A Simple Ball Milling and Thermal Oxidation Method for Synthesis of ZnO Nanowires Decorated with Cubic ZnO2 Nanoparticles
Abstract
:1. Introduction
- simplicity and effectiveness of ball milling in the production of doped Zn powders;
- low temperature required in the heating process for the growth of ZnO nanostructures;
- simplicity, scalability, and reproducibility of the overall process;
- very limited requirements for the annealing process.
2. Materials and Methods
2.1. Grinding by Ball Milling/Doped Powder Mixture Preparation
- (1)
- sample “A”: Zn powder only (ALFA AESAR 99.9% purity), grinding time 2 h;
- (2)
- sample “B-Sn”: Zn powder with 1 wt.% Sn (ACROS Organics, 99.5% purity), grinding time 2 h;
- (3)
- sample “C-Mg”: Zn powder with 2 wt.% Mg (ALFA AESAR, 99.8% purity), grinding time 6 h;
2.2. ZnO Nanostructures Synthesis by Thermal Annealing
2.3. Morphological and Structural Characterization
3. Results and Discussion
3.1. ZnO NWs Characterization
3.2. NWs Surface Analysis: A Preliminary Investigation of Cubic ZnO2 Nanoparticles Decorating ZnO NWs
- for ZnO: (0, 0, 1), (1, 0, 2), and (1, 1, 3) planes;
- for ZnO2: (2, 1, 0), (2, 2.0), and (3, 1, 0) planes.
- the superpositions of various diffraction spots between two families of ZnO;
- the experimental error of the measurements of the electron diffractions (generally estimated of about a 2–3%);
- the intrinsic difficulty of obtaining individual single particles to be observed at high-resolution in such densely decorated nanowires.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briscoe, J.; Dunn, S. Piezoelectric nanogenerators—A review of nanostructured piezoelectric energy harvesters. Nano Energy 2014, 14, 15–29. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittal, R.; Ho, K.C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Araneo, R.; Bini, F.; Pea, M.; Notargiacomo, A.; Rinaldi, A.; Celozzi, S. Impact of non-linear piezoelectricity on the piezotronic effect of ZnO nanowires. IEEE Trans. Nanotechnol. 2016, 15, 512–520. [Google Scholar] [CrossRef]
- Rinaldi, A.; Araneo, R.; Celozzi, S.; Pea, M.; Notargiacomo, A. The clash of mechanical and electrical size-effects in ZnO nanowires and a double power law approach to elastic strain engineering of piezoelectric and piezotronic devices. Adv. Mater. 2014, 26, 5976–5985. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on Zinc Oxide nanowire arrays. Science (80-) 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.L. Piezotronics and piezo-phototronics for adaptive electronics and optoelectronics. Nat. Rev. Mater. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Araneo, R.; Bini, F.; Pea, M.; Notargiacomo, A.; Lovat, G.; Celozzi, S.; Rinaldi, A.; Lovat, G.; Celozzi, S. Current-voltage characteristics of ZnO nanowires under uniaxial loading. IEEE Trans. Nanotechnol. 2014, 13, 724–735. [Google Scholar] [CrossRef]
- Bykhovski, A.; Gelmont, B.; Shur, M.; Khan, A. Current-voltage characteristics of strained piezoelectric structures. J. Appl. Phys. 1995, 77, 1616. [Google Scholar] [CrossRef]
- Araneo, R.; Rinaldi, A.; Notargiacomo, A.; Bini, F.; Pea, M.; Celozzi, S.; Marinozzi, F.; Lovat, G. Design concepts, fabrication and advanced characterization methods of innovative piezoelectric sensors based on ZnO nanowires. Sensors 2014, 14, 23539–23562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, B.D.; Chan, Y.F.; Wang, N. Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 2002, 81, 757–759. [Google Scholar] [CrossRef]
- Pea, M.; Maiolo, L.; Pilloton, R.; Rinaldi, A.; Araneo, R.; Giovine, E.; Orsini, A.; Notargiacomo, A. ZnO nanowires strips growth: Template reliability and morphology study. Microelectron. Eng. 2014, 121, 147–152. [Google Scholar] [CrossRef]
- Rai, P.; Kwak, W.K.; Yu, Y.T. Solvothermal synthesis of ZnO nanostructures and their morphology-dependent gas-sensing properties. ACS Appl. Mater. Interfaces 2013. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Kumari, P.; Soin, N.; Roy, S.S.; McLaughlin, J.A. Microstructure and field emission characteristics of ZnO nanoneedles grown by physical vapor deposition. Mater. Chem. Phys. 2010, 123, 634–638. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Zhao, S.; Zhou, G.; Zhou, Y.; Qi, J. Synthesis of well-aligned ZnO nanowires by simple physical vapor deposition on c -oriented ZnO thin films without catalysts or additives. Appl. Phys. Lett. 2005, 86, 10–13. [Google Scholar] [CrossRef]
- Chang, P.-C.; Fan, Z.; Wang, D.; Tseng, W.-Y.; Chiou, W.-A.; Hong, J.; Lu, J.G. ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Pea, M.; Mussi, V.; Barucca, G.; Giovine, E.; Rinaldi, A.; Araneo, R.; Notargiacomo, A. Focused ion beam surface treatments of single crystal zinc oxide for device fabrication. Mater. Des. 2016, 112, 530–538. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphoslogies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhong, M.L.; Tang, C.M. Large-scale oxide nanostructures grown by thermal oxidation. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Jakarta, Indonesia, 29–31 March 2014; Institute of Physics Publishing: Bristol, UK, 2014; Volume 60. [Google Scholar]
- Bueno, C.; Maestre, D.; Díaz, T.; Juárez, H.; Pacio, M.; Cremades, A.; Piqueras, J. High-yield growth of Ti doped ZnO nano- and microstructures by a vapor-solid method. J. Alloys Compd. 2017, 726, 201–208. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Li, C.P.; Chen, H.; Chen, Y.Y. One-dimensional nanomaterials synthesized using high-energy ball milling and annealing process. Sci. Technol. Adv. Mater. 2006, 7, 839–846. [Google Scholar] [CrossRef]
- Florica, C.; Preda, N.; Costas, A.; Zgura, I.; Enculescu, I. ZnO nanowires grown directly on zinc foils by thermal oxidation in air: Wetting and water adhesion properties. Mater. Lett. 2016, 170, 156–159. [Google Scholar] [CrossRef]
- Wu, Z.-W.W.; Tyan, S.-L.L.; Chen, H.-H.H.; Huang, J.-C.-A.C.A.; Huang, Y.-C.C.; Lee, C.-R.R.; Mo, T.-S.S. Temperature-dependent photoluminescence and XPS study of ZnO nanowires grown on flexible Zn foil via thermal oxidation. Superlattices Microstruct. 2017, 107, 38–43. [Google Scholar] [CrossRef]
- Vishal, C.; Chinthalapati, S.K.R.; Kanala, R.K.; Raman, R.; Bojja, R.R.; Kanaparthi, R. Effect of Ball Milling and Oxidation on Dispersibility and Dispersion Stability of Multiwalled Carbon Nanotubes in High Viscous Heat Exchange Fluids. ChemistrySelect 2020, 5, 7031–7039. [Google Scholar] [CrossRef]
- Wang, H.B.; Ma, F.; Zhou, L.; Qin, Y.; Sun, Y.S.; Xu, Y.K.; Chen, Y.N.; Xu, K.W.; Ma, D.Y. Polar surface dominated octagonal Sn doped ZnO nanowires and their room-temperature photoluminance properties. Appl. Surf. Sci. 2019, 476, 265–270. [Google Scholar] [CrossRef]
- Kennedy, O.W.; White, E.R.; Shaffer, M.S.P.; Warburton, P.A. Vapour-liquid-solid growth of ZnO-ZnMgO core-shell nanowires by gold-catalysed molecular beam epitaxy. Nanotechnology 2019, 30, 194001. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, T.; Zhao, Y.; Zang, W.; Xing, L.; Xue, X. High-performance self-powered/active humidity sensing of Fe-doped ZnO nanoarray nanogenerator. Sens. Actuators B Chem. 2015, 213, 382–389. [Google Scholar] [CrossRef]
- Chen, T.; Xing, G.Z.; Zhang, Z.; Chen, H.Y.; Wu, T. Tailoring the photoluminescence of ZnO nanowires using Au nanoparticles. Nanotechnology 2008, 19, 435711. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant improvement of the performance of ZnO nanowire photodetectors by Au nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhong, X.; Li, G.; Han, C.; Wei, D.; Li, S. Bimetallic Au/Pd nanoparticles decorated ZnO nanowires for NO2 detection. Sens. Actuators B Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Feng, Y.; Li, Z. Efficient photocatalytic performance enhancement in Co-doped ZnO nanowires coupled with CuS nanoparticles. Appl. Surf. Sci. 2018, 428, 154–164. [Google Scholar] [CrossRef]
- Park, S. Enhancement of hydrogen sensing response of ZnO nanowires for the decoration of WO3 nanoparticles. Mater. Lett. 2019, 234, 315–318. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.H.; Wang, M.; Kroner, L.; Paul, H.; Fecht, H.-J.; Bednarcik, J.; Stahl, K.; Zhang, Z.L.; Wiedwald, U.; et al. Synthesis, Thermal Stability and Properties of ZnO2 Nanoparticles. J. Phys. Chem. C 2009, 113, 1320–1324. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhang, Q.; Wang, H.; Zhao, Z. ZnO2-promoted ZnO as an efficient photocatalyst for the photoreduction of carbon dioxide in the presence of water. Catal. Commun. 2018, 103, 24–28. [Google Scholar] [CrossRef]
- Bergs, C.; Brück, L.; Rosencrantz, R.R.; Conrads, G.; Elling, L.; Pich, A. Biofunctionalized zinc peroxide (ZnO2) nanoparticles as active oxygen sources and antibacterial agents. RSC Adv. 2017, 7, 38998–39010. [Google Scholar] [CrossRef] [Green Version]
- Prommalikit, C.; Mekprasart, W.; Pecharapa, W. Effect of Milling Speed and Time on Ultrafine ZnO Powder by High Energy Ball Milling Technique. J. Phys. Conf. Ser. 2019, 1259, 012023. [Google Scholar] [CrossRef] [Green Version]
- Lutterotti, L.; Ceccato, R.; Maschio, R.D.; Pagani, E. Quantitative Analysis of Silicate glass in Ceramic Materials by the Rietveld Method. Mater. Sci. Forum 1998, 87, 278–281. [Google Scholar] [CrossRef]
- Lutterotti, L.; Scardi, P. Simultaneous structure and size-strain refinement by the Rietveld method. J. Appl. Crystallogr. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Glushenkov, A.; Chen, Y. Synthesis of ZnO nanowires using ball-milling and annealing method. Mater. Forum 2006, 30, 1–6. [Google Scholar]
- Staddelmann, P. Jems—Electron Microscopy Software; JAVA Version; JEMS SWISS: Jongny, Switzerland, 2019. [Google Scholar]
- Wriedt, H.A. The O-Zn (Oxygen-Zinc) System. Bull. Alloy Phase Diagr. 1987, 8, 166. [Google Scholar] [CrossRef]

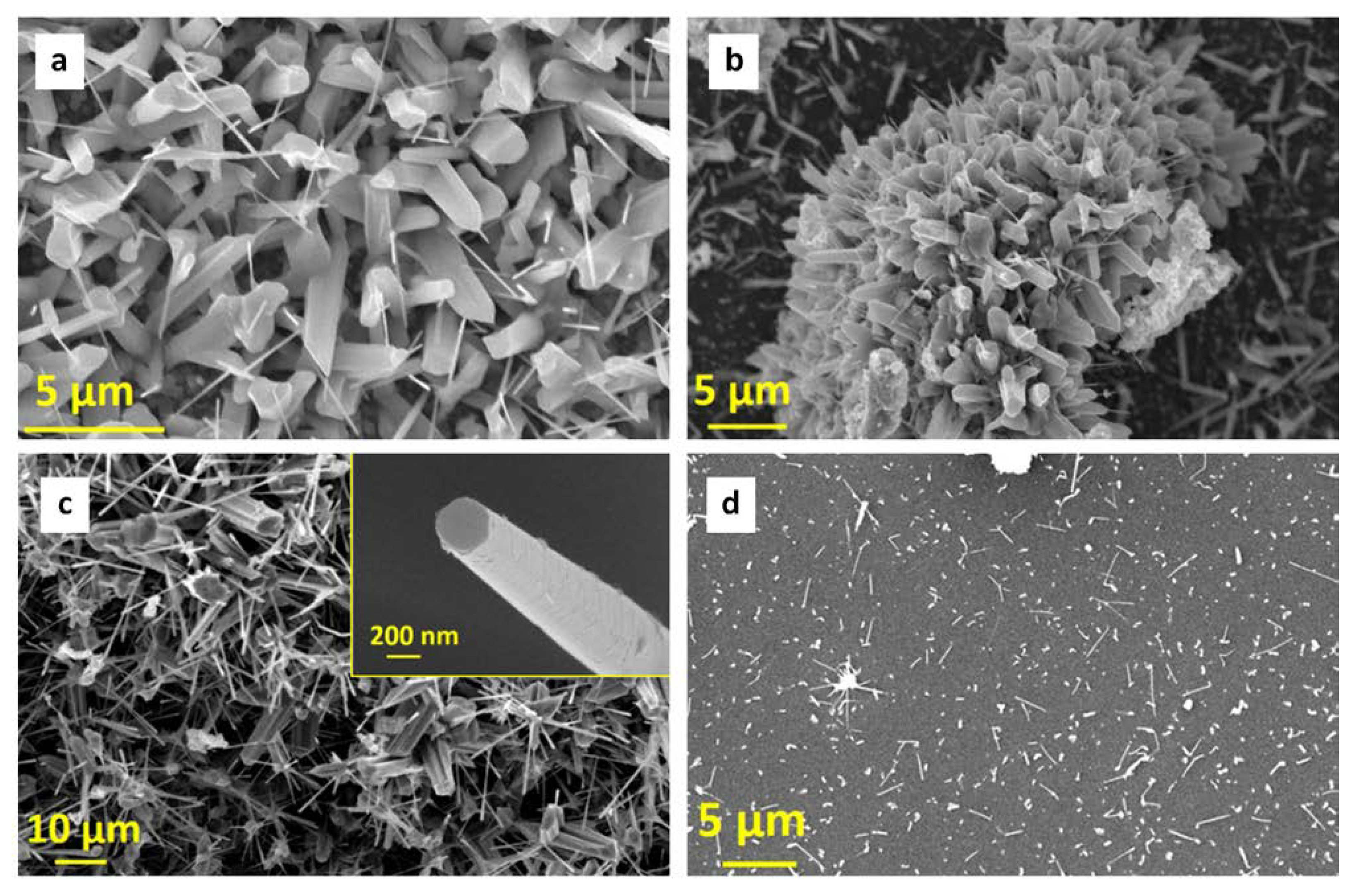


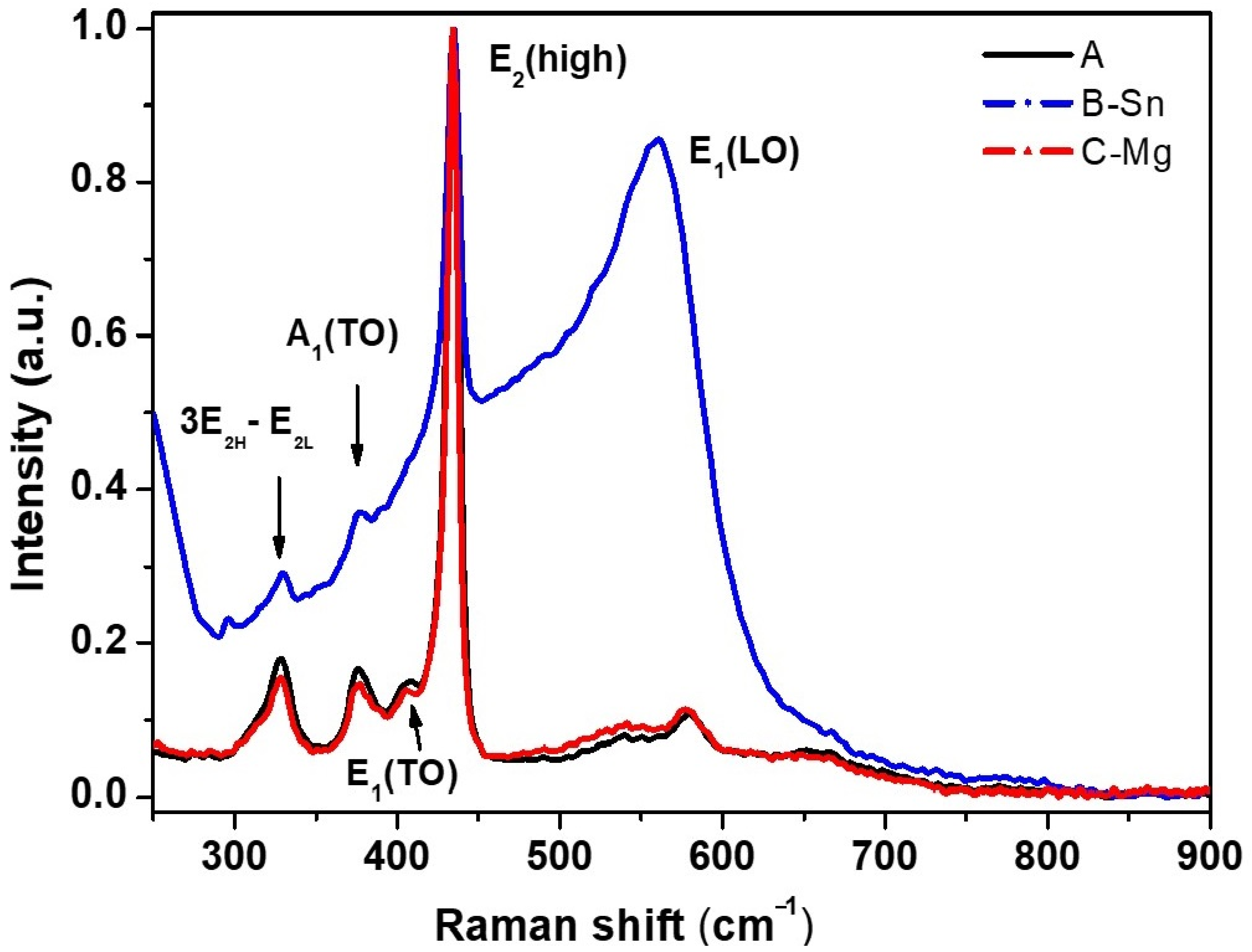


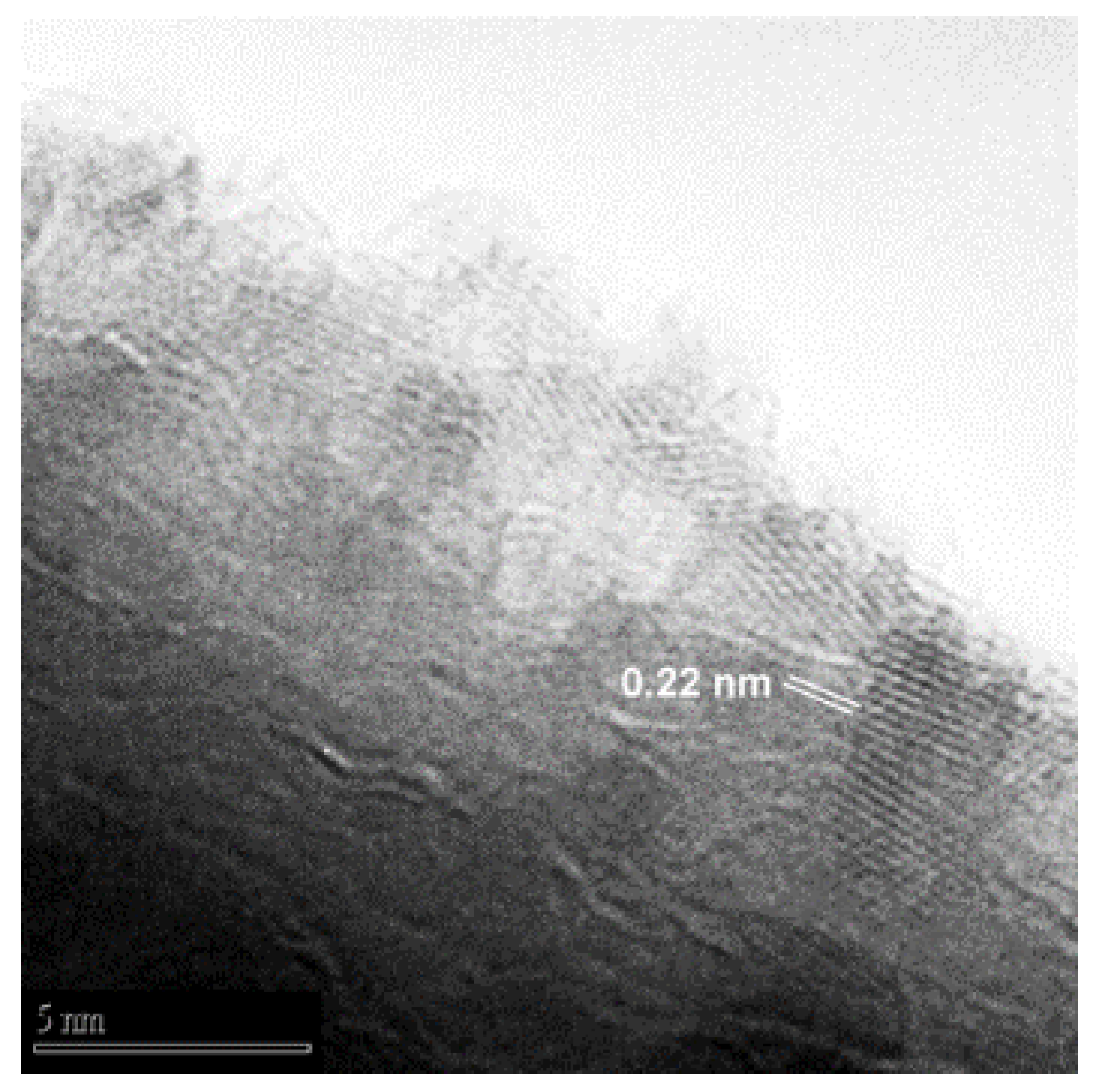
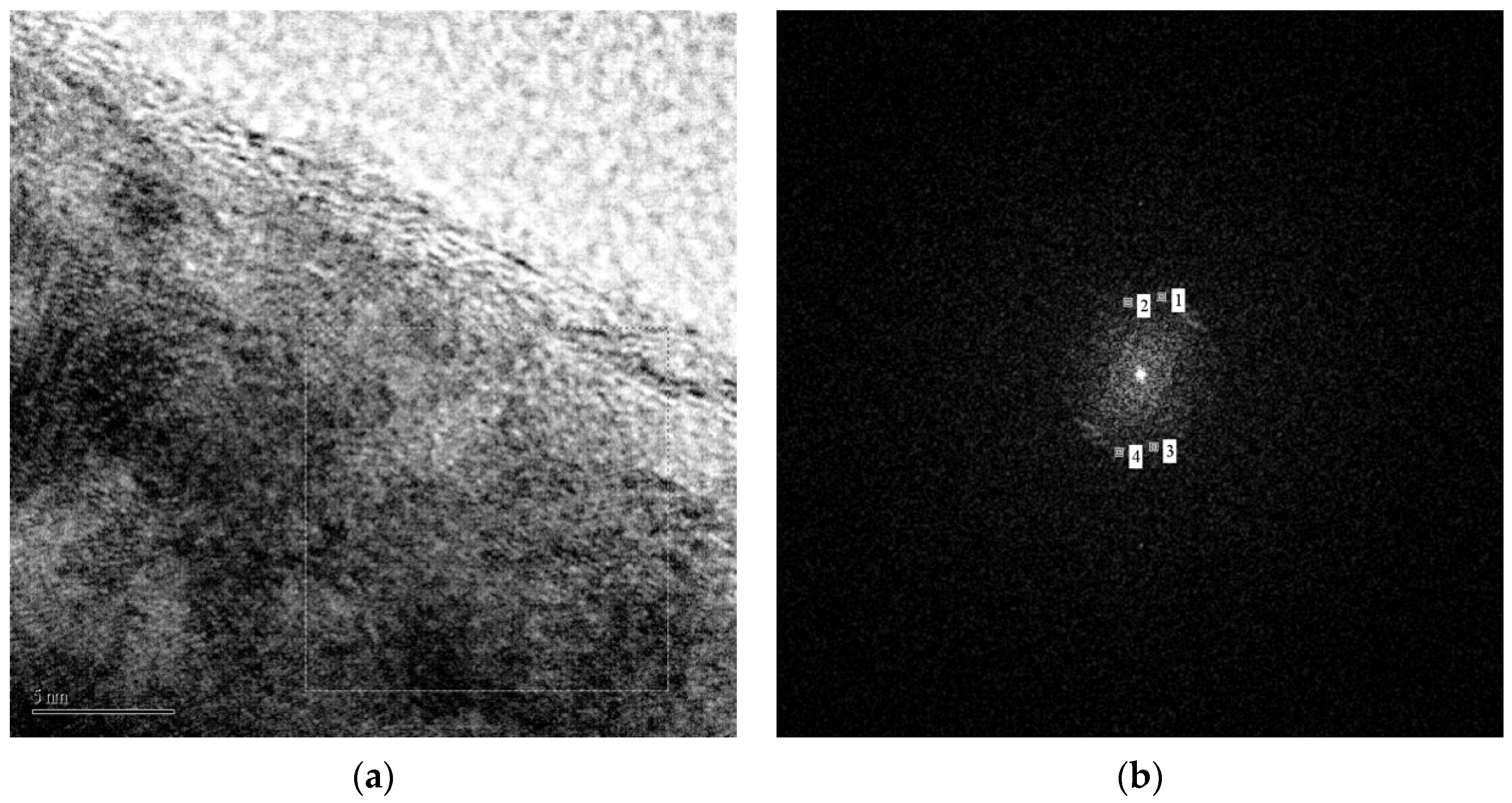


| Sample | Phase | a, Å | b, Å | Crystallite size, Å | r.m.s. Microstrain | Wt. % |
|---|---|---|---|---|---|---|
| A | ZnO | 3.2433 | 5.1937 | >1000 | 5 × 10−4 | 73.0 |
| Zn | 2.6611 | 4.9399 | >1000 | 4 × 10−4 | 27.0 | |
| B-Sn | ZnO | 3.2456 | 5.2178 | >1000 | 5 × 10−4 | 72.0 |
| Zn | 2.6663 | 4.9594 | >1000 | 4 × 10−4 | 26.3 | |
| Sn | 5.8400 | 3.1855 | 1000 | 7 × 10−4 | 1.2 | |
| C-Mg | ZnO | 3.2510 | 5.2059 | >1000 | 7 × 10−4 | 88.7 |
| Zn | 2.6658 | 4.9555 | 950 | 5 × 10−4 | 8.1 | |
| Mg | 4.2257 | / | 1000 | 1 × 10−4 | 3.2 |
| Diffractogram Results | ||
|---|---|---|
| Spot# | d-Spacing (nm) | Reciprocal Position (1/nm) |
| 1 | 0.2259 | 4.428 |
| 2 | 0.2476 | 4.038 |
| 3 | 0.2476 | 04.038 |
| 4 | 0.2259 | 4.428 |
| Miller’s Indices | d-Spacing (nm) | ||
|---|---|---|---|
| h | k | l | |
| 0 | 0 | 1 | 0.5207 |
| 1 | 0 | 0 | 0.28146 |
| 0 | 0 | 2 | 0.26035 |
| 1 | 0 | 1 | 0.2476 |
| 1 | 0 | 2 | 0.19112 |
| 0 | 0 | 3 | 0.17357 |
| 1 | 1 | 0 | 0.1625 |
| 1 | 1 | 1 | 0.15512 |
| 1 | 0 | 3 | 0.14773 |
| 2 | 0 | 0 | 0.14073 |
| 1 | 1 | 2 | 0.13785 |
| 2 | 0 | 1 | 0.13585 |
| 0 | 0 | 4 | 0.13017 |
| 2 | 0 | 2 | 0.1238 |
| 1 | 1 | 3 | 0.11862 |
| Miller’s Indices | d-Spacing (nm) | ||
|---|---|---|---|
| h | k | l | |
| 1 | 1 | 1 | 0.28123 |
| 2 | 0 | 0 | 0.24355 |
| 2 | 1 | 0 | 0.21784 |
| 2 | 1 | 1 | 0.19886 |
| 2 | 2 | 0 | 0.17222 |
| 3 | 0 | 0 | 0.16237 |
| 2 | 2 | 1 | 0.16237 |
| 3 | 1 | 0 | 0.15403 |
| 3 | 1 | 1 | 0.14687 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, A.; Pea, M.; Notargiacomo, A.; Ferrone, E.; Garroni, S.; Pilloni, L.; Araneo, R. A Simple Ball Milling and Thermal Oxidation Method for Synthesis of ZnO Nanowires Decorated with Cubic ZnO2 Nanoparticles. Nanomaterials 2021, 11, 475. https://doi.org/10.3390/nano11020475
Rinaldi A, Pea M, Notargiacomo A, Ferrone E, Garroni S, Pilloni L, Araneo R. A Simple Ball Milling and Thermal Oxidation Method for Synthesis of ZnO Nanowires Decorated with Cubic ZnO2 Nanoparticles. Nanomaterials. 2021; 11(2):475. https://doi.org/10.3390/nano11020475
Chicago/Turabian StyleRinaldi, Antonio, Marialilia Pea, Andrea Notargiacomo, Eloisa Ferrone, Sebastiano Garroni, Luciano Pilloni, and Rodolfo Araneo. 2021. "A Simple Ball Milling and Thermal Oxidation Method for Synthesis of ZnO Nanowires Decorated with Cubic ZnO2 Nanoparticles" Nanomaterials 11, no. 2: 475. https://doi.org/10.3390/nano11020475
APA StyleRinaldi, A., Pea, M., Notargiacomo, A., Ferrone, E., Garroni, S., Pilloni, L., & Araneo, R. (2021). A Simple Ball Milling and Thermal Oxidation Method for Synthesis of ZnO Nanowires Decorated with Cubic ZnO2 Nanoparticles. Nanomaterials, 11(2), 475. https://doi.org/10.3390/nano11020475









