Luminescent Yb3+,Er3+-Doped α-La(IO3)3 Nanocrystals for Neuronal Network Bio-Imaging and Nanothermometry
Abstract
:1. Introduction
2. Materials and Methods
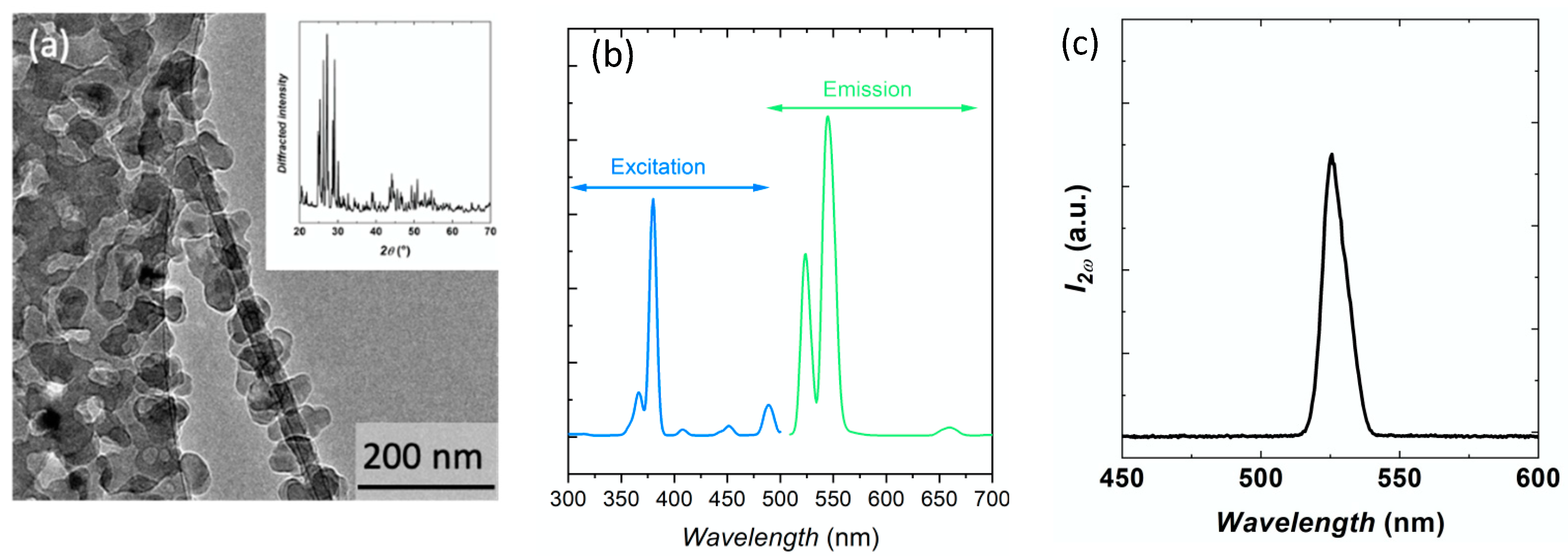
3. Results
3.1. Temperature Dependent PL of Nanocrystals
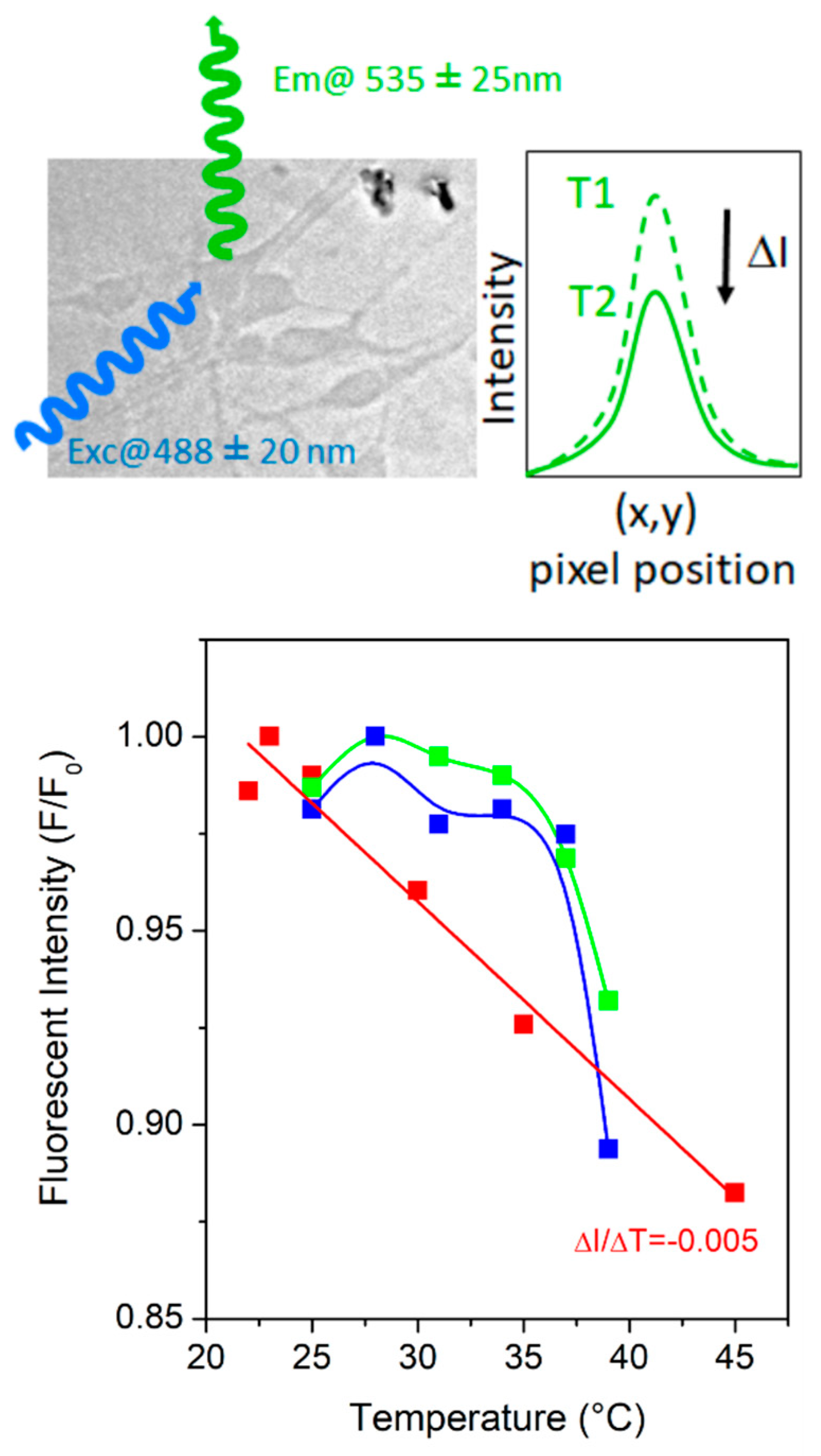
3.2. Impact of the Nanocrystals on Cultured Neurons
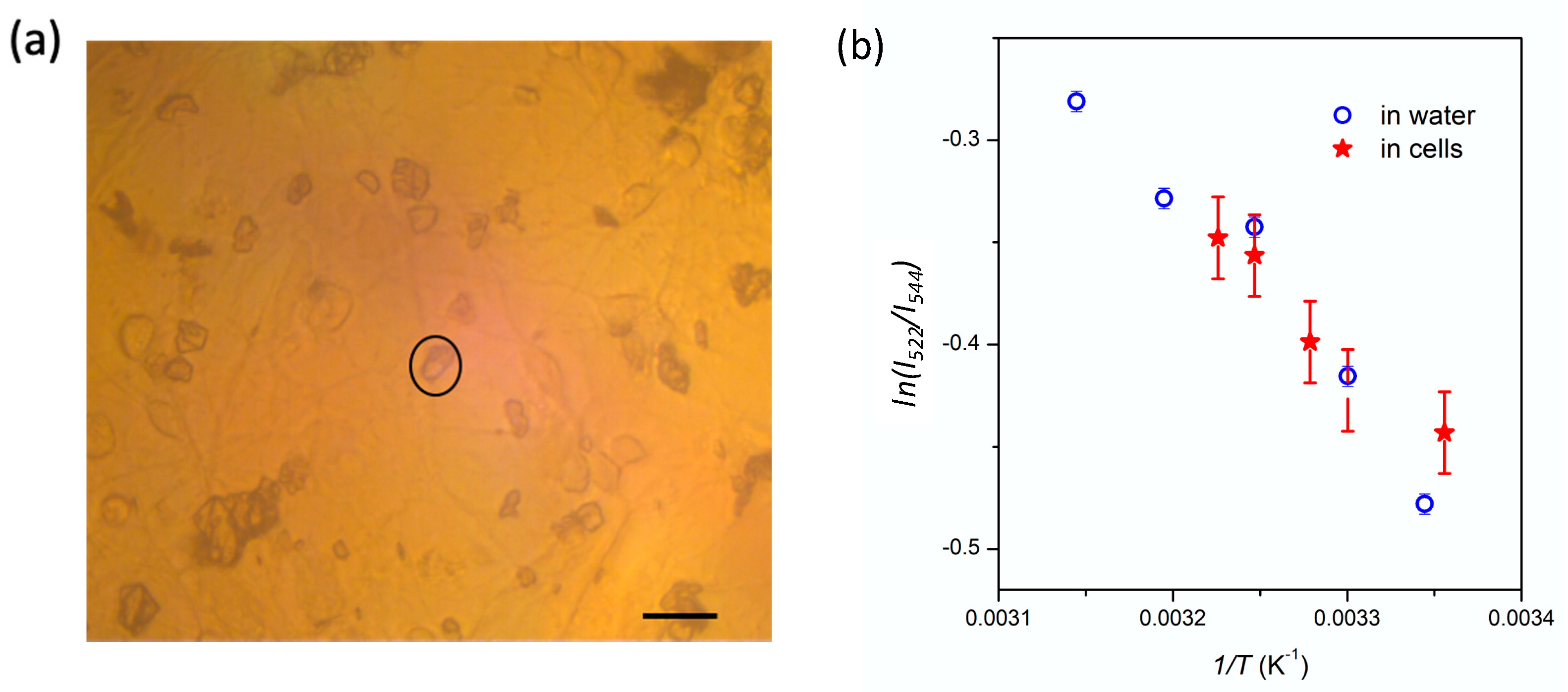
3.3. Temperature Dependence of the Nanocrystals PL in Neuronal Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McCabe, K.; Hernandez, M. Molecular Thermometry. Pediatric Res. 2010, 67, 469–475. [Google Scholar] [CrossRef]
- Baffou, G.; Rigneault, H.; Marguet, D.; Jullien, L. A critique of methods for temperature imaging in single cells. Nat. Methods 2014, 11, 899–901. [Google Scholar] [CrossRef]
- Ramires, M.L.V.; Nieto de Castro, C.A. Standard Reference Data for the Thermal Conductivity of Water. J. Phys. Chem. Ref. Data 1995, 24, 1377. [Google Scholar] [CrossRef]
- Bai, T.; Gu, N. Micro/nanoscale thermometry for cellular thermal sensing. Small 2016, 12, 4590–4610. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millan, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, D.A.; Morrisroe, E.; McCoey, J.M.; Lombard, A.H.; Mendis, D.C.; Treussart, F.; Hall, L.T.; Petrou, S.; Hollenberg, L.C. Non-neurotoxic nanodiamond probes for intraneuronal temperature mapping. ACS Nano 2017, 11, 12077–12086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanimoto, R.; Hiraiwa, T.; Nakai, Y.; Shindo, Y.; Oka, K.; Hiroi, N.; Funahashi, A. Detection of temperature difference in neuronal cells. Sci. Rep. 2016, 6, 22071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Donner, J.S.; Thompson, S.A.; Kreuzer, M.P.; Baffou, G.; Quidant, R. Mapping intracellular temperature using green fluorescent protein. Nano Lett. 2012, 12, 2107–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.M.; Yang, H.; Lin, L. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano 2011, 5, 5067–5071. [Google Scholar] [CrossRef]
- Gan, Z.; Hao, Y.; Shan, Y. Temperature-Dependent Dual Emission from Sucrose-Derived Carbon Nanodots: A Ratiometric Fluorescent Thermometer. ChemNanoMat 2016, 2, 171–175. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, X.; Cao, W.; Sheng, Y.; Liu, C.; Yu, L.; Di, Y.; Chen, Z.; Dong, L.; Gan, Z. Fluorescence enhancement of perovskite nanocrystals using photonic crystals. J. Mater. Chem. C 2021, 9, 908–915. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry. Adv. Opt. Mater. 2018, 7, 1801239. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Vedda, A.; Cantarelli, I.X.; Pedroni, M.; Piccinelli, F.; Bettinelli, M.; Speghini, A.; Quintanilla, M.; Vetrone, F.; Rocha, U.; et al. 1.3 μm Emitting SrF2:Nd3+ Nanoparticles for High Contrast In Vivo Imaging in the Second Biological Window. Nano Res. 2015, 8, 649–665. [Google Scholar] [CrossRef]
- Rocha, U.; Jacinto da Silva, C.; Ferreira Silva, W.; Guedes, I.; Benayas, A.; Martinez Maestro, L.; Acosta Elias, M.; Bovero, E.; van Veggel, F.C.J.M.; Garcia Sole, J.A.; et al. Subtissue Thermal Sensing Based on Neodymium-Doped LaF3 Nanoparticles. ACS Nano 2013, 7, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Liu, S.; Law, W.-C.; Wu, F.; Swihart, M.T.; Agren, H.; Prasad, P.N. Core/Shell NaGdF4:Nd3+/NaGdF4 Nanocrystals with Efficient Near-Infrared to Near-Infrared Downconversion Photoluminescence for Bioimaging Applications. ACS Nano 2012, 6, 2969–2977. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.F.; Shi, J.P.; Li, J.L.; Li, P.H.; Zhang, H.W. Luminescence enhancement of CaF2:Nd3+ nanoparticles in the second near-infrared window for in vivo imaging through Y3+ doping. J. Mater. Chem. B 2018, 6, 1238. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, Q.; Zhang, Y.; Li, F.; Li, Y.; Li, C.; Wang, Q.; Shi, L.; Lin, H. Neodymium-doped NaHoF4 nanoparticles as near-infrared luminescent/T2-weighted MR dual-modal imaging agents in vivo. J. Mater. Chem. B 2017, 5, 504. [Google Scholar] [CrossRef]
- Dantelle, G.; Matulionyte, M.; Testemale, D.; Cantarano, A.; Ibanez, A.; Vetrone, F. Nd3+-doped Gd3Sc2Al3O12 nanocrystals: Towards efficient nanoprobes for temperature sensing. Phys. Chem. Chem. Phys. 2019, 21, 11132–11141. [Google Scholar] [CrossRef] [PubMed]
- Del Rosal, B.; Pérez-Delgado, A.; Misiak, M.; Bednarkiewicz, A.; Vanetsev, A.S.; Orlovskii, Y.; Jovanović, D.J.; Dramićanin, M.D.; Rocha, U.; Kumar, K.U.; et al. Neodymium-doped nanoparticles for infrared fluorescence bioimaging: The role of the host. J. Appl. Phys. 2015, 118, 143104. [Google Scholar] [CrossRef] [Green Version]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Cantarano, A.; Yao, J.; Matulionyte, M.; Lifante, J.; Benayas, A.; Ortgies, D.; Vetrone, F.; Ibanez, A.; Gérardin, C.; Jaque, D.; et al. Autofluorescence-free in vivo imaging using polymer-stabilized Nd3+-doped YAG nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 51273–51284. [Google Scholar] [CrossRef]
- Suta, M.; Antić, Ž.; Ðorđević, V.; Kuzman, S.; Dramićanin, M.D.; Meijerink, A. Making Nd3+ a Sensitive Luminescent Thermometer for Physiological Temperatures—An Account of Pitfalls in Boltzmann Thermometry. Nanomaterials 2020, 10, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skripka, A.; Morinvil, A.; Matulionyte, M.; Cheng, T.; Vetrone, F. Advancing Neodymium Single-Band Nanothermometry. Nanoscale 2019, 11, 11322–11330. [Google Scholar] [CrossRef]
- Huang, P.; Zheng, W.; Tu, D.; Shang, X.; Zhang, M.; Li, R.; Xu, J.; Liu, Y.; Chen, X. Unraveling the Electronic Structures of Neodymium in LiLuF4 Nanocrystals for Ratiometric Temperature Sensing. Adv. Sci. 2019, 6, 1802282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabhadra, S.; Debasu, M.L.; Brites, C.D.S.; Ferreira, R.A.S.; Carlos, L.D. Upconverting Nanoparticles Working as Primary Thermometers In Different Media. J. Phys. Chem. C 2017, 121, 13962−13968. [Google Scholar] [CrossRef]
- Nigoghossian, K.; Messaddeq, Y.; Boudreau, D.; Ribeiro, S.J.L. UV and Temperature-Sensing Based on NaGdF4:Yb3+:Er3+@SiO2–Eu(tta)3. ACS Omega 2017, 2, 2065–2071. [Google Scholar] [CrossRef] [Green Version]
- Suta, M.; Meijerink, A. A Theoretical Framework for Ratiometric Single Ion Luminescent Thermometers—Thermodynamic and Kinetic Guidelines for Optimized Performance. Adv. Theory Simul. 2020, 3, 2000176. [Google Scholar] [CrossRef]
- Regny, S.; Riporto, J.; Mugnier, Y.; Le Dantec, R.; Kodjikian, S.; Pairis, S.; Gautier-Luneau, I.; Dantelle, G. Microwave synthesis and up-conversion properties of SHG-active α-(La,Er)(IO3)3 nanocrystals. Inorg. Chem. 2019, 58, 1647–1654. [Google Scholar] [CrossRef]
- Regny, S.; Ledrillet, K.; Riporto, J.; Gautier-Luneau, I.; Mugnier, Y.; Le Dantec, R.; Dantelle, G. Dual-emitting Yb3+,Er3+-doped α-La(IO3)3 iodate nanocrystals: Up-Conversion Emission & Second Harmonic Generation. MRS Commun. 2019, 9, 1221–1226. [Google Scholar]
- Bonacina, L. Nonlinear Nanomedecine: Harmonic Nanoparticles toward Targeted Diagnosis and Therapy. Mol. Pharm. 2013, 10, 783−792. [Google Scholar] [CrossRef] [PubMed]
- Regny, S.; Suffren, Y.; Leynaud, O.; Gautier-Luneau, I.; Dantelle, G. Evidence of reaction intermediates in microwave-assisted synthesis of SHG-active α-La(IO3)3. Cryst. Eng. Comm. 2020, 22, 2517–2525. [Google Scholar] [CrossRef]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Fluorescence Upconversion Properties of Er3+-Doped TiO2 and BaTiO3 Nanocrystallites. Chem. Mater. 2003, 15, 3650–3655. [Google Scholar] [CrossRef]
- Dantelle, G.; Mortier, M.; Patriarche, G.; Vivien, D. Er3+-doped PbF2: Comparison between nanocrystals in glass-ceramics and bulk single crystals. J. Solid State Chem. 2006, 179, 1995–2003. [Google Scholar] [CrossRef]
- Aigouy, L.; Saïdi, E.; Lalouat, L.; Labéguerie-Egéa, J.; Mortier, M.; Löw, P.; Bergaud, C. AC thermal imaging of a microwire with a fluorescent nanocrystal: Influence of the near field on the thermal contrast. J. Appl. Phys. 2009, 106, 074301. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarron, A.; Juarranz de la Fuente, A.; Sanz-Rodrıguez, F.; Martinez Maestro, L.; Martın Rodriguez, E.; Jaque, D.; Garcıa Sole, J.; Capobianco, J.A. Temperature Sensing Using Fluorescent Nanothermometers. ACS Nano 2010, 4, 3254. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Liu, D.P.; Wang, X.J.; Yang, T.; Miao, S.M.; Li, C.R. Optical thermometry through infrared excited green upconversionemissions in Er3+–Yb3+ codoped Al2O3. Appl. Phys. Lett. 2007, 90, 181117. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.M.; Gálico, D.A.; Mazali, I.O.; Sigoli, F.A. Self-supported films of poly(methylmethacrylate) (PMMA) containing TmIII-doped up-converting core@shell nanoparticles as high sensitivity temperature optical probe. Sens. Actuators A 2019, 291, 1–6. [Google Scholar] [CrossRef]
- Lin, M.; Xie, L.; Wang, Z.; Richards, B.S.; Gao, G.; Zhong, J. Facile synthesis of mono-disperse sub-20 nm NaY(WO4)2:Er3+,Yb3+ upconversion nanoparticles: A new choice for nanothermometry. J. Mater. Chem. C 2019, 7, 2971–2977. [Google Scholar] [CrossRef]
- Bastos, A.R.N.; Brites, C.D.S.; Rojas-Gutierrez, P.A.; DeWolf, C.; Ferreira, R.A.S.; Capobianco, J.A.; Carlos, L.D. Thermal Properties of Lipid Bilayers Determined Using Upconversion Nanothermometry. Adv. Funct. Mater. 2019, 29, 1905474. [Google Scholar] [CrossRef] [Green Version]
- Bednarkiewicz, A.; Marciniak, L.; Carlos, L.D.; Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 2020, 12, 14405–14421. [Google Scholar] [CrossRef] [PubMed]
- Veliev, F.; Briançon-Marjollet, A.; Bouchiat, V.; Delacour, C. Impact of crystalline quality on neuronal affinity of pristine graphene. Biomaterials 2016, 86, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Gan, Z.; Wu, X.; Zhang, J.; Zhu, X.; Chu, P.K. In Situ Thermal Imaging and Absolute Temperature Monitoring by Luminescent Diphenylalanine Nanotubes. Biomacromolecules 2013, 14, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, K.; Yang, J.-M.; Lin, L.; Yang, H. Single quantum dots as local temperature markers. Nano Lett 2007, 7, 3102–3105. [Google Scholar] [CrossRef]
- Zohar, O.; Ikeda, M.; Shinagawa, H.; Inoue, H.; Nakamura, H.; Elbaum, D.; Alkon, D.L.; Yoshioka, T. Thermal Imaging of Receptor-Activated Heat Production in Single Cells. Biophys. J. 1989, 74, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Moreau, D.; Lefort, C.; Burke, R.; Leveque, P.; O’Connor, R.P. Rhodamine B as an optical thermometer in cells focally exposed to infrared laser light or nanosecond pulsed electric fields. Biomed. Opt. Exp. 2015, 6, 4105–4117. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Joshi, R.P.; Schoenbach, K.H. Synergistic effects of local temperature enhancements on cellular responses in the context of high-intensity, ultrashort electric pulses. Med. Biol. Eng. Comput. 2011, 49, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, I.; Kusano, K.; Byrne, P. Rapid mechanical and thermal changes in the garfish olfactory nerve associated with a propagated impulse. Biophys. J. 1989, 55, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Del Rosal, B.; Ximendes, E.; Rocha, U.; Jaque, D. In Vivo Luminescence Nanothermometry: From Materials to Applications. Adv. Funct. Mater. 2017, 5, 1600508. [Google Scholar] [CrossRef]
- Donner, J.S.; Thompson, S.A.; Alonso-Ortega, C.; Morales, J.; Rico, L.G.; Santos, S.I.C.O.; Quidant, R. Imaging of Plasmonic Heating in a Living Organism. ACS Nano 2013, 7, 8666–8672. [Google Scholar] [CrossRef]
- Del Rosal, B.; Carrasco, E.; Ren, F.; Benayas, A.; Vetrone, F.; Sanz-Rodríguez, F.; Ma, F.; Juarranz, A.; Jaque, D. Infrared-Emitting QDs for Thermal Therapy with Real-Time Subcutaneous Temperature Feedback. Adv. Funct. Mater. 2016, 26, 6060–6068. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, S.; Xiao, Z.; Lai, F.; Huang, J.; Fu, J.; Ye, X.; You, W. A universal strategy to enhance the absolute sensitivity for temperature detection in bright Er3+/Yb3+ doped double perovskite Gd2ZnTiO6 phosphors. Mater. Chem. Front. 2020, 4, 1182. [Google Scholar] [CrossRef]
- Rodríguez-Sevilla, P.; Zhang, Y.; Haro-González, P.; Sanz-Rodríguez, F.; Jaque, F.; García Solé, J.; Liu, X.; Jaque, D. Thermal Scanning at the Cellular Level by an Optically Trapped Upconverting Fluorescent Particle. Adv. Mater. 2016, 28, 2421–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, A.M.; Suta, M.; Rijckaert, H.; Abalymov, A.; Van Driessche, I.; Skirtach, A.G.; Meijerink, A.; Van Der Voort, P. Visible and NIR Upconverting Er3+–Yb3+ Luminescent Nanorattles and Other Hybrid PMO-Inorganic Structures for In Vivo Nanothermometry. Adv. Funct. Mater. 2020, 30, 2003101. [Google Scholar] [CrossRef]
- Xu, M.; Zou, X.; Su, Q.; Yuan, W.; Cao, C.; Wang, Q.; Zhu, X.; Feng, W.; Li, F. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat. Comm. 2018, 9, 2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, M.; Arai, Y.; Kotera, I.; Okabe, K.; Kamei, Y.; Nagai, T. Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PLoS ONE 2017, 12, e0172344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic Fluorescent Nanogel Thermometer for Intracellular Thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef] [PubMed]


| Samples, Ref. | Sr (% K−1) | δT (K) | Type of Cells |
|---|---|---|---|
| La(IO3)3:Er3+,Yb3+ † | 1.2 | <1 | Cultured neurons |
| Fluorescent proteins [55,61] | 0.4 | 0.5 | Cultured HeLa cells |
| NaYF4:Nd3+ [18] | 0.1 | <1 | HeLa cancer cells |
| Quantum dots [56] | 1.0 | <1 | Cancer cells |
| NaYF4:Er3+,Yb3+ [58] | 1.6 | <1 | HeLa cancer cells |
| Luminescent nanogels [62] | NP | 0.3–0.5 | Living COS7 cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantelle, G.; Reita, V.; Delacour, C. Luminescent Yb3+,Er3+-Doped α-La(IO3)3 Nanocrystals for Neuronal Network Bio-Imaging and Nanothermometry. Nanomaterials 2021, 11, 479. https://doi.org/10.3390/nano11020479
Dantelle G, Reita V, Delacour C. Luminescent Yb3+,Er3+-Doped α-La(IO3)3 Nanocrystals for Neuronal Network Bio-Imaging and Nanothermometry. Nanomaterials. 2021; 11(2):479. https://doi.org/10.3390/nano11020479
Chicago/Turabian StyleDantelle, Géraldine, Valérie Reita, and Cécile Delacour. 2021. "Luminescent Yb3+,Er3+-Doped α-La(IO3)3 Nanocrystals for Neuronal Network Bio-Imaging and Nanothermometry" Nanomaterials 11, no. 2: 479. https://doi.org/10.3390/nano11020479
APA StyleDantelle, G., Reita, V., & Delacour, C. (2021). Luminescent Yb3+,Er3+-Doped α-La(IO3)3 Nanocrystals for Neuronal Network Bio-Imaging and Nanothermometry. Nanomaterials, 11(2), 479. https://doi.org/10.3390/nano11020479







