Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Flame Synthesis of Nanocatalysts
2.2. Characterization of Nanocatalysts
2.3. Catalytic Evaluation
3. Results
3.1. Characterization of the FSP-Made BiVO4-Based Photocatalysts
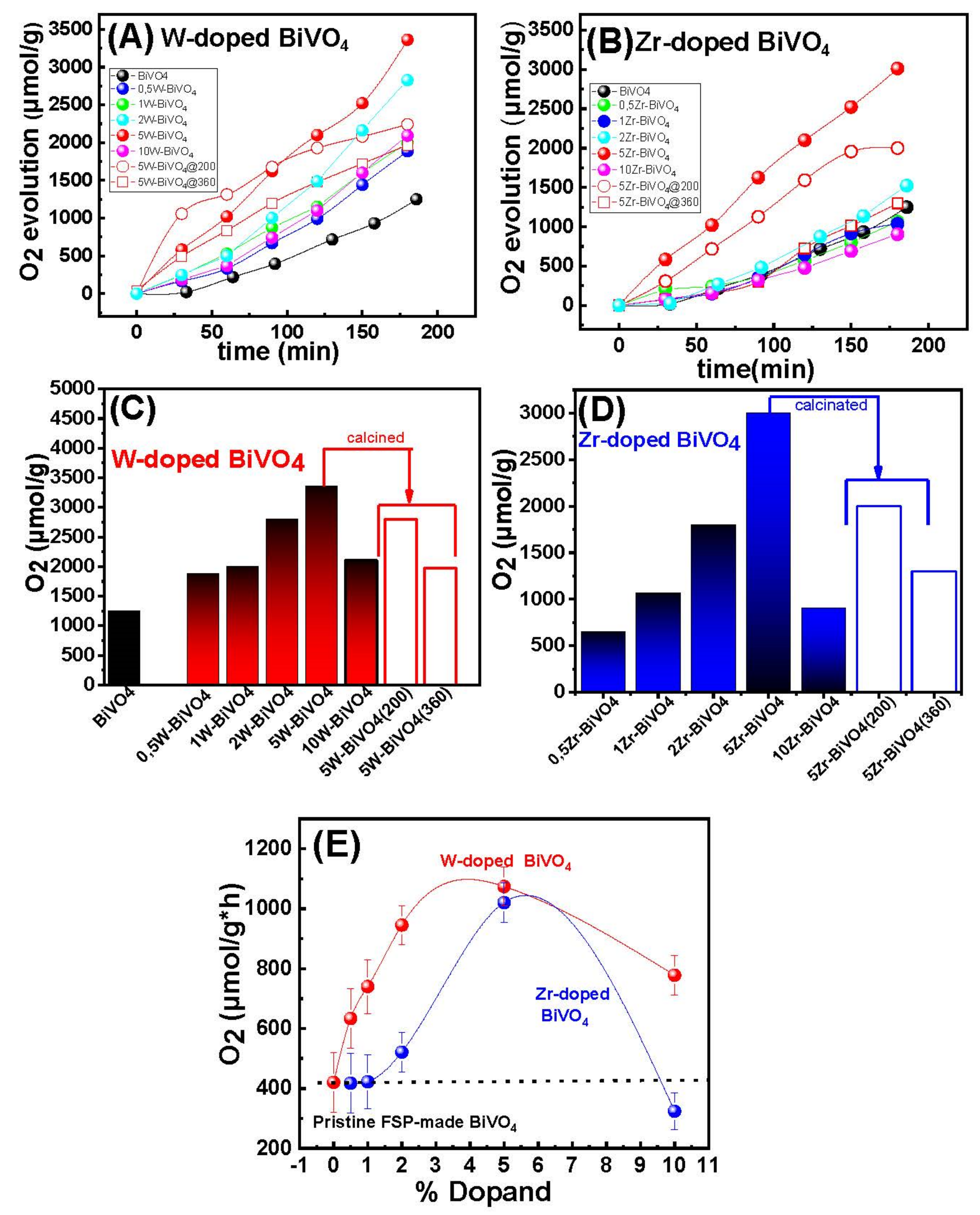
3.2. Catalytic Results
3.2.1. Photocatalytic O2-Evolution from H2O
3.2.2. Comparison with Literature
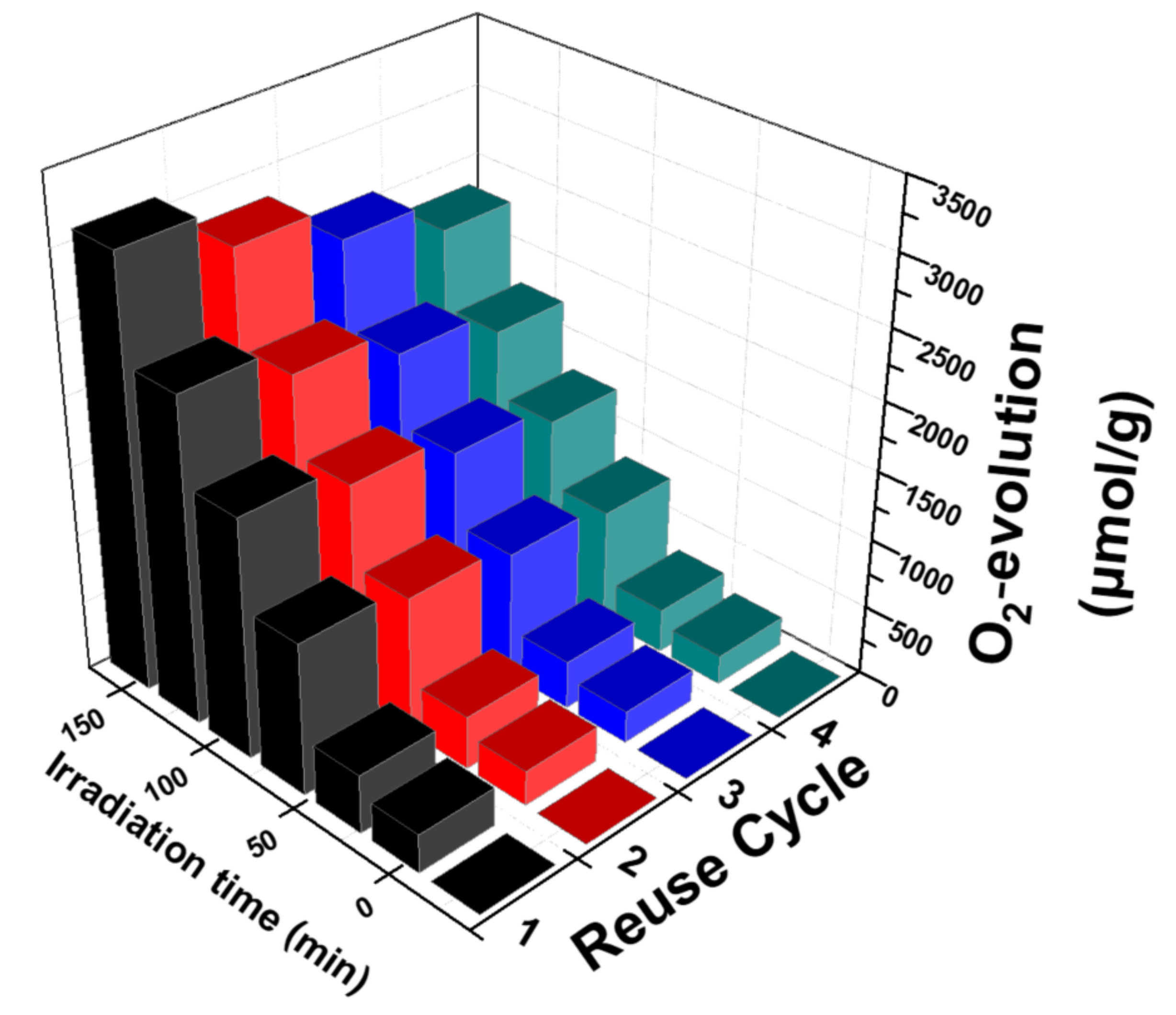
3.2.3. Reusability of the Nanocatalysts
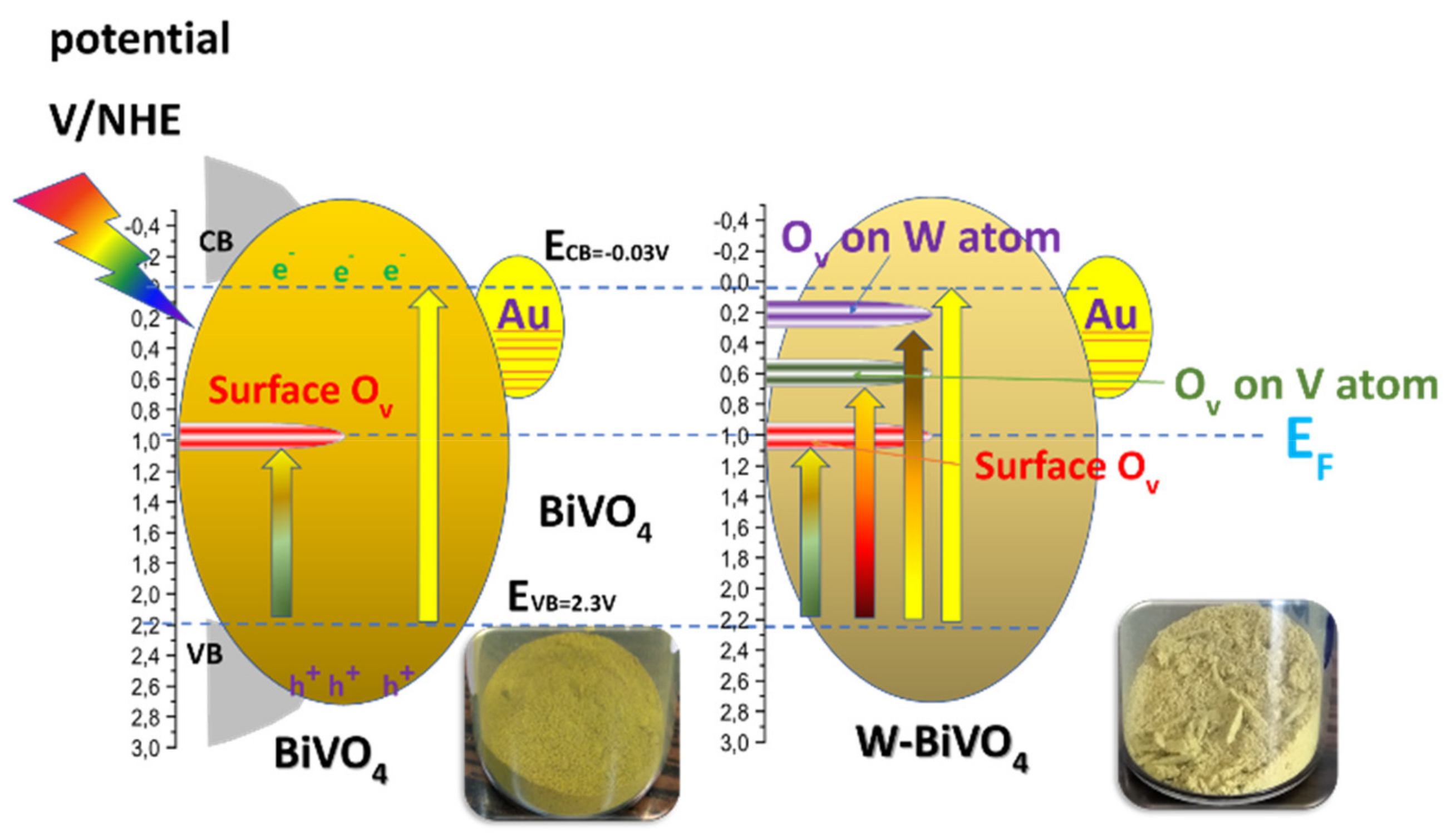
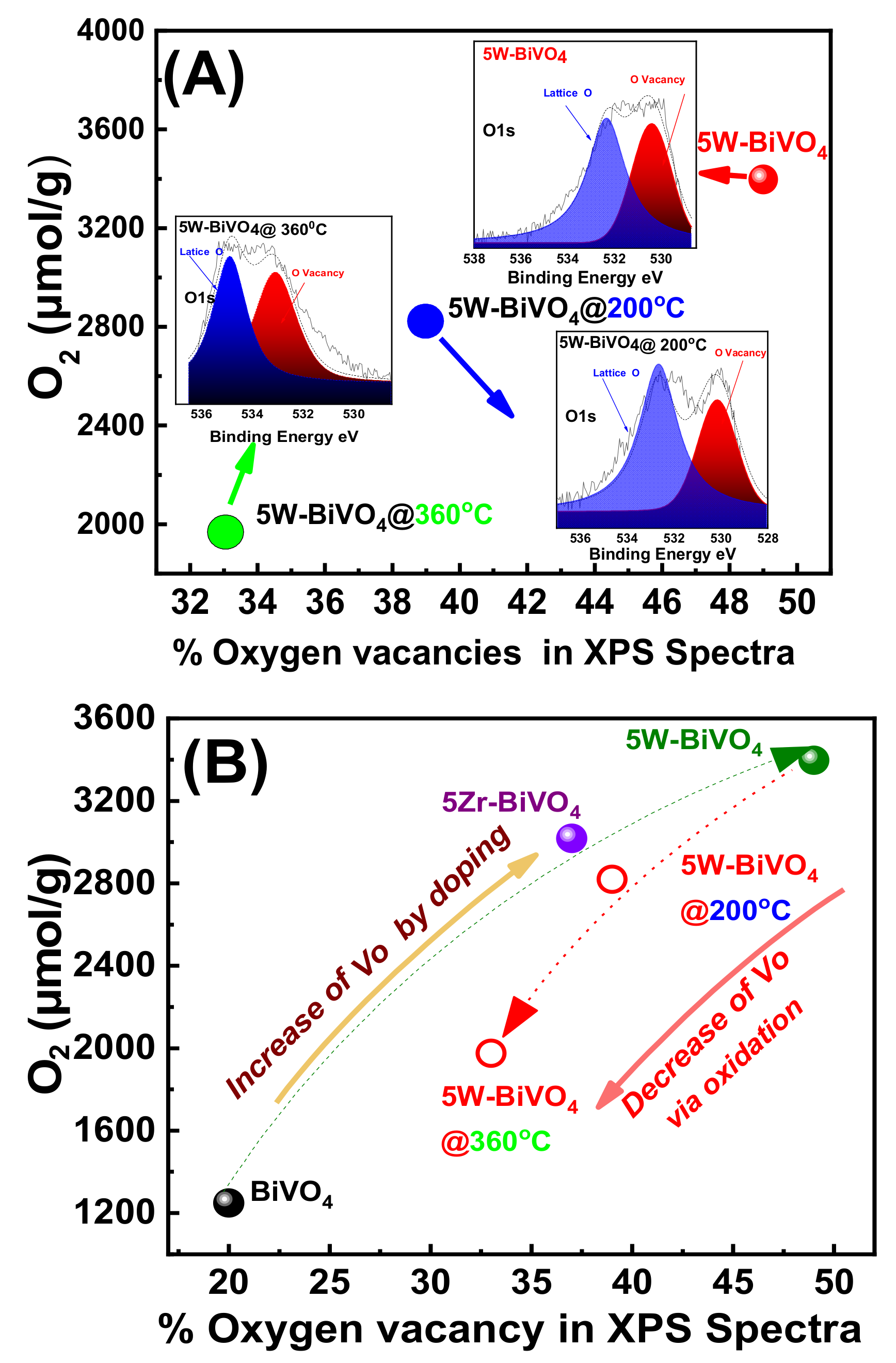
4. On the Mechanism of Photocatalytic Oxygen Evolution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujushima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Kato, H.; Hori, M.; Konta, R.; Shimodaira, Y.; Kudo, A. Construction of Z-scheme type heterogeneous photocatalysis systems for water splitting into H2 and O 2 under visible light irradiation. Chem. Lett. 2004, 33, 1348–1349. [Google Scholar] [CrossRef]
- Abdi, F.F.; Savenije, T.J.; May, M.M.; Dam, B.; Van De Krol, R. The Origin of Slow Carrier Transport in BiVO 4 Thin Film Photoanodes. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y.; Liu, Y.; Wang, P.; Li, Y.; Huang, B. Doping strategy to promote the charge separation in BiVO4 photoanodes. Appl. Catal. B Environ. 2017, 211, 258–265. [Google Scholar] [CrossRef]
- Ding, K.; Chen, B.; Fang, Z.; Zhang, Y.; Chen, Z. Why the photocatalytic activity of Mo-doped BiVO4 is enhanced: A comprehensive density functional study. Phys. Chem. Chem. Phys. 2014, 16, 13465–13476. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Liu, H.; Wang, G. Preparation of tungsten-doped BiVO4 and enhanced photocatalytic activity. J. Nanopart. Res. 2015. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Fan, W.; Shen, H.; Shi, W. A simple flame strategy for constructing W-doped BiVO4 photoanodes with enhanced photoelectrochemical water splitting. Int. J. Energy Res. 2020, 44, 10821–10831. [Google Scholar] [CrossRef]
- Ngoipala, A.; Ngamwongwan, L.; Fongkaew, I.; Jungthawan, S.; Hirunsit, P.; Limpijumnong, S.; Suthirakun, S. On the Enhanced Reducibility and Charge Transport Properties of Phosphorus-Doped BiVO4 as Photocatalysts: A Computational Study. J. Phys. Chem. C 2020, 124, 4352–4362. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Serendipitous Assembly of Mixed Phase BiVO4 on B-Doped g-C3N4: An Appropriate p-n Heterojunction for Photocatalytic O2 evolution and Cr(VI) reduction. Inorg. Chem. 2019, 58, 12480–12491. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Jing, T.; Huang, B.; Zhang, X.; Qin, X.; Dai, Y.; Whangbo, M.H. Enhancing the Photocatalytic Activity of BiVO4 for Oxygen Evolution by Ce Doping: Ce3+ Ions as Hole Traps. J. Phys. Chem. C 2016, 120, 2058–2063. [Google Scholar] [CrossRef]
- Ikeda, S.; Kawaguchi, T.; Higuchi, Y.; Kawasaki, N.; Harada, T. Effects of Zirconium Doping into a Monoclinic Scheelite BiVO 4 Crystal on Its Structural, Photocatalytic, and Photoelectrochemical Properties. Front. Chem. 2018, 6, 2–7. [Google Scholar] [CrossRef]
- Yin, W.; Wei, S.; Al-jassim, M.M.; Turner, J.; Yan, Y. Doping properties of monoclinic BiVO4 studied by first-principles density-functional theory. Phys. Rev. B 2011, 155102, 1–11. [Google Scholar]
- Parmar, K.P.S.; Kang, H.J.; Bist, A.; Dua, P.; Jang, J.S.; Lee, J.S. Photocatalytic and photoelectrochemical water oxidation over metal-doped monoclinic BiVO4 photoanodes. ChemSusChem 2012, 5, 1926–1934. [Google Scholar] [CrossRef]
- Camenzind, A.; Caseri, W.R.; Pratsinis, S.E. Flame-made nanoparticles for nanocomposites. Nano Today 2010, 5, 48–65. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1327. [Google Scholar] [CrossRef]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Mädler, L.; Kudo, A.; Amal, R. Flame preparation of visible-light-responsive BiVO 4 oxygen evolution photocatalysts with subsequent activation via aqueous route. ACS Appl. Mater. Interfaces 2011, 3, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Strohbeen, P.J.; Lee, D.; Zhou, C.; Kawasaki, J.K.; Choi, K.-S.; Liu, M.; Galli, G. The Role of Surface Oxygen Vacancies in BiVO 4. Chem. Mater. 2020, 32, 2899–2909. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, J.; Yao, X.; Chen, S.; Chen, Z. Clarifying the Roles of Oxygen Vacancy in W-Doped BiVO4 for Solar Water Splitting. ACS Appl. Energy Mater. 2018, 1, 3410–3419. [Google Scholar] [CrossRef]
- Moularas, C.; Georgiou, Y.; Adamska, K.; Deligiannakis, Y. Thermoplasmonic Heat Generation Efficiency by Nonmonodisperse Core–Shell Ag 0@SiO 2 Nanoparticle Ensemble. J. Phys. Chem. C 2019, 123, 22499–22510. [Google Scholar] [CrossRef]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and their evaluation as non-noble metal catalysts for efficient reduction of 4-nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar]
- Wu, J.M.; Chen, Y.; Pan, L.; Wang, P.; Cui, Y.; Kong, D.C.; Wang, L.; Zhang, X.; Zou, J.J. Multi-layer monoclinic BiVO4 with oxygen vacancies and V4+ species for highly efficient visible-light photoelectrochemical applications. Appl. Catal. B Environ. 2018, 221, 187–195. [Google Scholar] [CrossRef]
- Liu, G.; Li, F.; Zhu, Y.; Li, J.; Sun, L. Cobalt doped BiVO4 with rich oxygen vacancies for efficient photoelectrochemical water oxidation. RSC Adv. 2020, 10, 28523–28526. [Google Scholar] [CrossRef]
- Walsh, A.; Yan, Y.; Huda, M.N.; Al-Jassim, M.M.; Wei, S.H. Band edge electronic structure of BiVO 4: Elucidating the role of the Bi s and V d orbitals. Chem. Mater. 2009, 21, 547–551. [Google Scholar] [CrossRef]
- Chen, S.H.; Jiang, Y.S.; Lin, H.Y. Easy Synthesis of BiVO4 for Photocatalytic Overall Water Splitting. ACS Omega 2020, 5, 8927–8933. [Google Scholar] [CrossRef]
- Yoshino, S.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-Schematic Solar Water Splitting Using Fine Particles of H2-Evolving (CuGa)0.5ZnS2 Photocatalyst Prepared by a Flux Method with Chloride Salts. ACS Appl. Energy Mater. 2020, 3, 5684–5692. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R.; Ng, Y.H. Exploring the Different Roles of Particle Size in Photoelectrochemical and Photocatalytic Water Oxidation on BiVO4. ACS Appl. Mater. Interfaces 2016, 8, 28607–28614. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Geng, Z.; Ren, T.; Yang, Z. The improvement of photocatalysis O2 production over BiVO4 with amorphous FeOOH shell modification. Sci. Rep. 2019, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Thalluri, S.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Green-synthesized W- and Mo-doped BiVO4 oriented along the {040} facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B Environ. 2016, 180, 630–636. [Google Scholar] [CrossRef]
- Qiu, W.; Huang, Y.; Tang, S.; Ji, H.; Tong, Y. Thin-Layer Indium Oxide and Cobalt Oxyhydroxide Cobalt-Modified BiVO4 Photoanode for Solar-Assisted Water Electrolysis. J. Phys. Chem. C 2017, 121, 17150–17159. [Google Scholar] [CrossRef]
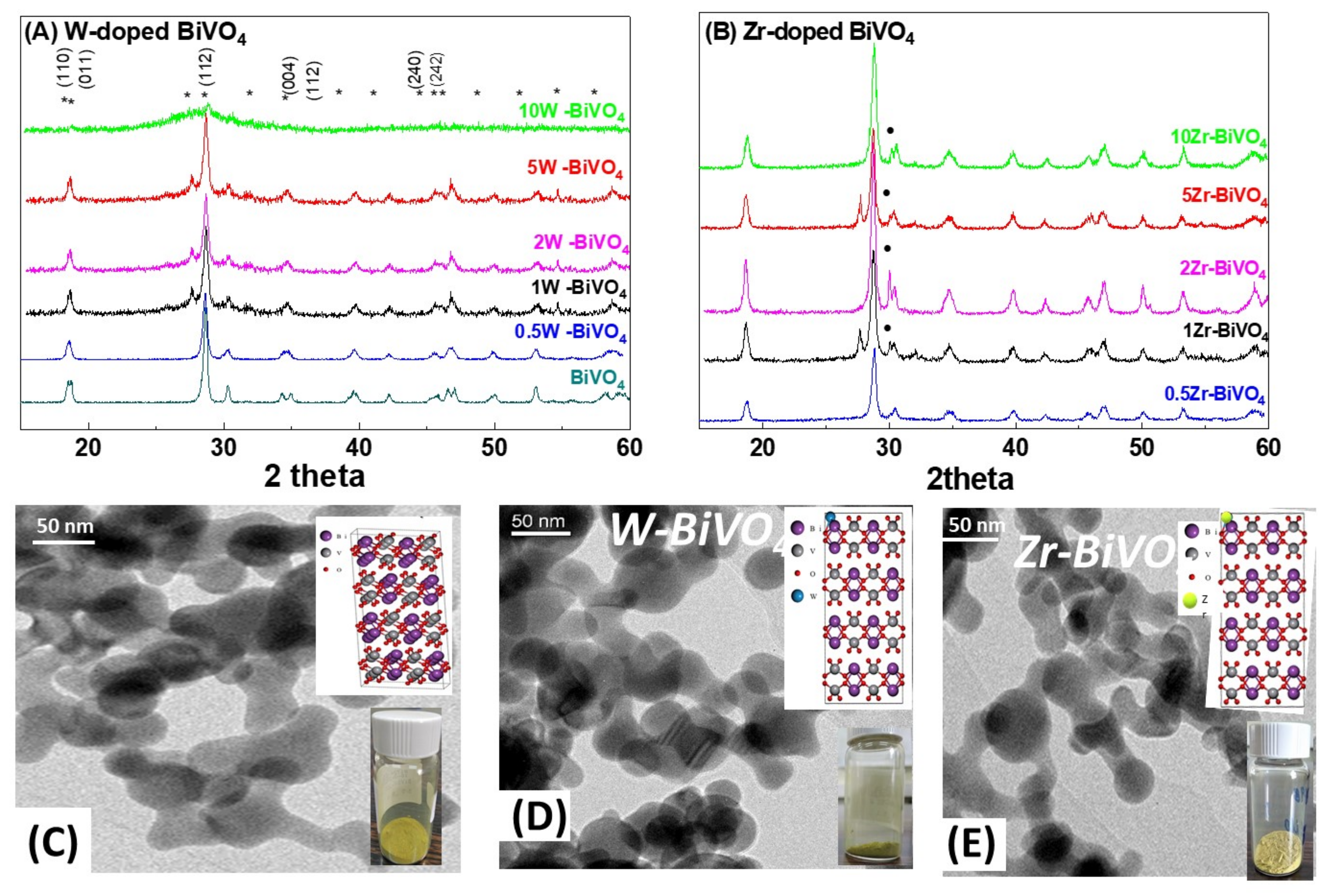


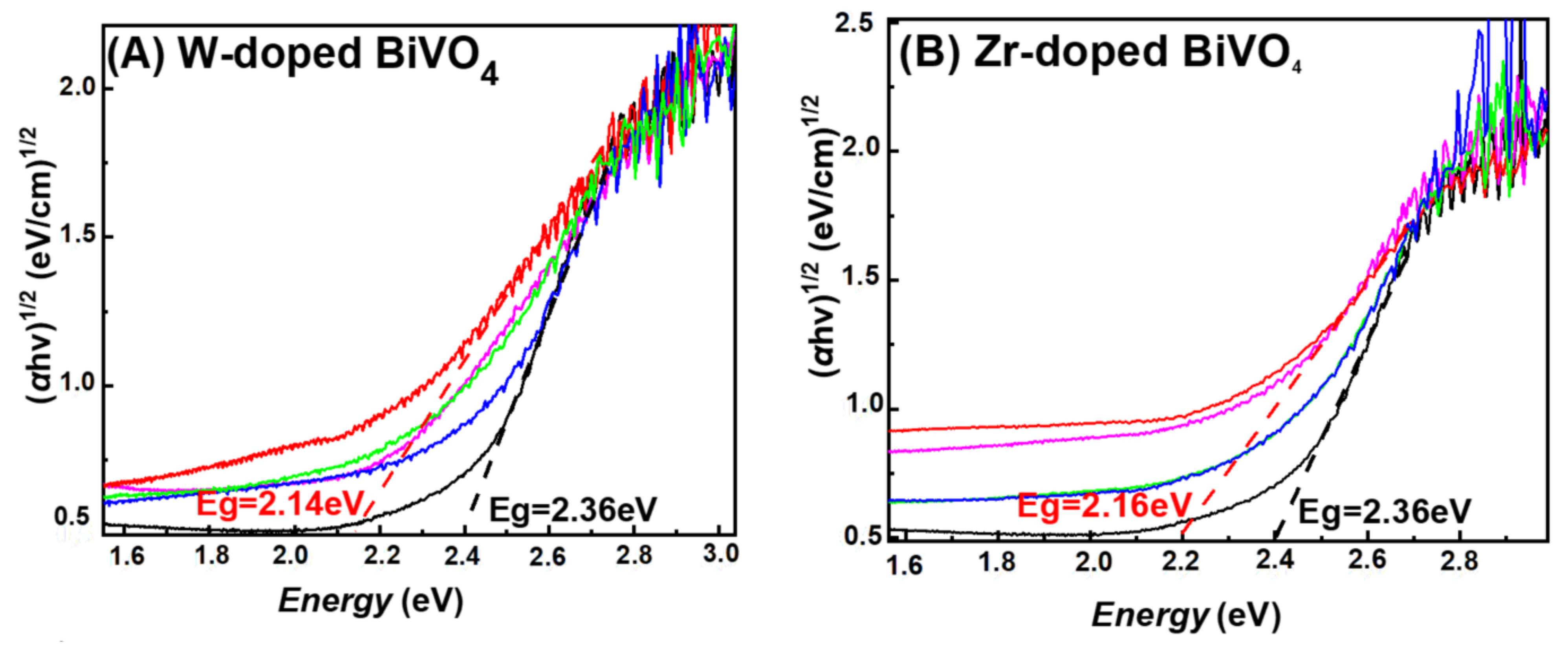
| Material | Nominal Dopant Content in the FSP Precursor (%w:w) | Dopant Content Measured by XRF | XRD Size (nm ± 0.5) | SSA (m2g−1 ± 0.8) | Band-Gap Eg (eV) |
|---|---|---|---|---|---|
| BiVO4 | - | - | 22.1 | 37.7 | 2.36 |
| W-Doped nanocatalysts | |||||
| 0.5W-BiVO4 | 0.5 | Not detectable | 17.5 | 45.0 | 2.31 |
| 1W-BiVO4 | 1 | 0.6 | 19.8 | 46.8 | 2.25 |
| 2W-BiVO4 | 2 | 1.8 | 15.3 | 45.0 | 2.21 |
| 5W-BiVO4 | 5 | 4.4 | 17.1 | 47.2 | 2.14 |
| 10W-BiVO4 | 10 | 7.8 | - | 49.0 | 2.13 |
| Zr-Doped nanocatalysts | |||||
| 0.5Zr-BiVO4 | 0.5 | Not detectable | 17.8 | 47.1 | 2.27 |
| 1Zr-BiVO4 | 1 | 0.4 | 20.2 | 45.6 | 2.25 |
| 2Zr-BiVO4 | 2 | 1.7 | 19.8 | 45.7 | 2.20 |
| 5Zr-BiVO4 | 5 | 3.4 | 20.5 | 46.0 | 2.16 |
| 10Zr-BiVO4 | 10 | 8.3 | - | 47.2 | 2.15 |
| Material | O2-Evolution (μmol/g) after 3 h Reaction | O2-Evolution Rate (μmol/g × h) |
|---|---|---|
| BiVO4 | 1249 (±20) | 420 (±5) |
| W-Doped nanocatalysts | ||
| 0.5W-BiVO4 | 1842 (±20) | 633 (±5) |
| 1W-BiVO4 | 2002 (±20) | 667 (±5) |
| 2W-BiVO4 | 2827 (±20) | 945 (±5) |
| 5W-BiVO4 | 3326 (±20) | 1020 (±5) |
| 10W-BiVO4 | 2100 (±20) | 778 (±5) |
| Zr-Doped nanocatalysts | ||
| 0.5Zr-BiVO4 | 600 (±20) | 235 (±5) |
| 1Zr-BiVO4 | 1039 (±20) | 337 (±5) |
| 2Zr-BiVO4 | 1512 (±20) | 521 (±5) |
| 5Zr-BiVO4 | 3018 (±20) | 974 (±5) |
| 10 Zr-BiVO4 | 907 (±20) | 324 (±5) |
| Calcined nanocatalysts | ||
| 5W-BiVO4@200 | 2804 (±20) | 1001 (±5) |
| 5W-BiVO4@360 | 1973 (±20) | 720 (±5) |
| 5Zr-BiVO4@200 | 1988 (±20) | 710 (±5) |
| 5Zr-BiVO4@360 | 1299 (±20) | 448 (±5) |
| Photocatalyst | Light Source | Synthesis Method | XRD Size (nm) | Electron Acceptor | Activity (μmol/g*h) | Ref. |
|---|---|---|---|---|---|---|
| BiVO4 | 400 W medium-pressure halide lamp (Phillips HPA400, λmax = 360 nm | Hydrothermal method | 28.3 | AgNO3 | 2622 | [26] |
| BiVO4 | 300 W Xe lamp with a 420 nm cut off filter | Flame Synthesis of BiVO4 and Acid Modification | 72.0 | AgNO3 | 333 | [27] |
| BiVO4 | 300 W Xe lamp with a 420 nm cut off filter | Solid-liquid state reaction in HNO3 | 91.0 | AgNO3 | 800 | [28] |
| Ce-BiVO4 | 300 W Xe lamp | Hydrothermal method | Not referred | AgNO3 | 775 | [14] |
| A-FeOOH/BiVO4 (8 wt %) | 300 W Xe lamp with a 420 nm cut off filter | Precipitation method | Not referred | NaIO4 | 1206 | [29] |
| Zr-BiVO4 | Perkin Elmer CERMAX LX-300BUV Xe lamp | Precipitation method | Not referred | AgNO3 | 700 | [13] |
| W(0.9%w/w)-BiVO4 | Simulated plasma (Lumix model LIFI STA-40) | Hydrothermal method | 92.0 | AgNO3 | 942 | [30] |
| BiVO4 | 125W Hg λmax = 440 nm | FSP | 22.1 | AuHCl4 | 420 | This work |
| 5W-BiVO4 | 125W Hg λmax = 440 nm | FSP | 17.1 | AuHCl4 | 1074 | This work |
| 5Zr-BiVO4 | 125W Hg λmax = 440 nm | FSP | 20.5 | AuHCl4 | 1020 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials 2021, 11, 501. https://doi.org/10.3390/nano11020501
Stathi P, Solakidou M, Deligiannakis Y. Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials. 2021; 11(2):501. https://doi.org/10.3390/nano11020501
Chicago/Turabian StyleStathi, Panagiota, Maria Solakidou, and Yiannis Deligiannakis. 2021. "Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution" Nanomaterials 11, no. 2: 501. https://doi.org/10.3390/nano11020501
APA StyleStathi, P., Solakidou, M., & Deligiannakis, Y. (2021). Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials, 11(2), 501. https://doi.org/10.3390/nano11020501








