Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Studies
2.1.1. Determination of PCX IC50 Value on 4T1 Cell Line
2.1.2. Anticancer Activity of Amphiphilic Cyclodextrin Nanoparticles
2.1.3. Migration Assay
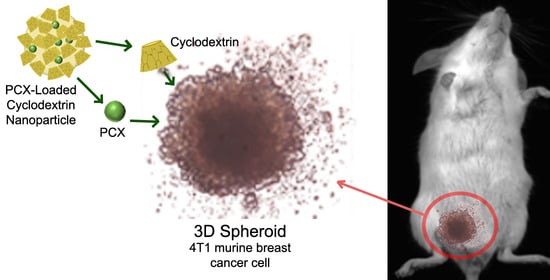
2.1.4. 3D Tumor Culture Studies
2.2. In Vivo Studies
2.2.1. Antitumoral Activities of Amphiphilic Cyclodextrin Nanoparticles
2.2.2. Biodistribution of Amphiphilic Cyclodextrin Nanoparticles
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of the Nanoparticles
3.2. Cell Culture Studies
3.2.1. Determination IC50 Value of Paclitaxel on 4T1 Cell Line
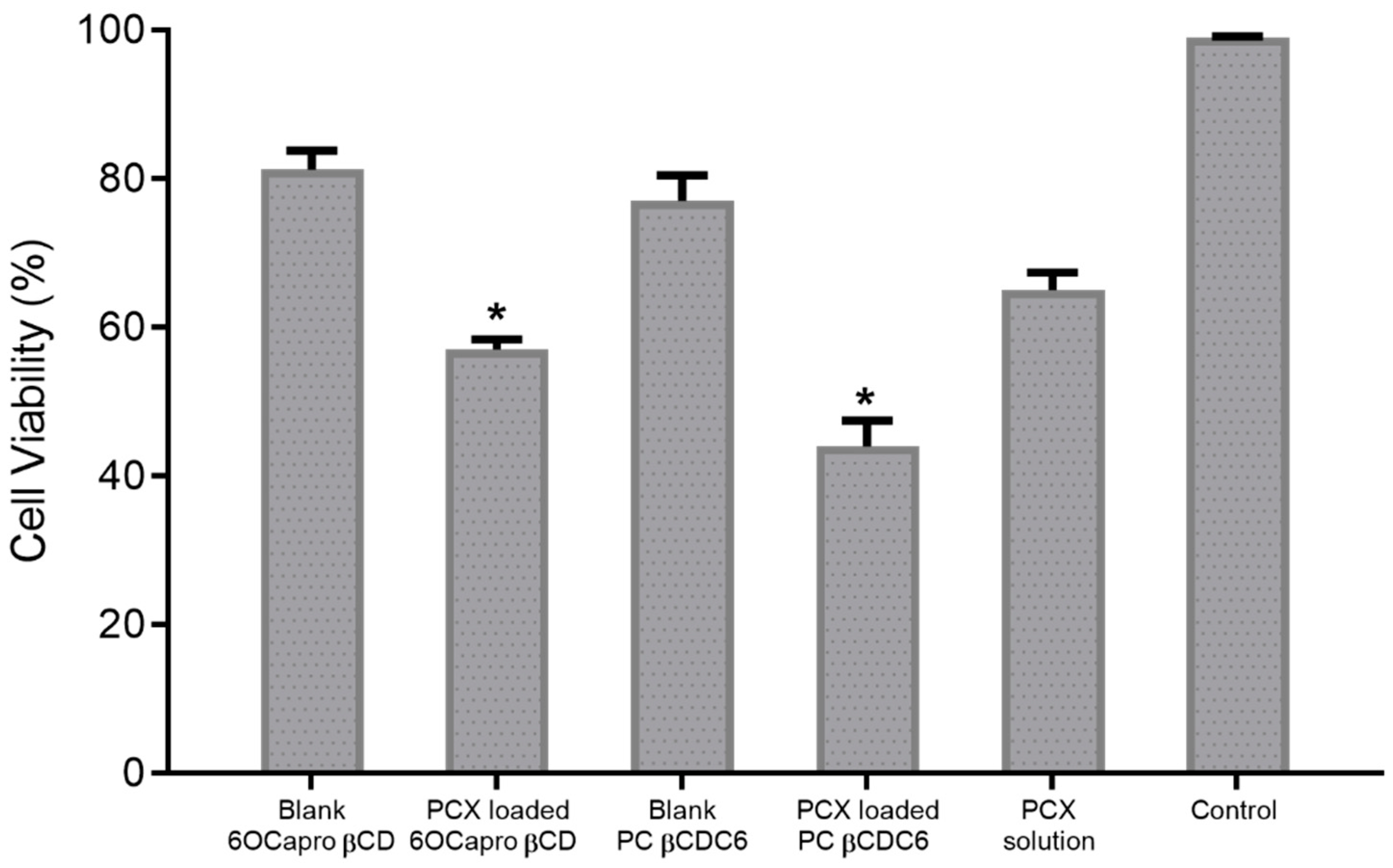
3.2.2. Anti-Cancer Activity of Amphiphilic Cyclodextrin Nanoparticles
3.2.3. Migration Assay
3.2.4. 3D Spheroid Culture
3.3. In Vivo Studies
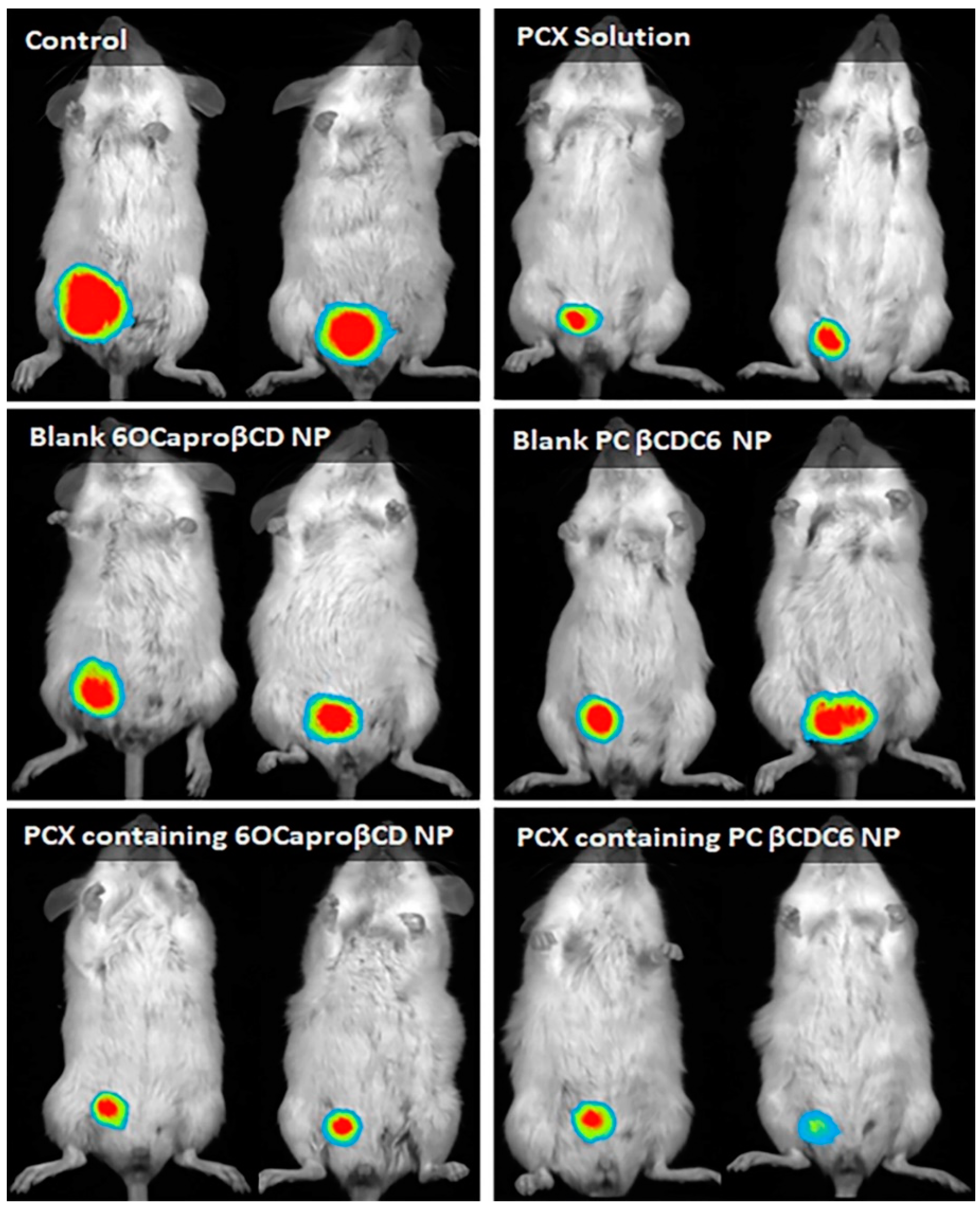
3.3.1. Antitumor Efficacy of Paclitaxel-Loaded Amphiphilic Cyclodextrin Nanoparticles
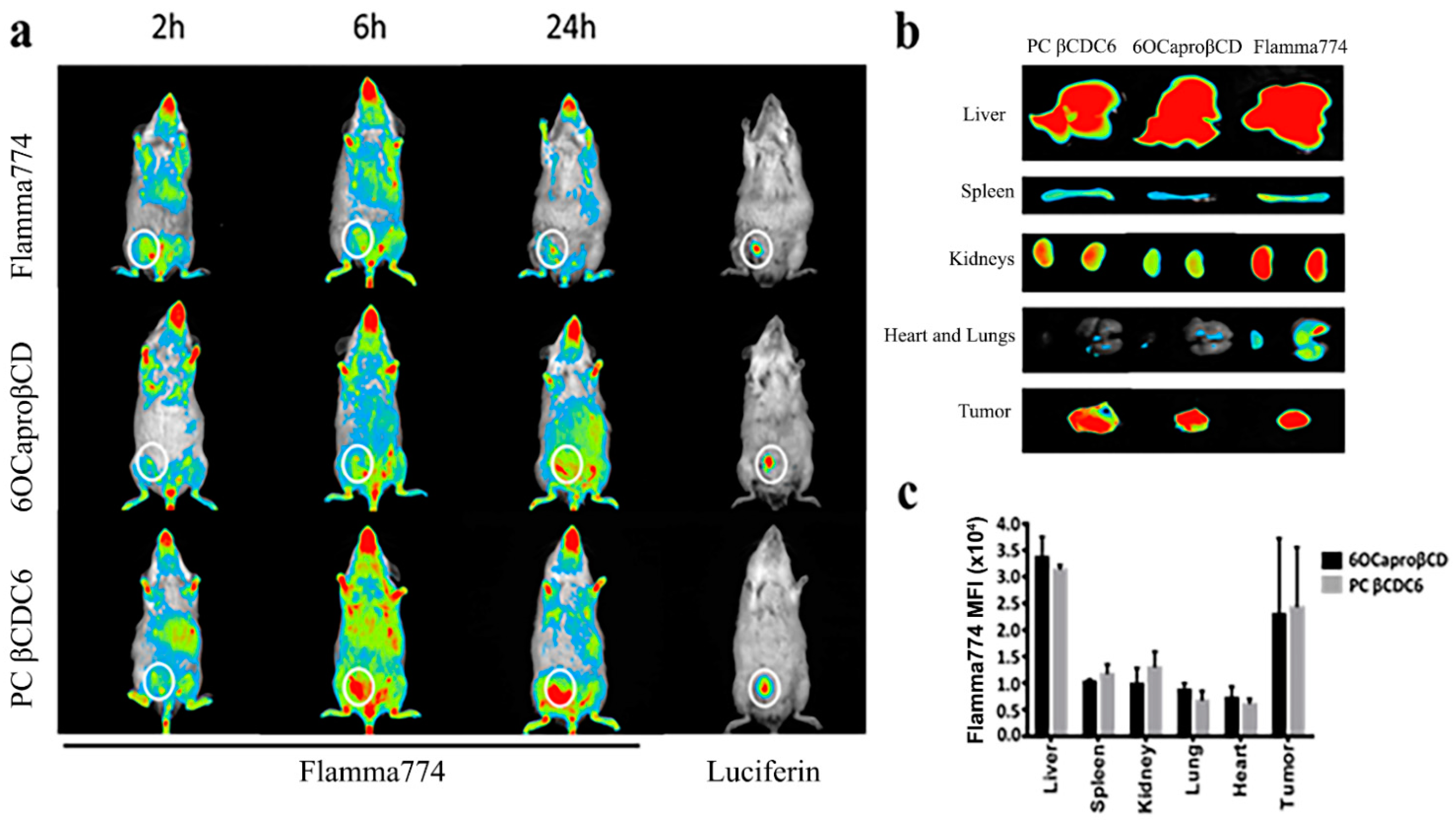
3.3.2. Biodistribution of Amphiphilic Cyclodextrin Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Watanabe, J.; Ishihara, K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J. Biomed. Mater. Res. Part A 2003, 65A, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Ishii, G.; Ochiai, A.; Neri, S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv. Drug Deliv. Rev. 2016, 99, 186–196. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose Response 2020, 18, 1559325820936161. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef]
- Bilensoy, E.; Gurkaynak, O.; Dogan, A.L.; Hincal, A.A. Safety and efficacy of amphiphilic beta-cyclodextrin nanoparticles for paclitaxel delivery. Int. J. Pharm. 2008, 347, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Erdogar, N.; Esendagli, G.; Nielsen, T.T.; Esendagli-Yilmaz, G.; Yoyen-Ermis, D.; Erdogdu, B.; Sargon, M.F.; Eroglu, H.; Bilensoy, E. Therapeutic efficacy of folate receptor-targeted amphiphilic cyclodextrin nanoparticles as a novel vehicle for paclitaxel delivery in breast cancer. J. Drug Target. 2018, 26, 66–74. [Google Scholar] [CrossRef]
- Varan, G.; Patrulea, V.; Borchard, G.; Bilensoy, E. Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model. Nanomaterials 2018, 8, 67. [Google Scholar] [CrossRef]
- Varan, G.; Akkin, S.; Demirturk, N.; Benito, J.M.; Bilensoy, E. Erlotinib Entrapped in Cholesterol-Depleting Cyclodextrin Nanoparticles Shows Improved Antitumoral Efficacy in 3D Spheroid Tumors of the Lung and the Liver. J. Drug Target. 2020, 1–15. [Google Scholar] [CrossRef]
- Ünal, H.; d’Angelo, I.; Pagano, E.; Borrelli, F.; Izzo, A.; Ungaro, F.; Quaglia, F.; Bilensoy, E. Core–shell hybrid nanocapsules for oral delivery of camptothecin: Formulation development, in vitro and in vivo evaluation. J. Nanoparticle Res. 2015, 17. [Google Scholar] [CrossRef]
- Unal, H.; Ozturk, N.; Bilensoy, E. Formulation development, stability and anticancer efficacy of core-shell cyclodextrin nanocapsules for oral chemotherapy with camptothecin. Beilstein. J. Org. Chem. 2015, 11, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Aktas, Y.; Benito, J.M.; Bilensoy, E. Cyclodextrin nanoparticle bound oral camptothecin for colorectal cancer: Formulation development and optimization. Int. J. Pharm. 2020, 584, 119468. [Google Scholar] [CrossRef]
- Quaglia, F.; Ostacolo, L.; Mazzaglia, A.; Villari, V.; Zaccaria, D.; Sciortino, M.T. The intracellular effects of non-ionic amphiphilic cyclodextrin nanoparticles in the delivery of anticancer drugs. Biomaterials 2009, 30, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Cannavà, C.; Crupi, V.; Ficarra, P.; Guardo, M.; Majolino, D.; Mazzaglia, A.; Stancanelli, R.; Venuti, V. Physico-chemical characterization of an amphiphilic cyclodextrin/genistein complex. J. Pharm. Biomed. Anal. 2010, 51, 1064–1068. [Google Scholar] [CrossRef]
- Bondi, M.L.; Scala, A.; Sortino, G.; Amore, E.; Botto, C.; Azzolina, A.; Balasus, D.; Cervello, M.; Mazzaglia, A. Nanoassemblies Based on Supramolecular Complexes of Nonionic Amphiphilic Cyclodextrin and Sorafenib as Effective Weapons to Kill Human HCC Cells. Biomacromolecules 2015, 16, 3784–3791. [Google Scholar] [CrossRef]
- Massaro, M.; Piana, S.; Colletti, C.G.; Noto, R.; Riela, S.; Baiamonte, C.; Giordano, C.; Pizzolanti, G.; Cavallaro, G.; Milioto, S.; et al. Multicavity halloysite-amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cells. J. Mater. Chem. B 2015, 3, 4074–4081. [Google Scholar] [CrossRef]
- Evans, J.C.; Malhotra, M.; Fitzgerald, K.A.; Guo, J.; Cronin, M.F.; Curtin, C.M.; O’Brien, F.J.; Darcy, R.; O’Driscoll, C.M. Formulation and Evaluation of Anisamide-Targeted Amphiphilic Cyclodextrin Nanoparticles To Promote Therapeutic Gene Silencing in a 3D Prostate Cancer Bone Metastases Model. Mol. Pharm. 2017, 14, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.C.; Malhotra, M.; Sweeney, K.; Darcy, R.; Nelson, C.C.; Hollier, B.G.; O’Driscoll, C.M. Folate-targeted amphiphilic cyclodextrin nanoparticles incorporating a fusogenic peptide deliver therapeutic siRNA and inhibit the invasive capacity of 3D prostate cancer tumours. Int. J. Pharm. 2017, 532, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Aranda, C.; Urbiola, K.; Méndez Ardoy, A.; García Fernández, J.M.; Ortiz Mellet, C.; de Ilarduya, C.T. Targeted gene delivery by new folate–polycationic amphiphilic cyclodextrin–DNA nanocomplexes in vitro and in vivo. Eur. J. Pharm. Biopharm. 2013, 85, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; Lomazzi, M.; Franceschi, V.; Sansone, F.; Ortiz Mellet, C.; Donofrio, G.; Casnati, A.; Garcia Fernandez, J.M. Cyclodextrin- and calixarene-based polycationic amphiphiles as gene delivery systems: A structure-activity relationship study. Org. Biomol. Chem. 2015, 13, 1708–1723. [Google Scholar] [CrossRef]
- Conte, C.; Scala, A.; Siracusano, G.; Leone, N.; Patanè, S.; Ungaro, F.; Miro, A.; Sciortino, M.T.; Quaglia, F.; Mazzaglia, A. Nanoassembly of an amphiphilic cyclodextrin and Zn(ii)-phthalocyanine with the potential for photodynamic therapy of cancer. RSC Adv. 2014, 4, 43903–43911. [Google Scholar] [CrossRef]
- Sortino, S.; Mazzaglia, A.; Monsù Scolaro, L.; Marino Merlo, F.; Valveri, V.; Sciortino, M.T. Nanoparticles of cationic amphiphilic cyclodextrins entangling anionic porphyrins as carrier-sensitizer system in photodynamic cancer therapy. Biomaterials 2006, 27, 4256–4265. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Micali, N.; Villari, V.; Zagami, R. A novel potential nanophototherapeutic based on the assembly of an amphiphilic cationic β-cyclodextrin and an anionic porphyrin. J. Porphyr. Phthalocyanines 2017, 21, 398–405. [Google Scholar] [CrossRef]
- Varan, G.; Benito, J.M.; Mellet, C.O.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein. J. Nanotechnol. 2017, 8, 1457–1468. [Google Scholar] [CrossRef]
- Ercan, A.; Celebier, M.; Varan, G.; Oncul, S.; Nenni, M.; Kaplan, O.; Bilensoy, E. Global omics strategies to investigate the effect of cyclodextrin nanoparticles on MCF-7 breast cancer cells. Eur. J. Pharm. Sci. 2018, 123, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Oncul, S.; Ercan, A.; Benito, J.M.; Ortiz Mellet, C.; Bilensoy, E. Cholesterol-Targeted Anticancer and Apoptotic Effects of Anionic and Polycationic Amphiphilic Cyclodextrin Nanoparticles. J. Pharm. Sci. 2016, 105, 3172–3182. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Moscoso, A.; Balbuena, P.; Gomez-Garcia, M.; Ortiz Mellet, C.; Benito, J.M.; Le Gourrierec, L.; Di Giorgio, C.; Vierling, P.; Mazzaglia, A.; Micali, N.; et al. Rational design of cationic cyclooligosaccharides as efficient gene delivery systems. Chem. Commun. 2008, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; You, X.; Chen, L.; Huang, J.; Wang, L.; Wu, J.; Guan, S. Biotherapeutic Nanoparticles of Poly(Ferulic Acid) Delivering Doxorubicin for Cancer Therapy. J. Biomed. Nanotechnol. 2019, 15, 1734–1743. [Google Scholar] [CrossRef]
- Contini, C.; Schneemilch, M.; Gaisford, S.; Quirke, N. Nanoparticle–membrane interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef]
- Huang, B.; Tan, Z.; Bohinc, K.; Zhang, S. Interaction between nanoparticles and charged phospholipid membranes. Phys. Chem. Chem. Phys. 2018, 20, 29249–29263. [Google Scholar] [CrossRef]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. [Google Scholar] [CrossRef]
- Håkanson, M.; Textor, M.; Charnley, M. Engineered 3D environments to elucidate the effect of environmental parameters on drug response in cancer. Integr. Biol. 2011, 3, 31–38. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Tang-Schomer, M.; Pouli, D.; Mizzoni, C.; Raia, N.; Tai, A.; Arkun, K.; Wu, J.; Black, L.D.; Scheffler, B.; et al. 3D extracellular matrix microenvironment in bioengineered tissue models of primary pediatric and adult brain tumors. Nat. Commun. 2019, 10, 4529. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, A.; Kubbies, M. Diversity of cell-mediated adhesions in breast cancer spheroids. Int. J. Oncol. 2007, 31, 1403–1413. [Google Scholar] [CrossRef]
- Green, S.K.; Francia, G.; Isidoro, C.; Kerbel, R.S. Antiadhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol. Cancer 2004, 3, 149–159. [Google Scholar]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Han, Y.H.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Subcellular Performance of Nanoparticles in Cancer Therapy. Int. J. Nanomed. 2020, 15, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Todor, I.N.; Lukyanova, N.Y.; Chekhun, V.F. The lipid content of cisplatin- and doxorubicin-resistant MCF-7 human breast cancer cells. Exp. Oncol. 2012, 34, 97–100. [Google Scholar] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]







| Formulation | Particle Size (nm) ±SD | Zeta Potential (mV) ±SD | Encapsulation Efficacy (%) ± SD |
|---|---|---|---|
| Blank 6OCaproβCD nanoparticle | 104 ± 1.1 | −24 ± 0.3 | - |
| PCX-loaded 6OCaproβCD nanoparticle | 113 ± 4.0 | −29 ± 2.0 | 41 ± 2.3 |
| Blank PC βCDCD nanoparticle | 75 ± 2.1 | +61 ± 1.4 | - |
| PCX-loaded PC βCDCD nanoparticle | 82 ± 2.0 | +62 ± 1.1 | 64 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varan, G.; Varan, C.; Öztürk, S.C.; Benito, J.M.; Esendağlı, G.; Bilensoy, E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials 2021, 11, 515. https://doi.org/10.3390/nano11020515
Varan G, Varan C, Öztürk SC, Benito JM, Esendağlı G, Bilensoy E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials. 2021; 11(2):515. https://doi.org/10.3390/nano11020515
Chicago/Turabian StyleVaran, Gamze, Cem Varan, Süleyman Can Öztürk, Juan M. Benito, Güneş Esendağlı, and Erem Bilensoy. 2021. "Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo" Nanomaterials 11, no. 2: 515. https://doi.org/10.3390/nano11020515
APA StyleVaran, G., Varan, C., Öztürk, S. C., Benito, J. M., Esendağlı, G., & Bilensoy, E. (2021). Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials, 11(2), 515. https://doi.org/10.3390/nano11020515