Hydrophobic Epoxy Caged Silsesquioxane Film (EP-POSS): Synthesis and Performance Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
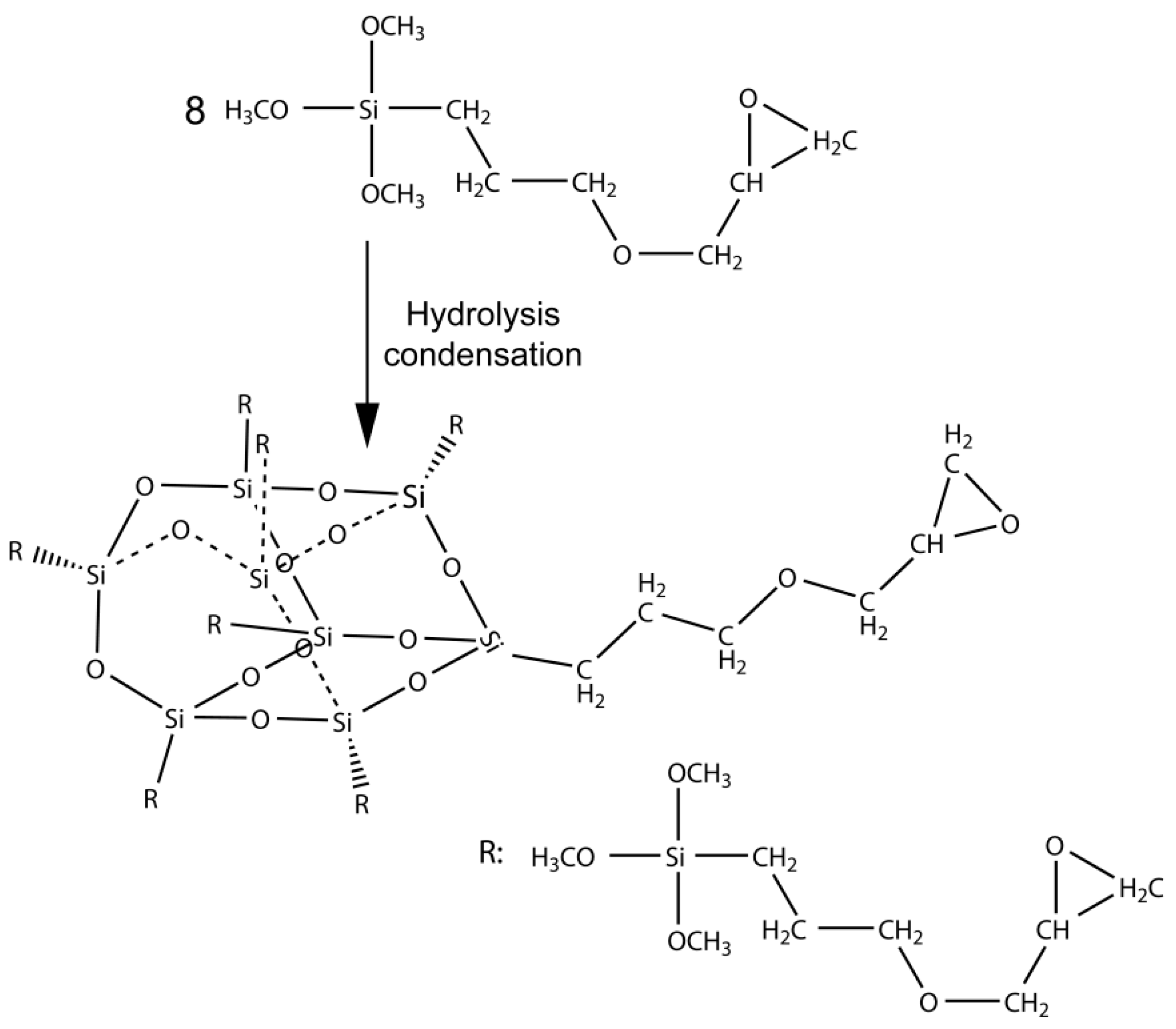
2.2. The Preparation of EP-POSS
2.3. Synthesis of Low-Viscosity POSS (POSS-L) and High-Viscosity POSS (POSS-H)
2.4. Preparation of the EP-POSS Composite
2.5. Synthesis of EP-POSS-SiO2 Nano-Composites
2.6. Characterization
3. Results
3.1. EP-POSS Synthesis Factors
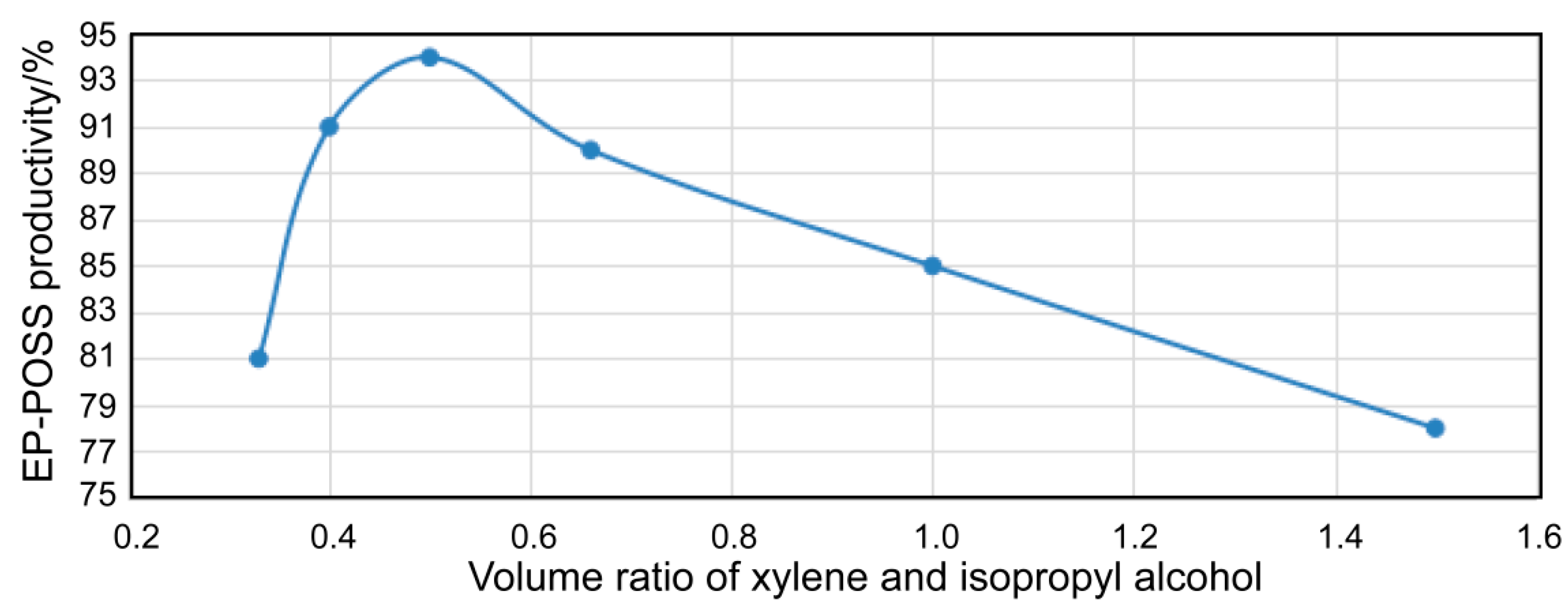
3.1.1. The Relationship between the Solvent Volume Ratio of Xylene and Isopropanol and the Yield of EP-POSS
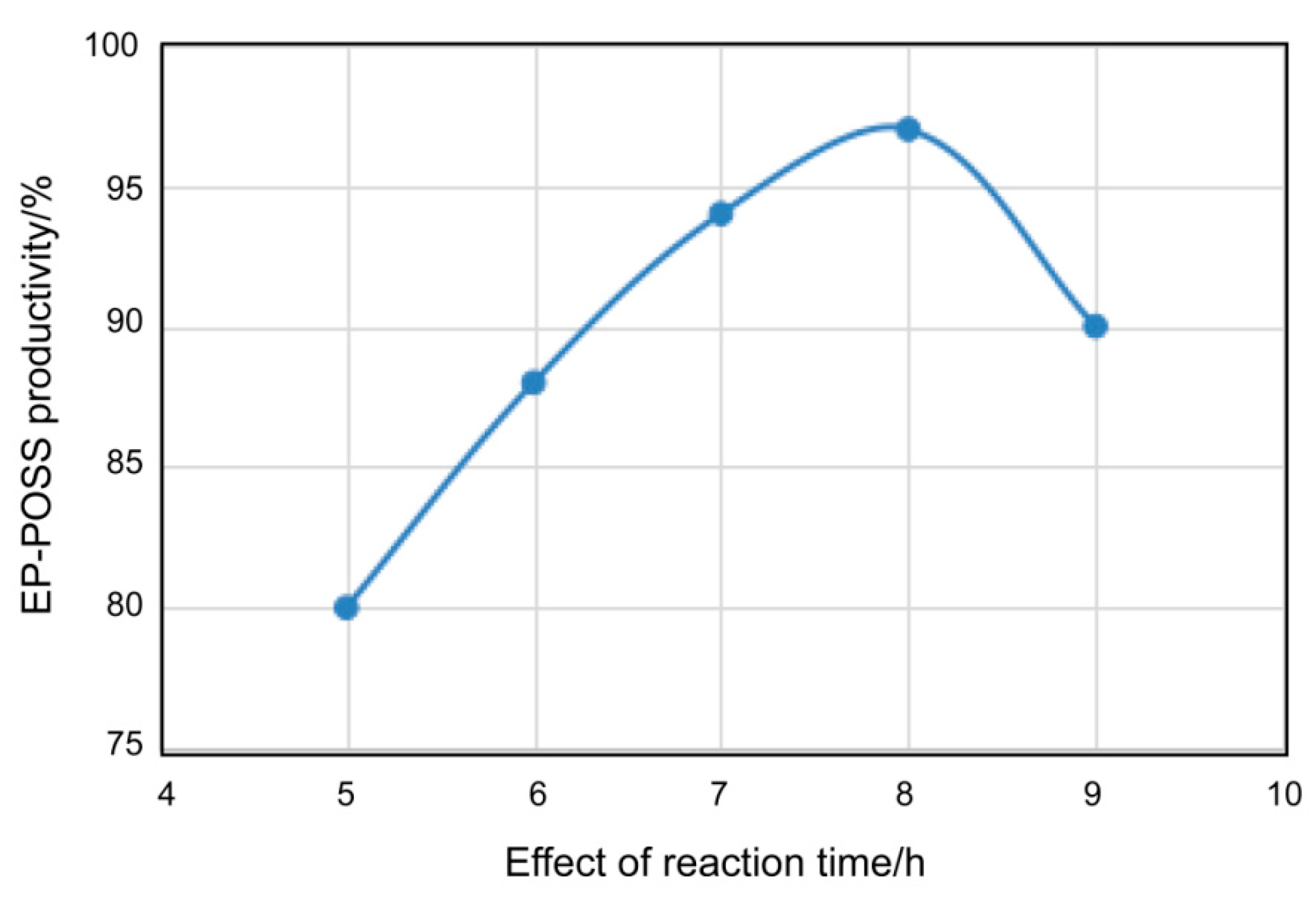
3.1.2. Relationship between Heating Time and EP-POSS Yield
3.2. Surface Structure Composition
3.3. Microscopic Morphology Analysis
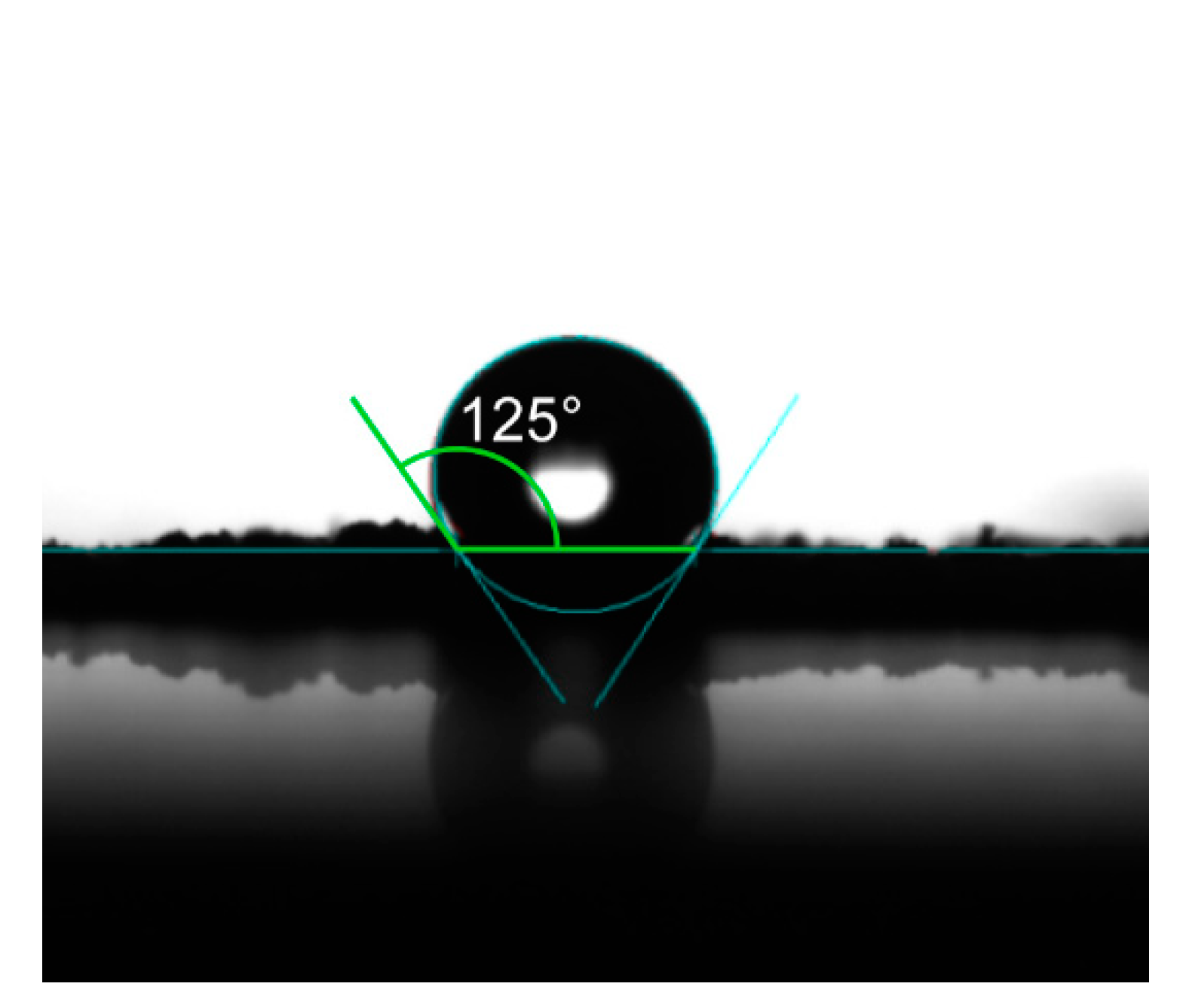
3.4. Hydrophobicity and Light Transmittance Testing
3.5. Thermal Performance
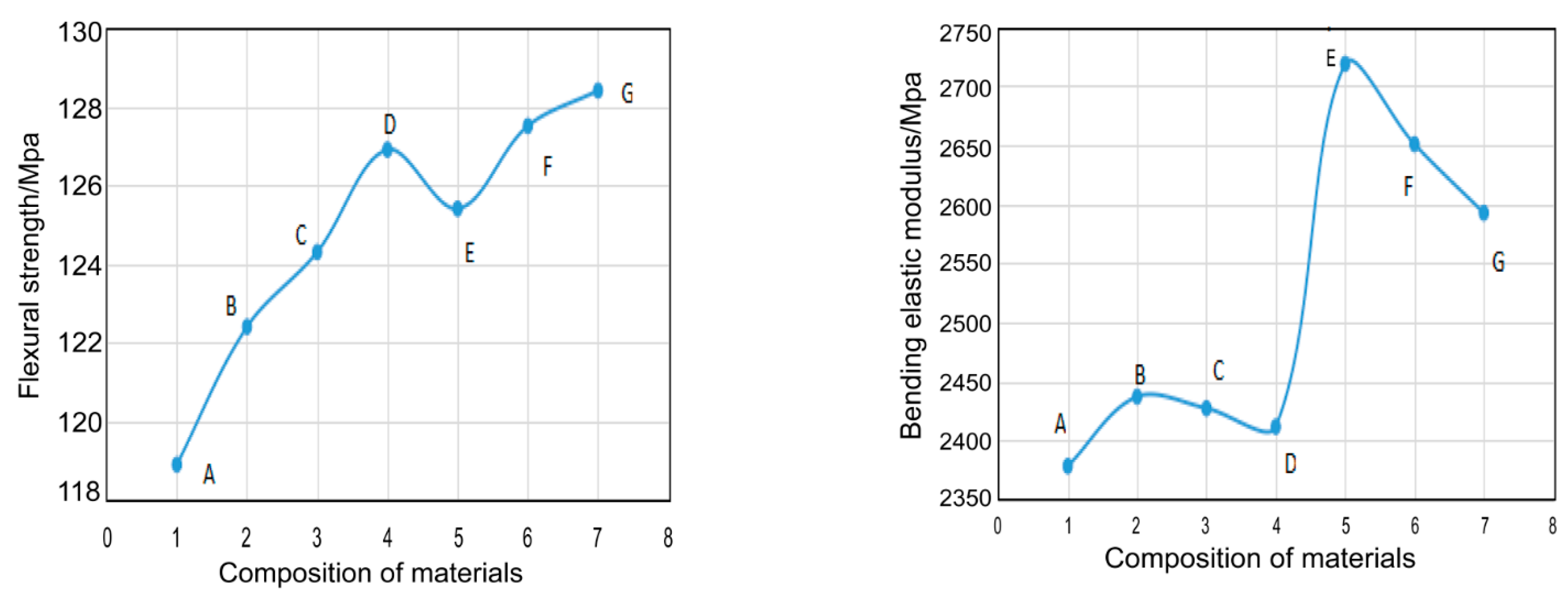
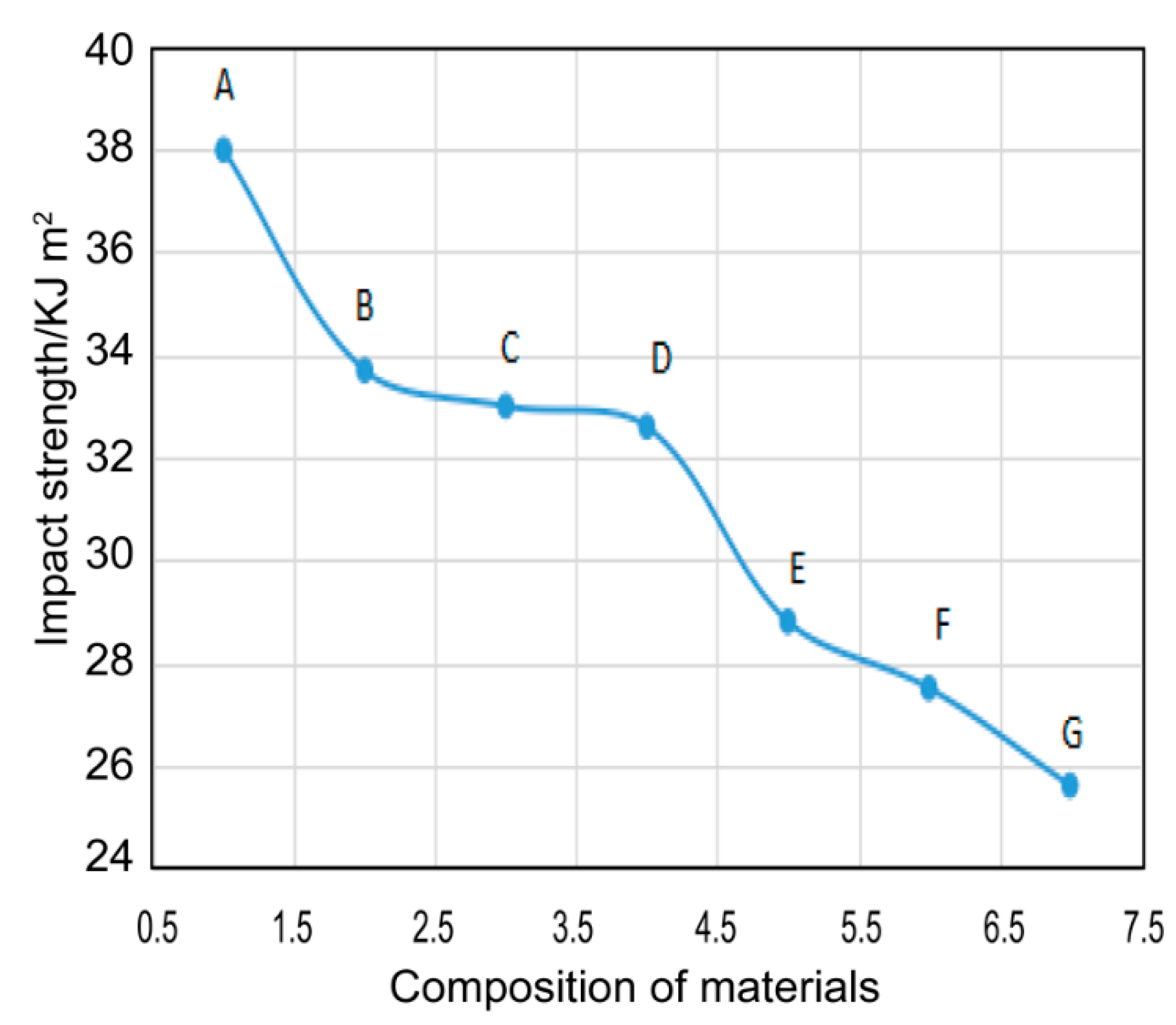
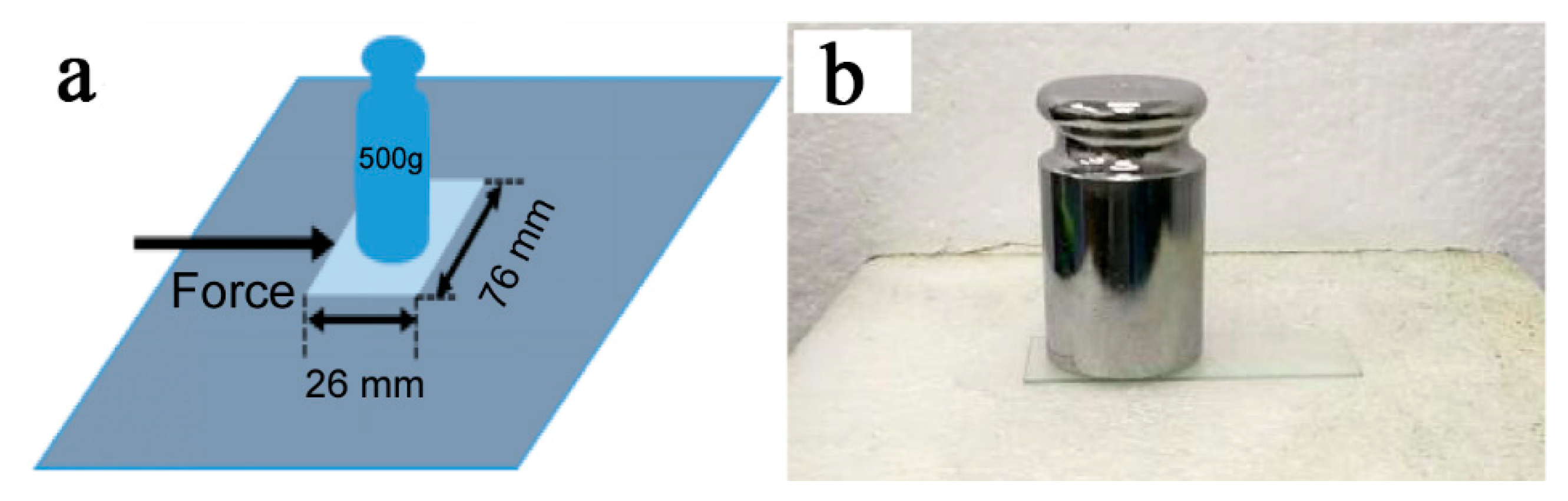
3.6. Mechanical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scott, D.W. Thermal Rearrangement of Branched-Chain Methylpolysiloxanes1. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S.; Nie, K. Morphology and thermal properties of inorganic–organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes. Polymer 2004, 45, 5557–5568. [Google Scholar] [CrossRef]
- Frank, K.L.; Exley, S.E.; Thornell, T.L.; Morgan, S.E.; Wiggins, J.S. Investigation of pre-reaction and cure temperature on multiscale dispersion in POSS–epoxy nanocomposites. Polymer 2012, 53, 4643–4651. [Google Scholar] [CrossRef]
- Matějka, L.; Murias, P.; Pleštil, J. Effect of POSS on thermomechanical properties of epoxy–POSS nanocomposites. Eur. Polym. J. 2012, 48, 260–274. [Google Scholar] [CrossRef]
- Jerman, I.; Šurca Vuk, A.; Koželj, M.; Švegl, F.; Orel, B. Influence of amino functionalised POSS additive on the corrosion properties of (3-glycidoxypropyl)trimethoxysilane coatings on AA 2024 alloy. Prog. Org. Coat. 2011, 72, 334–342. [Google Scholar] [CrossRef]
- Mishra, K.; Pandey, G.; Singh, R.P. Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS) as nano-reinforcement. Polym. Test. 2017, 62, 210–218. [Google Scholar] [CrossRef]
- Yang, H.; He, C.; Russell, T.P.; Wang, D. Epoxy-polyhedral oligomeric silsesquioxanes (POSS) nanocomposite vitrimers with high strength, toughness, and efficient relaxation. Giant 2020, 4, 100035. [Google Scholar] [CrossRef]
- Laine, R.M.; Roll, M.F. Polyhedral Phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Franczyk, A.; Marciniec, B. Synthesis of monofunctionalized POSS through hydrosilylation. J. Organomet. Chem. 2018, 872, 73–78. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Gao, Q.; Qi, S.; Wu, Z.; Wu, D.; Yang, W. Synthesis and characterization of functional ladder-like polysilsesquioxane and their hybrid films with polyimide. Thin Solid Film. 2011, 519, 6499–6507. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Zhao, X.; Jiang, P.; Yu, H. Flame retardancy and thermal stability of polyhedral oligomeric silsesquioxane nanocomposites. Fire Mater. 2013, 37, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, A.; Liang, G.; Ren, P.; Xie, J.; Wang, X. Thermo-oxygen degradation mechanisms of POSS/epoxy nanocomposites. Polym. Degrad. Stab. 2007, 92, 1986–1993. [Google Scholar] [CrossRef]
- Ramírez, C.; Rico, M.; Torres, A.; Barral, L.; López, J.; Montero, B. Epoxy/POSS organic–inorganic hybrids: ATR-FTIR and DSC studies. Eur. Polym. J. 2008, 44, 3035–3045. [Google Scholar] [CrossRef]
- Boček, J.; Matějka, L.; Mentlík, V.; Trnka, P.; Šlouf, M. Electrical and thermomechanical properties of epoxy-POSS nanocomposites. Eur. Polym. J. 2011, 47, 861–872. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sloan, R.; Wiggins, J.S. Epoxy hybrid networks with high mass fraction molecular-level dispersion of pendant polyhedral oligomeric silsesquioxane (POSS). Polymer 2017, 114, 298–310. [Google Scholar] [CrossRef]
- Jiri, B.; Martina, U.; Adam, S. Epoxy networks reinforced with polyhedral oligomeric silsesquioxanes: Structure and segmental dynamics as studied by Solid-State NMR. Macromolecules 2008, 41, 372–386. [Google Scholar] [CrossRef]
- Rashid, E.S.A.; Ariffin, K.; Kooi, C.C.; Akil, H.M. Preparation and properties of POSS/epoxy composites for electronic packaging applications. Mater. Des. 2009, 30, 1–8. [Google Scholar] [CrossRef]
- Lin, P.-H.; Khare, R. Molecular Simulation of Cross-Linked Epoxy and Epoxy−POSS Nanocomposite. Macromolecules 2009, 42, 4319–4327. [Google Scholar] [CrossRef]
- Chen, M.; Qu, Y.; Yang, L.; Gao, H. Structures and antifouling properties of low surface energy non-toxic antifouling coatings modified by nano-SiO2 powder. Sci. China Ser. B Chem. 2008, 51, 848–852. [Google Scholar] [CrossRef]
- Chi, B.; Hou, S.; Liu, G.; Deng, Y.; Zeng, J.; Song, H.; Liao, S.; Ren, J. Tuning hydrophobic-hydrophilic balance of cathode catalyst layer to improve cell performance of proton exchange membrane fuel cell (PEMFC) by mixing polytetrafluoroethylene (PTFE). Electrochim. Acta 2018, 277, 110–115. [Google Scholar] [CrossRef]
- Bryant, P.M.; Szili, E.J.; Whittle, T.; Park, S.-J.; Eden, J.G.; Al-Bataineh, S.; Steele, D.A.; Short, R.D.; Bradley, J.W. The use of a micro-cavity discharge array at atmospheric pressure to investigate the spatial modification of polymer surfaces. Surf. Coat. Technol. 2010, 204, 2279–2288. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Han, Y.; Liu, H.; Zou, H. Hydrophobically modified sustainable bio-based polyurethane for controllable release of coated urea. Eur. Polym. J. 2020. [Google Scholar] [CrossRef]
- Dai, M.; Zhai, Y.; Zhang, Y. A green approach to preparing hydrophobic, electrically conductive textiles based on waterborne polyurethane for electromagnetic interference shielding with low reflectivity. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- Shiraki, Y.; Yokoyama, H. Novel hydrophobic and oleophobic surfaces using polyurethane with hydrogenated polyisoprene soft segment. Mater. Today Commun. 2020, 24, 101243. [Google Scholar] [CrossRef]

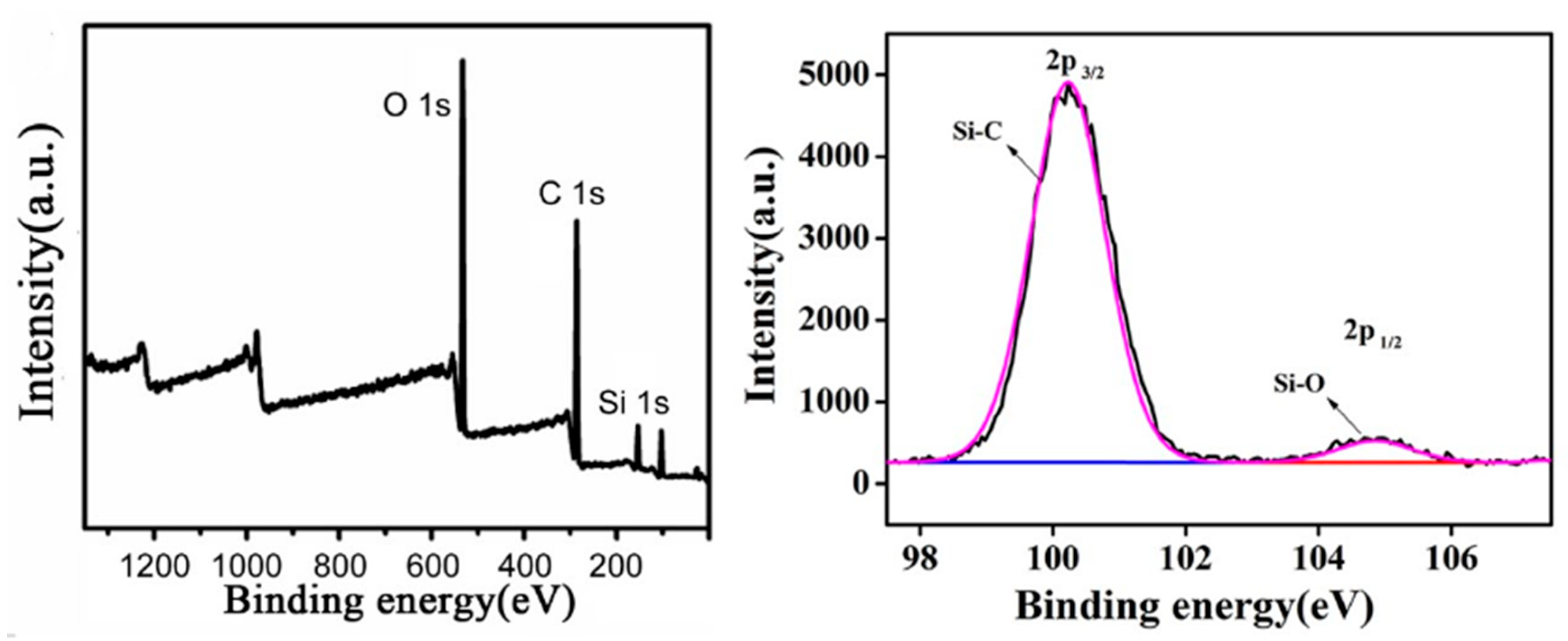
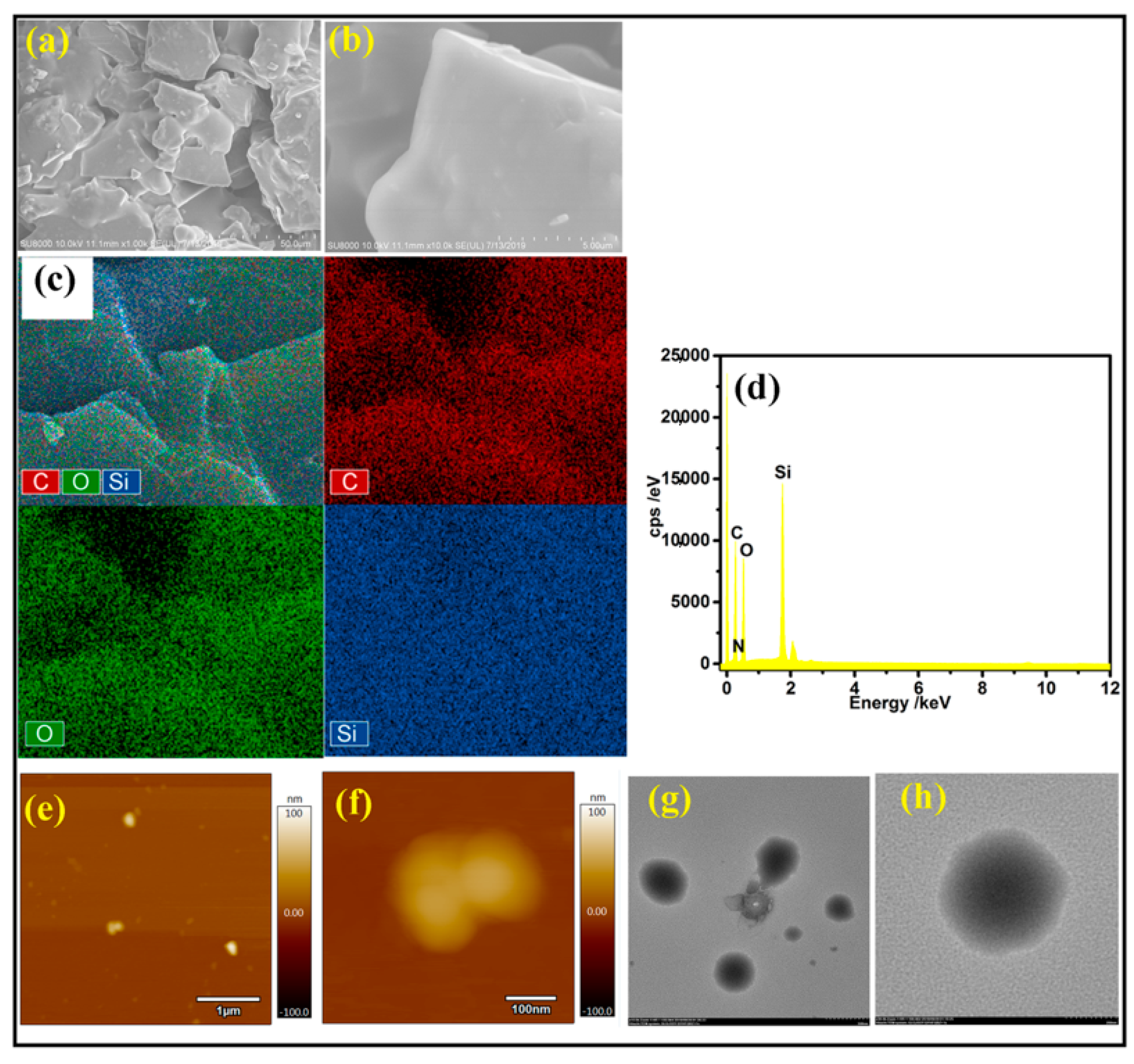


| Structure | Si–C | Si–O | C–H | C–O | C–N |
| Content/% | 7.30 | 0.54 | 64.61 | 27.47 | 0.12 |
| Composition of Materials | Bending Strength (MPa) | Bending Modulus (MPa) | Impact Strength (kJ·m−2) |
|---|---|---|---|
| EP | 118.9 | 2378.5 | 38.0 |
| EP-POSS(L) | 122.4 | 2437.3 | 33.7 |
| EP-POSS(M) | 124.3 | 2427.4 | 33.0 |
| EP-POSS(H) | 126.9 | 2411.8 | 32.6 |
| EP-SiO2-POSS(L) | 125.4 | 2718.6 | 28.8 |
| EP-SiO2-POSS(M) | 127.5 | 2650.8 | 27.5 |
| EP-SiO2-POSS(H) | 128.4 | 2592.6 | 25.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Wang, P.; Sun, L.; Wang, L. Hydrophobic Epoxy Caged Silsesquioxane Film (EP-POSS): Synthesis and Performance Characterization. Nanomaterials 2021, 11, 472. https://doi.org/10.3390/nano11020472
Fang Y, Wang P, Sun L, Wang L. Hydrophobic Epoxy Caged Silsesquioxane Film (EP-POSS): Synthesis and Performance Characterization. Nanomaterials. 2021; 11(2):472. https://doi.org/10.3390/nano11020472
Chicago/Turabian StyleFang, Yanhong, Ping Wang, Lifang Sun, and Linhong Wang. 2021. "Hydrophobic Epoxy Caged Silsesquioxane Film (EP-POSS): Synthesis and Performance Characterization" Nanomaterials 11, no. 2: 472. https://doi.org/10.3390/nano11020472





