Bioactive Icariin/β-CD-IC/Bacterial Cellulose with Enhanced Biomedical Potential
Abstract
1. Introduction
2. Materials and Experiments
2.1. Chemicals and Reagents
2.2. Preparation of Clean BC
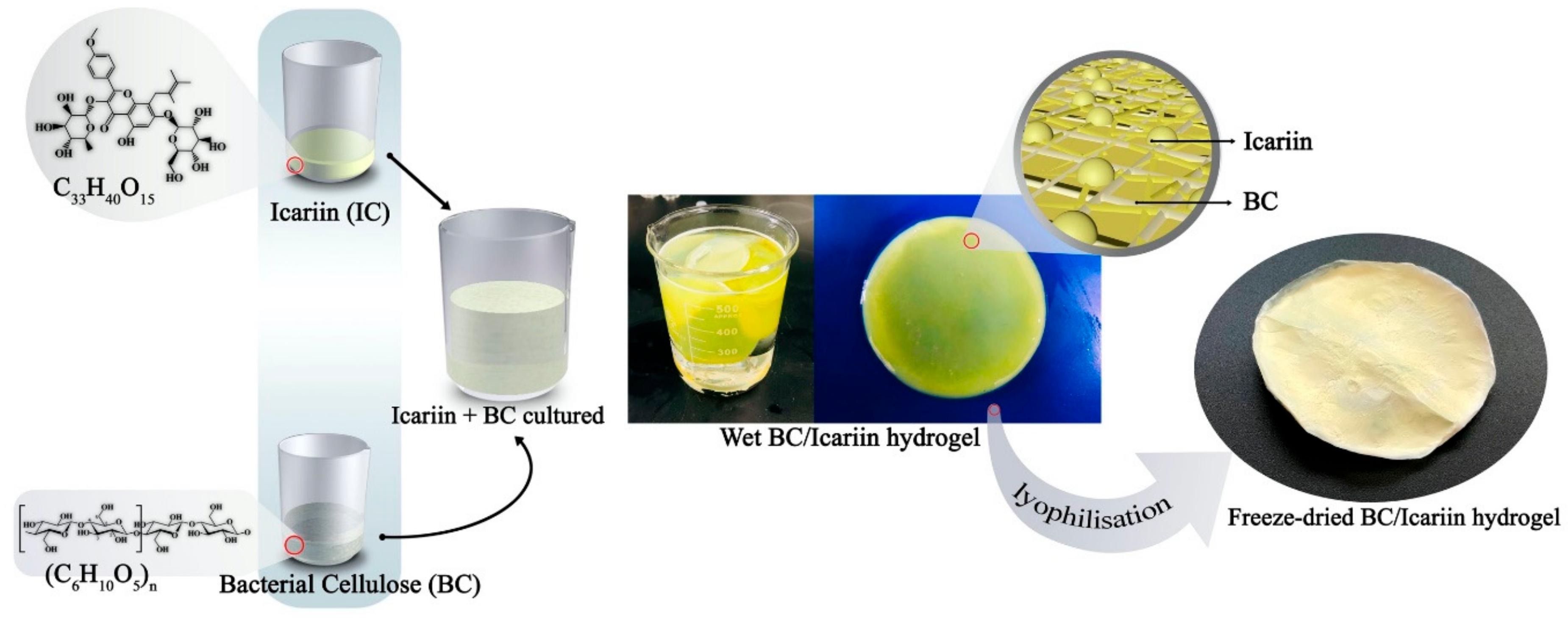
2.3. Preparation of Icariin-Bacterial Cellulose (ICBC)
2.4. Preparation of Icariin/Beta-Cyclodextrin Inclusion Complex/BC (Icariin/β-CD-IC/BC (IC/P/BC))
2.5. Preparation of Icariin/BC (ICr/BC) (In Situ Method)
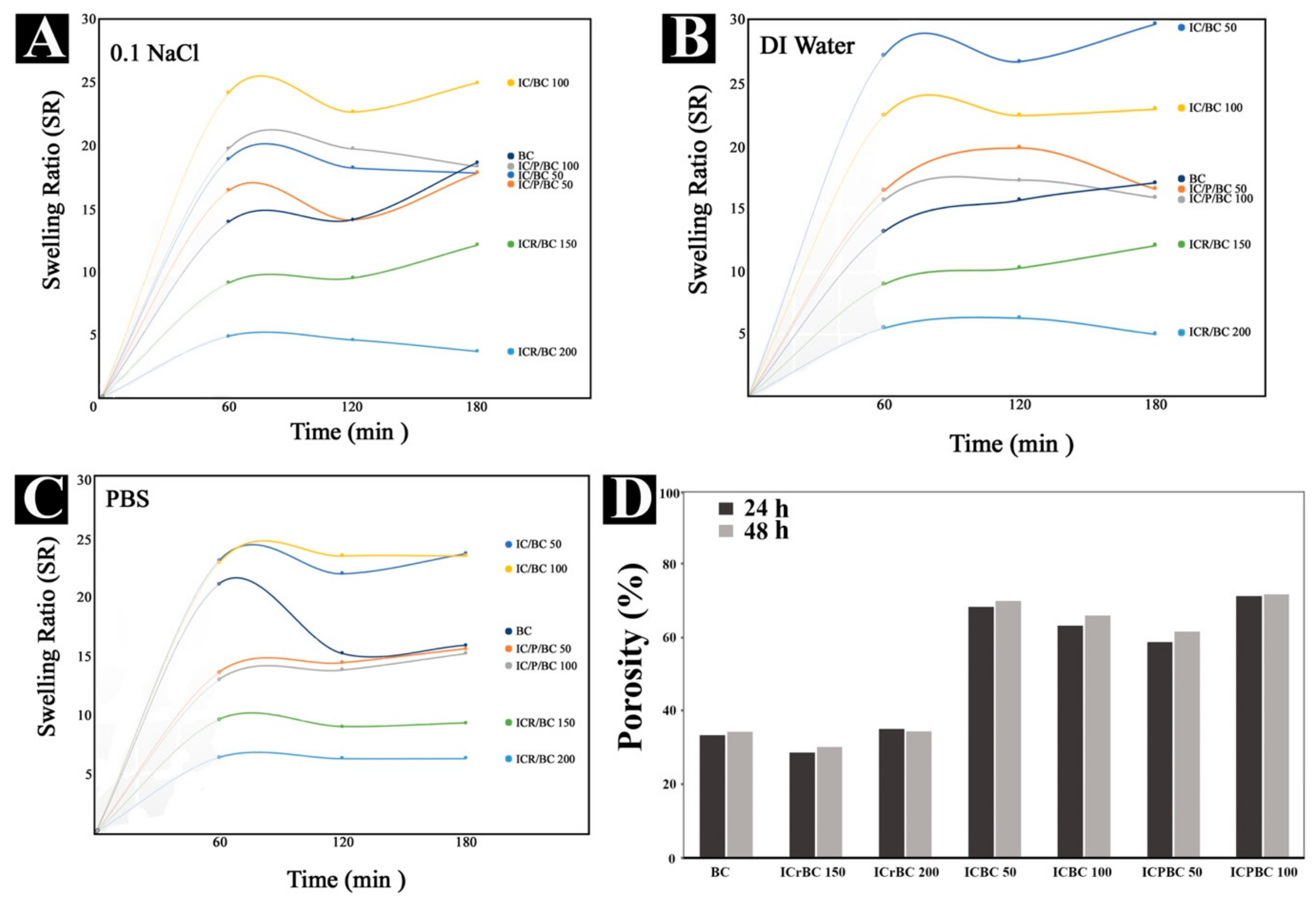
2.6. Investigating the Swelling Properties of the Hydrogels
2.7. The Porosity of Icariin/BC (ICBC) and Icariin/β-CD-IC/BC (IC/P/BC) Hydrogels
2.8. Characterization
2.9. Antioxidant Activities
2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.10. Mechanical Properties
2.11. In Vitro Drug Release Tests
2.12. Antibacterial Activity
2.13. Cytocompatibility Evaluation
3. Results and Discussion
3.1. Analysis of Swelling Ratios and Porosity
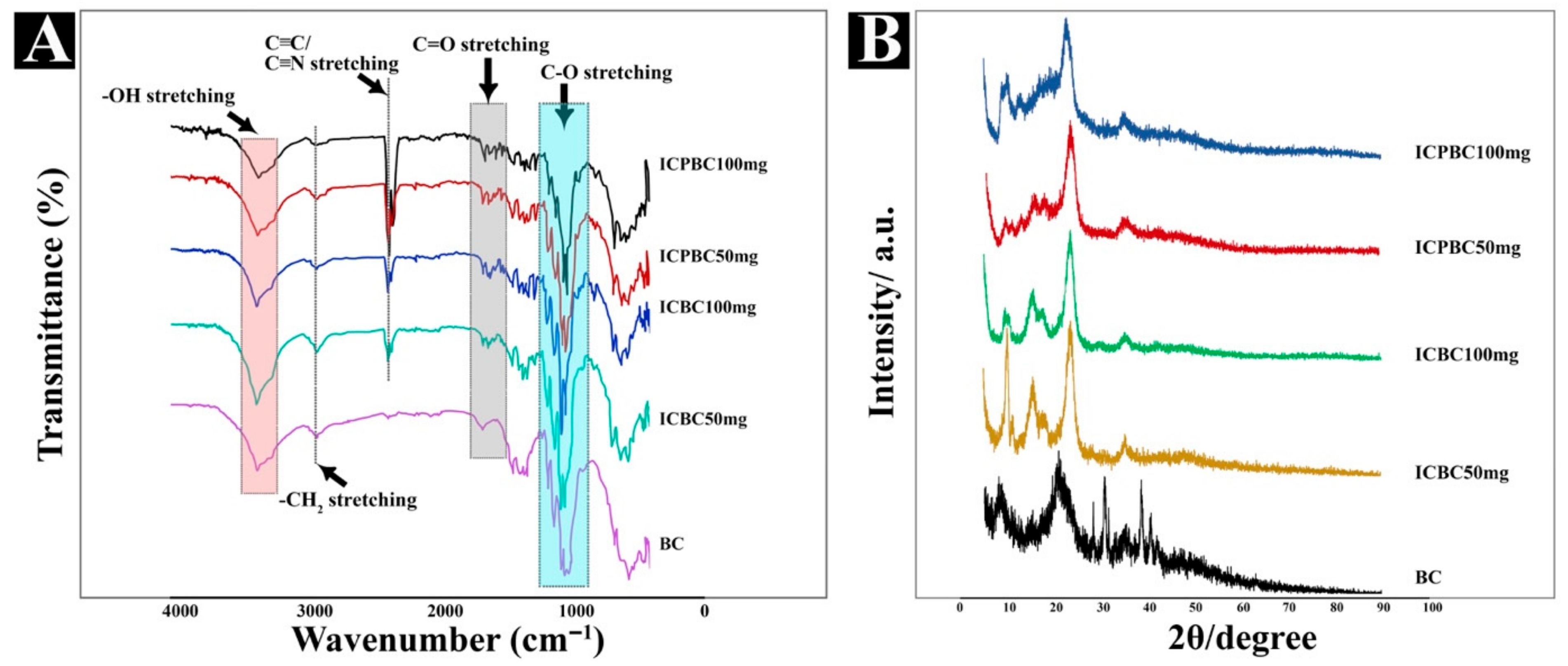
3.2. SEM and Complexation Mode Analysis
3.3. Compatibility and Physical States Analysis (FTIR, XRD & DSC)
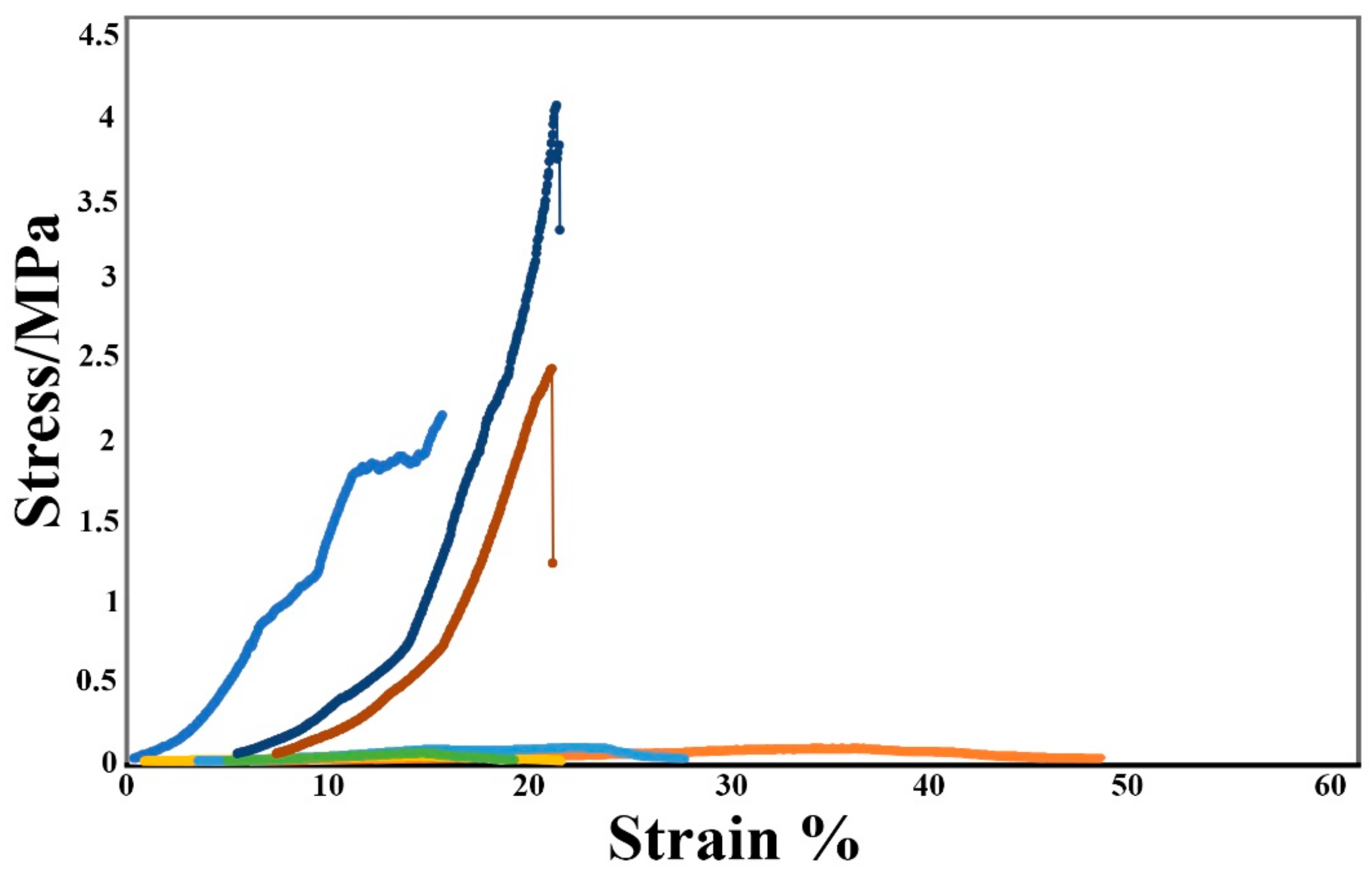
3.4. Mechanical Strength
3.5. In Vitro Drug Release
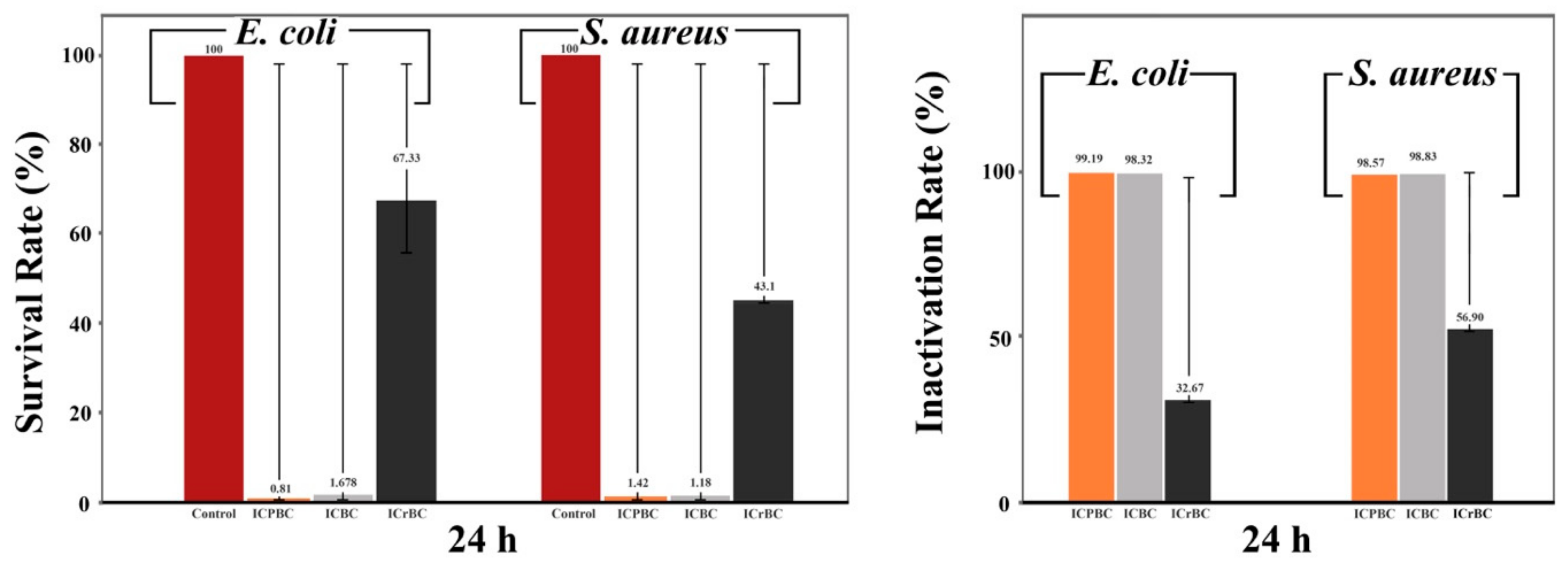
3.6. Antibacterial Activity
3.7. Antioxidant Activities
(DPPH) Radical Scavenging Activity
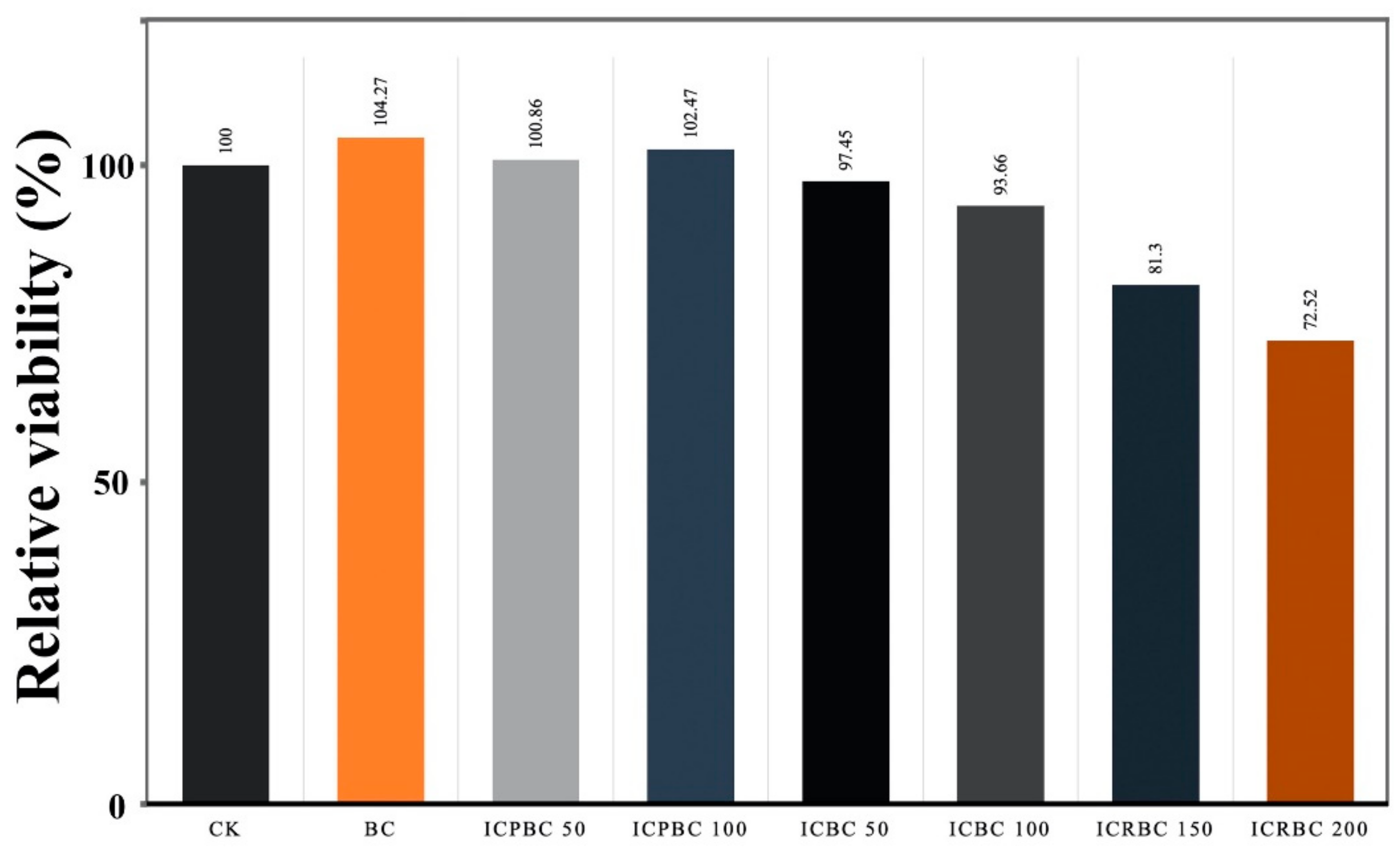
3.8. In Vitro Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niranjan, R.; Kaushik, M.; Selvi, R.T.; Prakash, J.; Venkataprasanna, K.; Prema, D.; Pannerselvam, B.; Venkatasubbu, G.D. PVA/SA/TiO2-CUR patch for enhanced wound healing application: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2019, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.J.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.L.; Plano, L.R.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Si, H.; Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Guo, R.; Wan, Y. One-Step In Situ Biosynthesis of Graphene Oxide-Bacterial Cellulose Nanocomposite Hydrogels. Macromol. Rapid Commun. 2014, 35, 1706–1711. [Google Scholar] [CrossRef]
- Chen, Y.; Mensah, A.; Wang, Q.; Li, D.; Qiu, Y.; Wei, Q. Hierarchical porous nanofibers containing thymol/beta-cyclodextrin: Physico-chemical characterization and potential biomedical applications. Mater. Sci. Eng. C 2020, 115, 111155. [Google Scholar] [CrossRef]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Bin Ariff, A.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Chen, C.; Bang, S.; Cho, Y.; Lee, S.; Lee, I.; Zhang, S.; Noh, I. Research trends in biomimetic medical materials for tissue engineering: 3D bioprinting, surface modification, nano/micro-technology and clinical aspects in tissue engineering of cartilage and bone. Biomater. Res. 2016, 20, 1–7. [Google Scholar] [CrossRef]
- Inoue, B.S.; Streit, S.; Schneider, A.L.D.S.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef]
- Chunshom, N.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of gallic acid/cyclodextrin inclusion complex in freeze-dried bacterial cellulose and poly(vinyl alcohol) hydrogel: Controlled-release characteristic and antioxidant properties. Mater. Chem. Phys. 2019, 232, 294–300. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2012, 21, 1–16. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Cavicchioli, M.; Amaral, T.S.D.; Junior, O.B.D.O.; Santos, D.M.; Petersen, A.L.D.O.A.; Celes, F.S.; Borges, V.D.M.; De Oliveira, C.I.; De Oliveira, P.F.; et al. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carbohydr. Polym. 2015, 128, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, H.; Esguerra, M.; Delbro, D.; Risberg, B.; Gatenholm, P. Engineering microporosity in bacterial cellulose scaffolds. J. Tissue Eng. Regen. Med. 2008, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Kramer, F.; Klemm, D.; Schumann, D.; Heßler, N.; Wesarg, F.; Fried, W.; Stadermann, D. Nanocellulose Polymer Composites as Innovative Pool for (Bio)Material Development. Macromol. Symp. 2006, 244, 136–148. [Google Scholar] [CrossRef]
- Vielreicher, M.; Kralisch, D.; Völkl, S.; Sternal, F.; Arkudas, A.; Friedrich, O. Bacterial nanocellulose stimulates mesenchymal stem cell expansion and formation of stable collagen-I networks as a novel biomaterial in tissue engineering. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef]
- Anton-Sales, I.; Beekmann, U.; Laromaine, A.; Roig, A.; Kralisch, D. Opportunities of Bacterial Cellulose to Treat Epithelial Tissues. Curr. Drug Targets 2019, 20, 808–822. [Google Scholar] [CrossRef]
- Esguerra, M.; Fink, H.; Laschke, M.W.; Jeppsson, A.; Delbro, D.; Gatenholm, P.; Menger, M.D.; Risberg, B. Intravital fluorescent microscopic evaluation of bacterial cellulose as scaffold for vascular grafts. J. Biomed. Mater. Res. Part A 2010, 93, 140–149. [Google Scholar] [CrossRef]
- Arief, Z.M.; Munshi, A.H.; Shawl, A.S. Evaluation of medicinal value of Epimedium elatum on the basis of pharmaco-logically active constituents, Icariin and Icariside-II. Pak. J. Pharm. Sci. 2015, 28, 1665–1669. [Google Scholar] [PubMed]
- Ning, H.; Xin, Z.-C.; Lin, G.; Banie, L.; Lue, T.F.; Lin, C. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology 2006, 68, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Pk, E.-B.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Wang, J.-H. The effect of icariin on immunity and its potential application. Am. J. Clin. Exp. Immunol. 2018, 7, 50–56. [Google Scholar]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Zhang, W.-P.; Bai, X.-J.; Zheng, X.-P.; Xie, X.-L.; Yuan, Z. Icariin Attenuates the Enhanced Prothrombotic State in Atherosclerotic Rabbits Independently of Its Lipid-Lowering Effects. Planta Med. 2013, 79, 731–736. [Google Scholar] [CrossRef]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and Its Metabolites as Potential Protective Phytochemicals Against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Lee, M.K.; Choi, Y.-J.; Sung, S.H.; Shin, D.-I.; Kim, J.; Kim, Y. Antihepatotoxic Activity of Icariin, a Major Constituent ofEpimedium koreanum. Planta Med. 1995, 61, 523–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Zhu, Z.; Gong, W.; Liu, X.; Hou, Q.; Sun, Y.; Chai, J.; Zou, L.; Zhou, T. Icariin Enhances Radiosensitivity of Colorectal Cancer Cells by Suppressing NF-κB Activity. Cell Biophys. 2014, 69, 303–310. [Google Scholar] [CrossRef]
- Guo, J.; Li, F.; Wu, Q.; Gong, Q.; Lu, Y.; Shi, J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine 2010, 17, 950–955. [Google Scholar] [CrossRef]
- Huang, J.-H.; Cai, W.; Zhang, X.; Shen, Z. Icariin Promotes Self-Renewal of Neural Stem Cells: An Involvement of Ex-tracellular Regulated Kinase Signaling Pathway. Chin. J. Integr. Med. 2014, 20, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.H.; Jia, X.; Hu, M. Intestinal Absorption Mechanisms of Prenylated Flavonoids Present in the Heat-Processed Epimedium koreanum Nakai (Yin Yanghuo). Pharm. Res. 2008, 25, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, X.; Liu, Y.; Huang, M.; Qu, D.; Chen, Y. Icariin combined with snailase shows improved intestinal hydrolysis and absorption in osteoporosis rats. Biomed. Pharmacother. 2017, 94, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-L.; Zhang, Y.; Meng, F.-C.; Lin, K.-M.; Wang, Q.-S. Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int. J. Nanomed. 2012, 7, 4239–4249. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, X.-C.; Chen, X.-Y.; Zhang, L.; Lu, C.-T.; Zhao, Y.-Z. Pharmacokinetics and tissue distribution profile of icariin propylene glycol-liposome intraperitoneal injection in mice. J. Pharm. Pharmacol. 2012, 64, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q.; Luo, X.-Y.; Sun, Y.-X.; Wu, W.; Liu, C.-M.; Liu, Z.-Q.; Liu, S.-Y. The antioxidative effect of icariin in human erythrocytes against free-radical-induced haemolysis. J. Pharm. Pharmacol. 2004, 56, 1557–1562. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Fenert, B.; Głód, B.K. Cyclodextrins-Based Nanocomplexes for Encapsulation of Bioactive Compounds in Food, Cosmetics, And Pharmaceutical Products: Principles of Supramolecular Complexes Formation, Their In-fluence On the Antioxidative Properties of Target Chemicals, And Recent Advances in Selected Industrial Applications. In Novel Approaches of Nanotechnology in Food; Elsevier Inc.: Bucharest, Romania, 2016. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Hu, Y.-N.; Luo, Z.-G.; Chen, W.; Chen, H.; Peng, X.-C. Preparation and properties of octenyl succinate β-cyclodextrin and its application as an emulsion stabilizer. Food Chem. 2017, 218, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Cyclodextrins and Their Inclusion Complexes; Akadémiai Kiadó: Budapest, Hungary, 1982; 296p. [Google Scholar]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Narh, C.; Frimpong, C.; Mensah, A.; Wei, Q. Rice Bran, an Alternative Nitrogen Source for Acetobacter xylinum Bacterial Cellulose Synthesis. BioResource 2018, 13, 4346–4363. [Google Scholar] [CrossRef]
- Gromet-Elhanan, Z.; Hestrin, S. Synthesis of Cellulose By Acetobacter Xylinum. J. Bacteriol. 1963, 85, 284–292. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Villar, J.C. The Effect of Carbon and Nitrogen Sources on Bacterial Cellulose Production and Properties from Gluconacetobacter sucrofermentans CECT 7291 Focused on its use in Degraded Paper Restoration. BioResource 2013, 8, 3630–3645. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Electrospun characteristics of gallic acid-loaded poly vinyl alcohol fibers: Release characteristics and antioxidant properties. J. Sci. Adv. Mater. Devices 2018, 3, 175–180. [Google Scholar] [CrossRef]
- Lu, H.; Qiu, Y.; Wang, Q.; Li, G.; Wei, Q. Nanocomposites prepared by electrohydrodynamics and their drug release properties. Mater. Sci. Eng. C 2018, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Sun, Y.; Zheng, Y.; He, W.; Yang, Y.-Y.; Xie, Y.-J.; Feng, Z.-X.; Qiao, K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237. [Google Scholar] [CrossRef]
- Chunshom, N.; Chuysinuan, P.; Techasakul, S.; Ummartyotin, S. Dried-state bacterial cellulose (Acetobacter xylinum) and polyvinyl-alcohol-based hydrogel: An approach to a personal care material. J. Sci. Adv. Mater. Devices 2018, 3, 296–302. [Google Scholar] [CrossRef]
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb(II) Ions from Aqueous Systems with Novel Green Bacterial Cellulose Graphene Oxide Composite. Materials 2019, 12, 218. [Google Scholar] [CrossRef]
- Abuçafy, M.P.; Caetano, B.L.; Chiari-Andréo, B.G.; Fonseca-Santos, B.; Santos, A.M.D.; Chorilli, M.; Chiavacci, L.A. Supramolecular cyclodextrin-based metal-organic frameworks as efficient carrier for anti-inflammatory drugs. Eur. J. Pharm. Biopharm. 2018, 127, 112–119. [Google Scholar] [CrossRef]
- Uppuluri, V.N.V.A.; Shanmugarajan, T.S. Icariin-Loaded Polyvinyl Alcohol/Agar Hydrogel: Development, Characterization, and In Vivo Evaluation in a Full-Thickness Burn Model. Int. J. Low. Extrem. Wounds 2019, 18, 323–335. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-T.; Lu, Z.; Chou, M. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef]
- Narh, C.; Badoe, W.; Howard, E.K.; Lin, N.X.; Mensah, A.; Wang, T.; Wang, Q.; Huang, F.; Wei, Q. Synthesized OH-radical rich bacteria cellulosic pockets with photodynamic bacteria inactivation properties against S. ureus and E. coli. Mater. Sci. Eng. C 2020, 116, 111230. [Google Scholar] [CrossRef] [PubMed]
- University of California Davis. Functional Groups. University of California, Davis. 2020. Available online: https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A%3A_Introductory_Biology_(Britt)/Readings/08%3A_Functional_Groups (accessed on 18 July 2020).
- Gupta, A.; Keddie, D.; Kannappan, V.; Gibson, H.; Khalil, I.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Wen, X.; Chen, Y.; Wang, Y.; Liu, J. Roles of the antioxidant properties of icariin and its phosphorylated derivative in the protection against duck virus hepatitis. BMC Veter. Res. 2014, 10, 226. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Crumling, M.A.; King, K.A.; Duncan, R.K. Cyclodextrins and Iatrogenic Hearing Loss: New Drugs with Significant Risk. Front. Cell. Neurosci. 2017, 11, 355. [Google Scholar] [CrossRef]





| Culture Constituents (Via Modification) | C:N (Carbon to Nitrogen) Ratio for D-MP | Nitrogen Index of Rice Bran (%) (Mean +/− SD) | Cultivation Period |
|---|---|---|---|
| Peptone (0.3 g) + D-Mannitol (2.5 g) + Rice Bran extract (0.5 g) | 8.3:1 | 0.238 +/− 0.035 | 7 Days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, A.; Chen, Y.; Asinyo, B.K.; Howard, E.K.; Narh, C.; Huang, J.; Wei, Q. Bioactive Icariin/β-CD-IC/Bacterial Cellulose with Enhanced Biomedical Potential. Nanomaterials 2021, 11, 387. https://doi.org/10.3390/nano11020387
Mensah A, Chen Y, Asinyo BK, Howard EK, Narh C, Huang J, Wei Q. Bioactive Icariin/β-CD-IC/Bacterial Cellulose with Enhanced Biomedical Potential. Nanomaterials. 2021; 11(2):387. https://doi.org/10.3390/nano11020387
Chicago/Turabian StyleMensah, Alfred, Yajun Chen, Benjamin K. Asinyo, Ebenezer Kofi Howard, Christopher Narh, Jieyu Huang, and Qufu Wei. 2021. "Bioactive Icariin/β-CD-IC/Bacterial Cellulose with Enhanced Biomedical Potential" Nanomaterials 11, no. 2: 387. https://doi.org/10.3390/nano11020387
APA StyleMensah, A., Chen, Y., Asinyo, B. K., Howard, E. K., Narh, C., Huang, J., & Wei, Q. (2021). Bioactive Icariin/β-CD-IC/Bacterial Cellulose with Enhanced Biomedical Potential. Nanomaterials, 11(2), 387. https://doi.org/10.3390/nano11020387






