Bacteriocin assisted food functional membrane for simultaneous exclusion and inactivation of Alicyclobacillus acidoterrestris in apple juice. J. Membr. Sci. 2021, 618, 118741. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Muriana, P. Inhibition of Listeria monocytogenes on Ready-to-Eat Meats Using Bacteriocin Mixtures Based on Mode-of-Action. Foods 2017, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, M.; Bhandari, B.; Cao, P. Microorganism control and product quality improvement of Twice-cooked pork dish using ZnO nanoparticles combined radio frequency pasteurization. LWT 2018, 95, 65–71. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Cao, P.; Adhikari, B. Effect of ZnO nanoparticles combined radio frequency pasteurization on the protein structure and water state of chicken thigh meat. LWT 2020, 134, 110168. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Bhandari, B.; Kachele, R. ZnO nanoparticles combined radio frequency heating: A novel method to control microorganism and improve product quality of prepared carrots. Innov. Food Sci. Emerg. Technol. 2017, 44, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Rokbani, H.; Daigle, F.; Ajji, A. Combined Effect of Ultrasound Stimulations and Autoclaving on the Enhancement of Antibacterial Activity of ZnO and SiO2/ZnO Nanoparticles. Nanomaterials 2018, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Stothard, P.; Banting, G.; Scott, C.; Huntley, K.; Ryu, K.; Otto, S.; Ashbolt, N.; Checkley, S.; Dong, T.; et al. Characterization of water treatment-resistant and multidrug-resistant urinary pathogenic Escherichia coli in treated wastewater. Water Res. 2020, 182, 115827. [Google Scholar] [CrossRef]
- Murphy, C.P.; Carson, C.; Smith, B.A.; Chapman, B.; Marrotte, J.; McCann, M.; Primeau, C.; Sharma, P.; Parmley, E.J. Factors potentially linked with the occurrence of antimicrobial resistance in selected bacteria from cattle, chickens and pigs: A scoping review of publications for use in modelling of antimicrobial resistance (IAM.AMR Project). Zoonoses Public Health 2018, 65, 957–971. [Google Scholar] [CrossRef]
- Phillips, C.A. Bacterial biofilms in food processing environments: A review of recent developments in chemical and biological control. Int. J. Food Sci. Technol. 2016, 51, 1731–1743. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Haas, B.; Gottschalk, M.; Grenier, D. Antimicrobial potential of bacteriocins in poultry and swine production. Vet. Res. 2017, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schofs, L.; Sparo, M.D.; Bruni, S.F.S. Gram-positive bacteriocins: Usage as antimicrobial agents in veterinary medicine. Vet. Res. Commun. 2020, 1–12. [Google Scholar] [CrossRef]
- Field, D.; Blake, T.; Mathur, H.; O’Connor, P.M.; Cotter, P.D.; Paul Ross, R.; Hill, C. Bioengineering nisin to overcome the nisin resistance protein. Mol. Microbiol. 2019, 111, 717–731. [Google Scholar]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated nisin: An engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar] [CrossRef]
- Tan, Z.; Luo, J.; Liu, F.; Zhang, Q.; Jia, S. Effects of pH, Temperature, Storage Time, and Protective Agents on Nisin Antibacterial Stability. In Advances in Applied Biotechnology; Springer Nature: Berlin/Heidelberg, Germany, 2015; pp. 305–312. [Google Scholar]
- Peng, X.; Zhu, L.; Wang, Z.; Zhan, X.-B. Enhanced stability of the bactericidal activity of nisin through conjugation with gellan gum. Int. J. Biol. Macromol. 2020, 148, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Guiotto, A.; Pozzobon, M.; Canevari, M.; Manganelli, R.; Scarin, M.; Veronese, F.M. PEGylation of the antimicrobial peptide nisin A: Problems and perspectives. Il Farmaco 2003, 58, 45–50. [Google Scholar] [CrossRef]
- Guiga, W.; Swesi, Y.; Galland, S.; Peyrol, E.; Degraeve, P.; Sebti, I. Innovative multilayer antimicrobial films made with Nisaplin® or nisin and cellulosic ethers: Physico-chemical characterization, bioactivity and nisin desorption kinetics. Innov. Food Sci. Emerg. Technol. 2010, 11, 352–360. [Google Scholar] [CrossRef]
- Crandall, A.D.; Montville, T.J. Nisin Resistance in Listeria monocytogenes ATCC 700302 Is a Complex Phenotype. Appl. Environ. Microbiol. 1998, 64, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Zhou, L.; Van Heel, A.J.; Montalban-Lopez, M.; Kuipers, O.P. Potentiating the Activity of Nisin against Escherichia coli. Front. Cell Dev. Biol. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.M. Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2019, 60, 1581–1592. [Google Scholar] [CrossRef]
- Hinds, L.M.; O’Donnell, C.P.; Akhter, M.; Tiwari, B.K. Principles and mechanisms of ultraviolet light emitting diode technology for food industry applications. Innov. Food Sci. Emerg. Technol. 2019, 56, 102153. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomašević, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Berdejo, D.; Pagán, E.; García-Gonzalo, D.; Pagán, R. Exploiting the synergism among physical and chemical processes for improving food safety. Curr. Opin. Food Sci. 2019, 30, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Wiedemann, I.; Benz, R.; Sahl, H.-G. Lipid II-Mediated Pore Formation by the Peptide Antibiotic Nisin: A Black Lipid Membrane Study. J. Bacteriol. 2004, 186, 3259–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novickij, V.; Stanevičienė, R.; Grainys, A.; Lukša, J.; Badokas, K.; Krivorotova, T.; Sereikaitė, J.; Novickij, J.; Servienė, E. Electroporation-assisted inactivation of Escherichia coli using nisin-loaded pectin nanoparticles. Innov. Food Sci. Emerg. Technol. 2016, 38, 98–104. [Google Scholar] [CrossRef]
- Novickij, V.; Zinkevičienė, A.; Stanevičienė, R.; Gruškienė, R.; Servienė, E.; Vepštaitė-Monstavičė, I.; Krivorotova, T.; Lastauskienė, E.; Sereikaitė, J.; Girkontaitė, I.; et al. Inactivation of Escherichia coli Using Nanosecond Electric Fields and Nisin Nanoparticles: A Kinetics Study. Front. Microbiol. 2018, 9, 3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novickij, V.; Stanevičienė, R.; Staigvila, G.; Gruškienė, R.; Sereikaitė, J.; Girkontaitė, I.; Novickij, J.; Servienė, E. Effects of pulsed electric fields and mild thermal treatment on antimicrobial efficacy of nisin-loaded pectin nanoparticles for food preservation. LWT 2020, 120, 108915. [Google Scholar] [CrossRef]
- Maglietti, F.H.; Michinski, S.D.; Olaiz, N.M.; Castro, M.A.; Suárez, C.A.; Marshall, G.R. The Role of Ph Fronts in Tissue Electroporation Based Treatments. PLoS ONE 2013, 8, e80167. [Google Scholar] [CrossRef]
- Guenther, E.; Klein, N.; Mikus, P.; Stehling, M.K.; Rubinsky, B. Electrical breakdown in tissue electroporation. Biochem. Biophys. Res. Commun. 2015, 467, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Schottroff, F.; Johnson, K.; Johnson, N.B.; Bédard, M.F.; Jaeger, H. Challenges and limitations for the decontamination of high solids protein solutions at neutral pH using pulsed electric fields. J. Food Eng. 2020, 268, 109737. [Google Scholar] [CrossRef]
- Ruzgys, P.; Novickij, V.; Novickij, J.; Šatkauskas, S. Influence of the electrode material on ROS generation and electroporation efficiency in low and high frequency nanosecond pulse range. Bioelectrochemistry 2019, 127, 87–93. [Google Scholar] [CrossRef]
- Rodaite-Riseviciene, R.; Saule, R.; Snitka, V.; Saulis, G. Release of Iron Ions From the Stainless Steel Anode Occurring During High-Voltage Pulses and Its Consequences for Cell Electroporation Technology. IEEE Trans. Plasma Sci. 2014, 42, 249–254. [Google Scholar] [CrossRef]
- Miklavcic, D.; Novickij, V.; Kranjc, M.; Polajzer, T.; Meglic, S.H.; Napotnik, T.B.; Romih, R.; Lisjak, D. Contactless electroporation induced by high intensity pulsed electromagnetic fields via distributed nanoelectrodes. Bioelectrochemistry 2020, 132, 107440. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.-H. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2016, 24, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, J.d.A.; Magro, M.; Spagnolo, S.; Bonaiuto, E.; Baratella, D.; Fasolato, L.; Vianello, F. Antimicrobial and magnetically removable tannic acid nanocarrier: A processing aid for Listeria monocytogenes treatment for food industry applications. Food Chem. 2018, 267, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, A.H.M.; Salimi, M.N. Superparamagnetic Nanoparticles for Drug Delivery. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 843–859. ISBN 9780128137581. [Google Scholar]
- Gruskiene, R.; Krivorotova, T.; Stanevičienė, R.; Ratautas, D.; Serviene, E.; Sereikaite, J. Preparation and characterization of iron oxide magnetic nanoparticles functionalized by nisin. Coll. Surf. B Biointerfaces 2018, 169, 126–134. [Google Scholar] [CrossRef]
- Novickij, V.; Stanevičiene, R.; Vepštaite-Monstaviče, I.; Gruškiene, R.; Krivorotova, T.; Sereikaite, J.; Novickij, J.; Serviene, E. Overcoming antimicrobial resistance in bacteria using bioactive magnetic nanoparticles and pulsed electromagnetic fields. Front. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Mössbauer Spectroscopy; Cambridge University Press: Cambridge, UK, 1986; ISBN 9780521261012.
- Mohan, V.; Wibisono, R.; De Hoop, L.; Summers, G.; Fletcher, G.C. Identifying Suitable Listeria innocua Strains as Surrogates for Listeria monocytogenes for Horticultural Products. Front. Microbiol. 2019, 10, 2281. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zou, M.; Liu, K.; Gu, Z.; Yang, R. Effect of mild thermal treatment on the polymerization behavior, conformation and viscoelasticity of wheat gliadin. Food Chem. 2018, 239, 984–992. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, J.; Zou, L.; Liu, C.; Liu, W. Unfolding and Inhibition of Polyphenoloxidase Induced by Acidic pH and Mild Thermal Treatment. Food Bioprocess Technol. 2019, 12, 1907–1916. [Google Scholar] [CrossRef]
- Li, M.; Qu, J.-H.; Peng, Y.-Z. Sterilization of Escherichia coli cells by the application of pulsed magnetic field. J. Environ. Sci. 2004, 16, 348–352. [Google Scholar]
- Bayraktar, V.N. Magnetic Field Effect on Yeast Saccharomyces Cerevisiae Activity at Grape Must Fermentation. Biotechnol. Acta 2013, 6, 125–137. [Google Scholar] [CrossRef]
- Santos, L.O.; Alegre, R.M.; Garcia-Diego, C.; Cuellar, J. Effects of magnetic fields on biomass and glutathione production by the yeast Saccharomyces cerevisiae. Process. Biochem. 2010, 45, 1362–1367. [Google Scholar] [CrossRef]
- Sahebjamei, H.; Abdolmaleki, P.; Ghanati, F. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 2006, 28, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Rowan, N.J.; Anderson, J.G. Effects of Above-Optimum Growth Temperature and Cell Morphology on Thermotolerance of Listeria monocytogenesCells Suspended in Bovine Milk. Appl. Environ. Microbiol. 1998, 64, 2065–2071. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Brackett, R.; Chen, J.; Beuchat, L.R. Mild heat treatment of lettuce enhances growth of Listeria monocytogenes during subsequent storage at 5 °C or 15 °C. J. Appl. Microbiol. 2002, 92, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Golneshan, A.A.; Lahonian, M. The effect of magnetic nanoparticle dispersion on temperature distribution in a spherical tissue in magnetic fluid hyperthermia using the lattice Boltzmann method. Int. J. Hyperth. 2011, 27, 266–274. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, H.; Lee, S.; Kim, S.; Choi, K.-H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015, 72, 25–36. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Physics responsible for heating efficiency and self-controlled temperature rise of magnetic nanoparticles in magnetic hyperthermia therapy. Prog. Biophys. Mol. Biol. 2018, 133, 9–19. [Google Scholar] [CrossRef]
- Puligundla, P.; Abdullah, S.A.; Choi, W.; Jun, S.; Oh, S.-E.; Ko, S. Potentials of Microwave Heating Technology for Select Food Processing Applications—A Brief Overview and Update. J. Food Process. Technol. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Prudêncio, C.V.; Mantovani, H.C.; Cecon, P.R.; Vanetti, M.C.D. Differences in the antibacterial activity of nisin and bovicin HC5 against Salmonella Typhimurium under different temperature and pH conditions. J. Appl. Microbiol. 2015, 118, 18–26. [Google Scholar] [CrossRef]
- Umeki, S.; Watanabe, T.; Shimabukuro, H.; Kato, T.; Yoshikawa, N.; Taniguchi, S.; Tohji, K. Change in Zeta Potential by Alternating Electromagnetic Treatment as Scale Prevention Process. Trans. Mater. Res. Soc. Jpn. 2007, 32, 611–614. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar]
- Jurgons, R.; Seliger, C.; Hilpert, A.; Trahms, L.; Odenbach, S.; Alexiou, C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2893–S2902. [Google Scholar] [CrossRef]
- Hwang, J.; Kwon, D.; Lee, S.; Jeon, S. Detection of Salmonella bacteria in milk using gold-coated magnetic nanoparticle clusters and lateral flow filters. RSC Adv. 2016, 6, 48445–48448. [Google Scholar] [CrossRef]
- Cao, M.; Li, Z.; Wang, J.; Ge, W.; Yue, T.; Li, R.; Colvin, V.L.; Yu, W.W. Food related applications of magnetic iron oxide nanoparticles: Enzyme immobilization, protein purification, and food analysis. Trends Food Sci. Technol. 2012, 27, 47–56. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Khan, M.S.; Ansari, F.A.; Alam, M.Z.; Ahmed, M.A.; Khan, M.S.; Baig, M.H.; et al. Low Temperature Synthesis of Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles and Their ROS Mediated Inhibition of Biofilm Formed by Food-Associated Bacteria. Front. Microbiol. 2018, 9, 2567. [Google Scholar] [CrossRef] [Green Version]






| Sub-spectrum | δ, mm/s | 2ε, mm/s | <B>, T | A, % |
|---|---|---|---|---|
| Magnetite A (Fe3+) or maghemite | 0.28 * | 0.02 ± 0.01 | 36.2 | 69 |
| Magnetite B (Fe2+ + Fe3+) | 0.66 * | −0.23 ± 0.03 | 33.2 | 27 |
| Superparamagnetic singlet | 0.43 ± 0.04 | - | - | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novickij, V.; Stanevičienė, R.; Gruškienė, R.; Badokas, K.; Lukša, J.; Sereikaitė, J.; Mažeika, K.; Višniakov, N.; Novickij, J.; Servienė, E. Inactivation of Bacteria Using Bioactive Nanoparticles and Alternating Magnetic Fields. Nanomaterials 2021, 11, 342. https://doi.org/10.3390/nano11020342
Novickij V, Stanevičienė R, Gruškienė R, Badokas K, Lukša J, Sereikaitė J, Mažeika K, Višniakov N, Novickij J, Servienė E. Inactivation of Bacteria Using Bioactive Nanoparticles and Alternating Magnetic Fields. Nanomaterials. 2021; 11(2):342. https://doi.org/10.3390/nano11020342
Chicago/Turabian StyleNovickij, Vitalij, Ramunė Stanevičienė, Rūta Gruškienė, Kazimieras Badokas, Juliana Lukša, Jolanta Sereikaitė, Kęstutis Mažeika, Nikolaj Višniakov, Jurij Novickij, and Elena Servienė. 2021. "Inactivation of Bacteria Using Bioactive Nanoparticles and Alternating Magnetic Fields" Nanomaterials 11, no. 2: 342. https://doi.org/10.3390/nano11020342
APA StyleNovickij, V., Stanevičienė, R., Gruškienė, R., Badokas, K., Lukša, J., Sereikaitė, J., Mažeika, K., Višniakov, N., Novickij, J., & Servienė, E. (2021). Inactivation of Bacteria Using Bioactive Nanoparticles and Alternating Magnetic Fields. Nanomaterials, 11(2), 342. https://doi.org/10.3390/nano11020342








