Carbon Nanotubes: Probabilistic Approach for Occupational Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hazard Identification
2.2. Dose–Response Assessment
2.3. Exposure Assessment
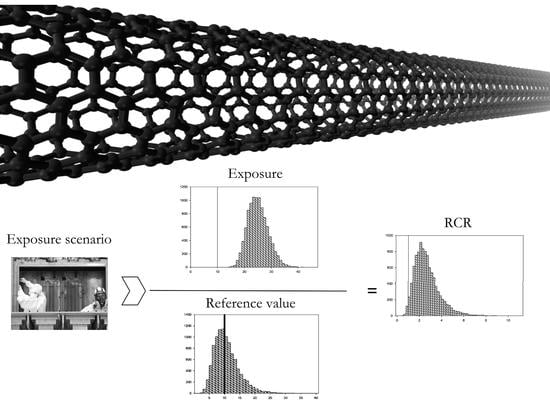
2.4. Risk Characterization and Uncertainty Analysis
3. Results
3.1. Hazard Identification
3.2. Dose–Response Assessment
3.3. Exposure Assessment
3.4. Risk Characterization and Uncertainty Analysis
3.5. Limitations of the Sudy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| AIC | Akaike’s information criterion |
| AM | arithmetic mean |
| BMC | benchmark concentration |
| BMCh | benchmark concentration-human |
| BMCL | benchmark concentration (95% CI Lower Bound) |
| BMCU | benchmark concentration (95% CI Upper Bound) |
| BMD | benchmark dose |
| BMDL | benchmark dose (95% CI lower bound) |
| BMDU | benchmark dose (95% CI upper bound) |
| CNTs | Carbon nanotubes |
| EFinter | interspecies extrapolation factor |
| EFintra | intraspecies extrapolation factor |
| ES | exposure scenario |
| EXP | exposure data distribution |
| GM | geometric mean |
| GNPs | graphene nanoplatelets |
| GSD | geometric standard deviation |
| HBGV | health-based guidance value |
| LOAEL | lowest-observed adverse effect level |
| MN | manufactured (engineered) nanomaterial |
| MWCNTs | multiwalled carbon nanotubes |
| MT | measurement technique |
| NOAEL | no-observed-adverse-effect level |
| NWH | non-working hours |
| OEL | occupational exposure level |
| RA | risk assessment |
| RCR | risk characterization ratios |
| REL | recommended exposure limit |
| SD | standard deviation |
| SWCNTs | single-walled carbon nanotubes |
| TWA | time-weighted average |
| UFi | uncertainty factor |
| WE | work events |
| WH | working hours |
References
- Leso, V.; Fontana, L.; Mauriello, M.; Iavicoli, I. Occupational Risk Assessment of Engineered Nanomaterials: Limits, Challenges and Opportunities. Curr. Nanosci. 2016, 13, 55–78. [Google Scholar] [CrossRef] [Green Version]
- Isigonis, P.; Afantitis, A.; Antunes, D.; Bartonova, A.; Beitollahi, A.; Bohmer, N.; Bouman, E.; Chaudhry, Q.; Cimpan, M.R.; Cimpan, E.; et al. Risk Governance of Emerging Technologies Demonstrated in Terms of its Applicability to Nanomaterials. Small 2020, 16, 2003303. [Google Scholar] [CrossRef]
- Schulte, P.A.; Leso, V.; Niang, M.; Iavicoli, I. Current state of knowledge on the health effects of engineered nanomaterials in workers: A systematic review of human studies and epidemiological investigations. Scand. J. Work. Environ. Health 2019, 45, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.P.; Hristozov, D.; Zabeo, A.; Koivisto, A.J.; Jensen, A.C.Ø.; Jensen, K.A.; Pang, C.; Marcomini, A.; Sonnemann, G. Probabilistic risk assessment of emerging materials: Case study of titanium dioxide nanoparticles. Nanotoxicology 2017, 11, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Spinazzè, A.; Cattaneo, A.; Borghi, F.; Del Buono, L.; Campagnolo, D.; Rovelli, S.; Cavallo, D.M. Probabilistic approach for the risk assessment of nanomaterials: A case study for graphene nanoplatelets. Int. J. Hyg. Environ. Health 2019, 222, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Risk Assessment in the Federal Government; National Academies Press: Washington, DC, USA, 1983; ISBN 978-0-309-03349-7.
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Guseva Canu, I.; Batsungnoen, K.; Maynard, A.; Hopf, N.B. State of knowledge on the occupational exposure to carbon nanotube. Int. J. Hyg. Environ. Health 2020, 225, 113472. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Hart, J.E.; Laden, F.; Garshick, E.; Smith, T.J. A retrospective assessment of occupational exposure to elemental carbon in the U.S. Trucking Industry. Environ. Health Perspect. 2011, 119, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, K.H.; More, S.; Mortensen, A.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, e04658. [Google Scholar]
- Slob, W.; Bakker, M.I.; Biesebeek, J.D.T.; Bokkers, B.G.H. Exploring the uncertainties in cancer risk assessment using the integrated probabilistic risk assessment (IPRA) approach. Risk Anal. 2014, 34, 1401–1422. [Google Scholar] [CrossRef]
- U.S. EPA Benchmark Dose Tools|US EPA. Available online: https://www.epa.gov/bmds (accessed on 1 September 2020).
- European Chemicals Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health; European Chemicals Agency: Helsinki, Finland, 2012. [Google Scholar]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef] [Green Version]
- Pauluhn, J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: Toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol. Sci. 2010, 113, 226–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Kondo, H.; Takeuchi, T.; Aiso, S.; Nishizawa, T.; Matsumoto, M.; Fukushima, S. Thirteen-week study of toxicity of fiber-like multi-walled carbon nanotubes with whole-body inhalation exposure in rats. Nanotoxicology 2015, 9, 413–422. [Google Scholar] [CrossRef]
- Pothmann, D.; Simar, S.; Schuler, D.; Dony, E.; Gaering, S.; Net, J.L.; Okazaki, Y.; Chabagno, J.M.; Bessibes, C.; Beausoleil, J.; et al. Lung inflammation and lack of genotoxicity in the comet and micronucleus assays of industrial multiwalled carbon nanotubes Graphistrength© C100 after a 90-day nose-only inhalation exposure of rats. Part. Fibre Toxicol. 2015, 12, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Hirohashi, M.; Kobayashi, N.; Ogami, A.; Horie, M.; Oyabu, T.; Myojo, T.; Hashiba, M.; Mizuguchi, Y.; Kambara, T.; et al. Pulmonary toxicity of well-dispersed single-wall carbon nanotubes after inhalation. Nanotoxicology 2012, 6, 766–775. [Google Scholar] [CrossRef]
- Osmond-McLeod, M.J.; Poland, C.A.; Murphy, F.; Waddington, L.; Morris, H.; Hawkins, S.C.; Clark, S.; Aitken, R.; McCall, M.J.; Donaldson, K. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part. Fibre Toxicol. 2011, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2015, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sargent, L.M.; Porter, D.W.; Staska, L.M.; Hubbs, A.F.; Lowry, D.T.; Battelli, L.; Siegrist, K.J.; Kashon, M.L.; Mercer, R.R.; Bauer, A.K.; et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Emerce, E.; Ghosh, M.; Öner, D.; Duca, R.C.; Vanoirbeek, J.; Bekaert, B.; Hoet, P.H.M.; Godderis, L. Carbon Nanotube- and Asbestos-Induced DNA and RNA Methylation Changes in Bronchial Epithelial Cells. Chem. Res. Toxicol. 2019, 32, 8501–8860. [Google Scholar] [CrossRef]
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014, 15, 1427–1428. [Google Scholar] [CrossRef]
- Hougaard, K.S.; Jackson, P.; Kyjovska, Z.O.; Birkedal, R.K.; De Temmerman, P.J.; Brunelli, A.; Verleysen, E.; Madsen, A.M.; Saber, A.T.; Pojana, G.; et al. Effects of lung exposure to carbon nanotubes on female fertility and pregnancy. A study in mice. Reprod. Toxicol. 2013, 41, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Health and Human Services, Centers for Disease Control and Prevention—National Institute for Occupational Safety and Health (NIOSH). Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers; NIOSH: Cincinnati, OH, USA, 2013. [Google Scholar]
- British Standards Institution. (BSI). Nanotechnologies—Part 2: Guide to Safe Handling and Disposal of Manufactured Nanomaterials; Report No. 978 0580 60; British Standards Institute: London, UK, 2007; Volume 2. [Google Scholar]
- Lee, J.H.; Sohn, E.K.; Ahn, J.S.; Ahn, K.; Kim, K.S.; Lee, J.H.; Lee, T.M.; Yu, I.J. Exposure assessment of workers in printed electronics workplace. Inhal. Toxicol. 2013, 25, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.S.; Viitanen, A.K.; Koivisto, A.J.; Kangas, A.; Huhtiniemi, M.; Hussein, T.; Vanhala, E.; Viana, M.; Querol, X.; Hameri, K. Characterization of exposure to carbon nanotubes in an industrial setting. Ann. Occup. Hyg. 2015, 59, 586–599. [Google Scholar] [PubMed]
| BMCa | BMCLa | BMCUa | BMCh |
|---|---|---|---|
| GM (± GSD) [mg/m3] | GM (± GSD) [µg/m3] | ||
| 14.1 (± 1.1) | 9.4 (± 1.0) | 20.4 (± 2.3) | 10.0 (± 1.5) |
| MTs | WEs | Exposure [µg/m3] | |
|---|---|---|---|
| Mean | SD | ||
| MT1 | WE1 | 0.83 | 0.18 |
| WE2 | 1.0 | 0.07 | |
| WE3 | 1.2 | 0.12 | |
| WH | 1.2 | 0.32 | |
| NWH | 0.92 | 0.31 | |
| MT2 | WE1 | 3.4 | 22 |
| WE2 | 25 | 3.80 | |
| WE3 | 6.1 | 12.0 | |
| WH | 6.7 | 22.0 | |
| NWH | 4.5 | 6 | |
| MT3 | WE1 | 0.81 | 0.82 |
| WE2 | 1.7 | 0.69 | |
| WE3 | n.a. | n.a. | |
| WH | 1.5 | 5.7 | |
| NWH | 0.42 | 0.21 | |
| MTs | WEs | Exposure [µg/m3] | |||||
|---|---|---|---|---|---|---|---|
| GM | GSD | Min | 5th Percentile | 95th Percentile | Max | ||
| MT1 | WE1 | 0.81 | 1.24 | 0.38 | 0.57 | 0.62 | 1.71 |
| WE2 | 1.00 | 1.07 | 0.77 | 0.89 | 0.91 | 1.29 | |
| WE3 | 1.19 | 1.10 | 0.83 | 1.01 | 1.05 | 1.71 | |
| WH | 1.16 | 1.30 | 0.46 | 0.76 | 0.82 | 3.23 | |
| NWH | 0.87 | 1.39 | 0.23 | 0.51 | 0.57 | 3.22 | |
| MT2 | WE1 | 0.53 | 7.08 | 0.00 | 0.02 | 0.04 | 1287 |
| WE2 | 24.8 | 1.16 | 14.49 | 19.44 | 20.4 | 44.5 | |
| WE3 | 2.76 | 3.53 | 0.01 | 0.35 | 0.53 | 372 | |
| WH | 2.65 | 2.76 | 0.002 | 0.15 | 0.26 | 493 | |
| NWH | 2.65 | 2.76 | 0.06 | 0.49 | 0.71 | 89.8 | |
| MT3 | WE1 | 0.56 | 2.30 | 0.02 | 0.15 | 0.19 | 12.8 |
| WE2 | 1.58 | 1.48 | 0.33 | 0.83 | 0.95 | 6.81 | |
| WE3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| WH | 0.38 | 5.10 | 0.001 | 0.03 | 0.04 | 137 | |
| NWH | 0.38 | 1.60 | 0.05 | 0.17 | 0.20 | 2.39 | |
| MT | WE | Risk Characterization Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GM | GSD | Min | 5th Percentile | 95th Percentile | Max | RCR ≥ 1 (95% CI) | Probability (%) RCR ≥ 1 | ||
| MT1 | WE1 | 0.08 | 1.54 | 0.02 | 0.08 | 0.08 | 0.35 | no | 0.0 |
| WE2 | 0.10 | 1.47 | 0.03 | 0.10 | 0.10 | 0.42 | no | 0.0 | |
| WE3 | 0.12 | 1.48 | 0.03 | 0.12 | 0.12 | 0.48 | no | 0.0 | |
| WH | 0.12 | 1.58 | 0.02 | 0.11 | 0.12 | 0.98 | no | 0.0 | |
| MT2 | WE1 | 0.05 | 7.37 | 0.00003 | 0.05 | 0.05 | 147 | no | 7.0 |
| WE2 | 2.47 | 1.50 | 0.53 | 2.45 | 2.49 | 10.7 | yes | 98.6 | |
| WE3 | 0.28 | 3.72 | 0.001 | 0.27 | 0.28 | 73.7 | no | 16.2 | |
| WH | 0.20 | 5.05 | 0.0002 | 0.20 | 0.21 | 72.8 | no | 16.4 | |
| MT3 | WE1 | 0.56 | 2.49 | 0.002 | 0.55 | 0.57 | 2.0 | no | 0.1 |
| WE2 | 0.16 | 1.72 | 0.03 | 0.16 | 0.16 | 1.42 | no | 0.1 | |
| WE3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.0 | |
| WH | 0.04 | 5.31 | 0.0001 | 0.04 | 0.04 | 21.0 | no | 2.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinazzè, A.; Zellino, C.; Borghi, F.; Campagnolo, D.; Rovelli, S.; Keller, M.; Fanti, G.; Cattaneo, A.; Cavallo, D.M. Carbon Nanotubes: Probabilistic Approach for Occupational Risk Assessment. Nanomaterials 2021, 11, 409. https://doi.org/10.3390/nano11020409
Spinazzè A, Zellino C, Borghi F, Campagnolo D, Rovelli S, Keller M, Fanti G, Cattaneo A, Cavallo DM. Carbon Nanotubes: Probabilistic Approach for Occupational Risk Assessment. Nanomaterials. 2021; 11(2):409. https://doi.org/10.3390/nano11020409
Chicago/Turabian StyleSpinazzè, Andrea, Carolina Zellino, Francesca Borghi, Davide Campagnolo, Sabrina Rovelli, Marta Keller, Giacomo Fanti, Andrea Cattaneo, and Domenico M. Cavallo. 2021. "Carbon Nanotubes: Probabilistic Approach for Occupational Risk Assessment" Nanomaterials 11, no. 2: 409. https://doi.org/10.3390/nano11020409
APA StyleSpinazzè, A., Zellino, C., Borghi, F., Campagnolo, D., Rovelli, S., Keller, M., Fanti, G., Cattaneo, A., & Cavallo, D. M. (2021). Carbon Nanotubes: Probabilistic Approach for Occupational Risk Assessment. Nanomaterials, 11(2), 409. https://doi.org/10.3390/nano11020409