Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Purification of AV-TeNPs
2.2. High-Resolution Transmission Electron Microscopy Analysis
2.3. X-ray Photoelectron Spectroscopy Analysis
2.4. Fourier-Transform Infrared and Raman Spectroscopy
2.5. Bacterial Culture Preparation and Antibacterial Analysis
2.6. In Vitro Cytotoxicity Assay with Biogenic AV-TeNPs
2.6.1. In Vitro Cell Culture
2.6.2. Metabolic Activity of the Cells Using MTS Assays
2.6.3. Metabolic Activity of the Cells Using PrestoBlue Assays
2.6.4. Cell Viability Using Live/Dead Assays
2.7. Reactive Oxygen Species (ROS) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Purification of AV-TeNPs
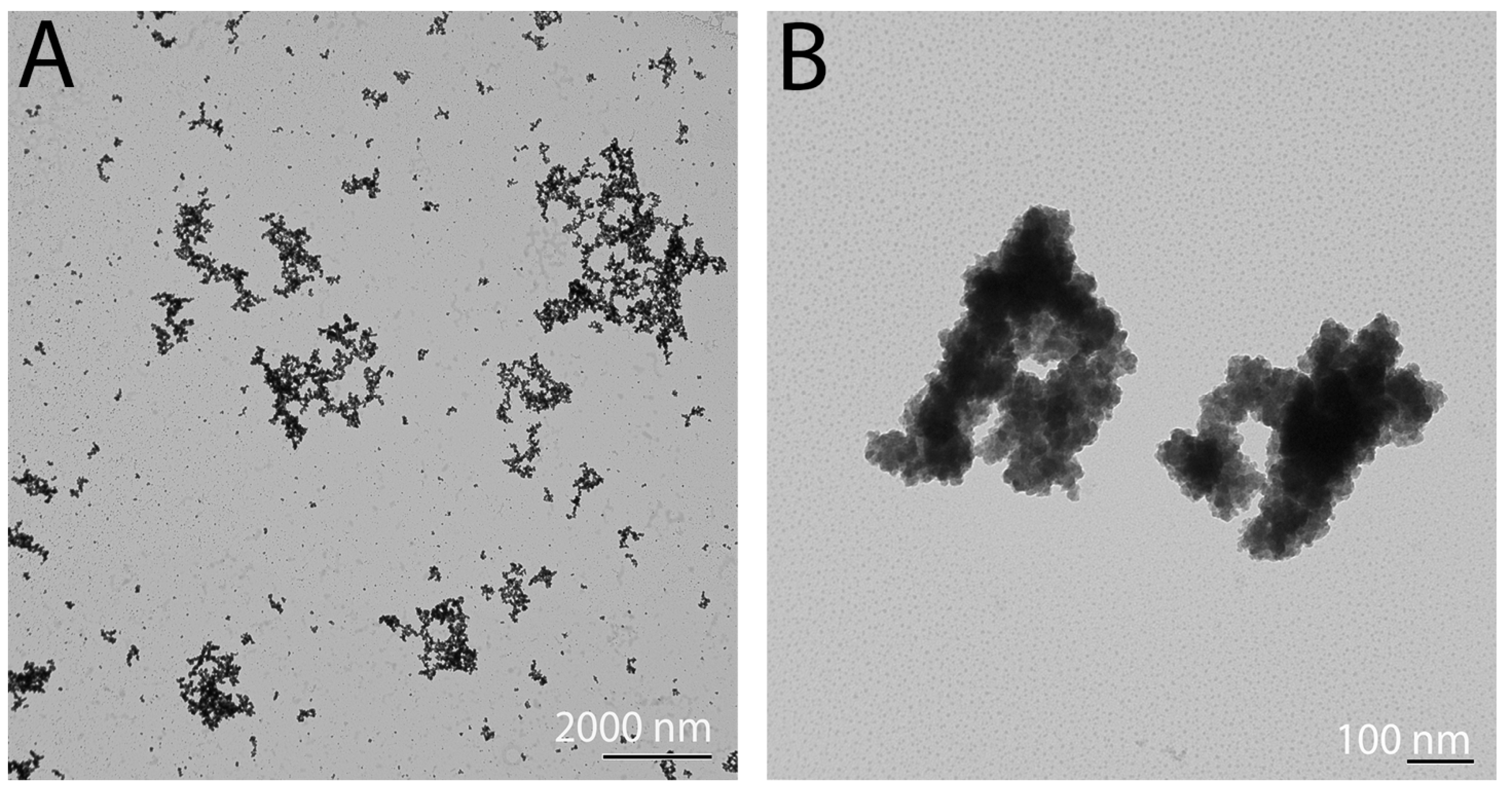
3.2. High-Resolution Transmission Electron Microscopy Analysis
3.3. Fourier-Transform Infrared and Raman Spectroscopy Analysis
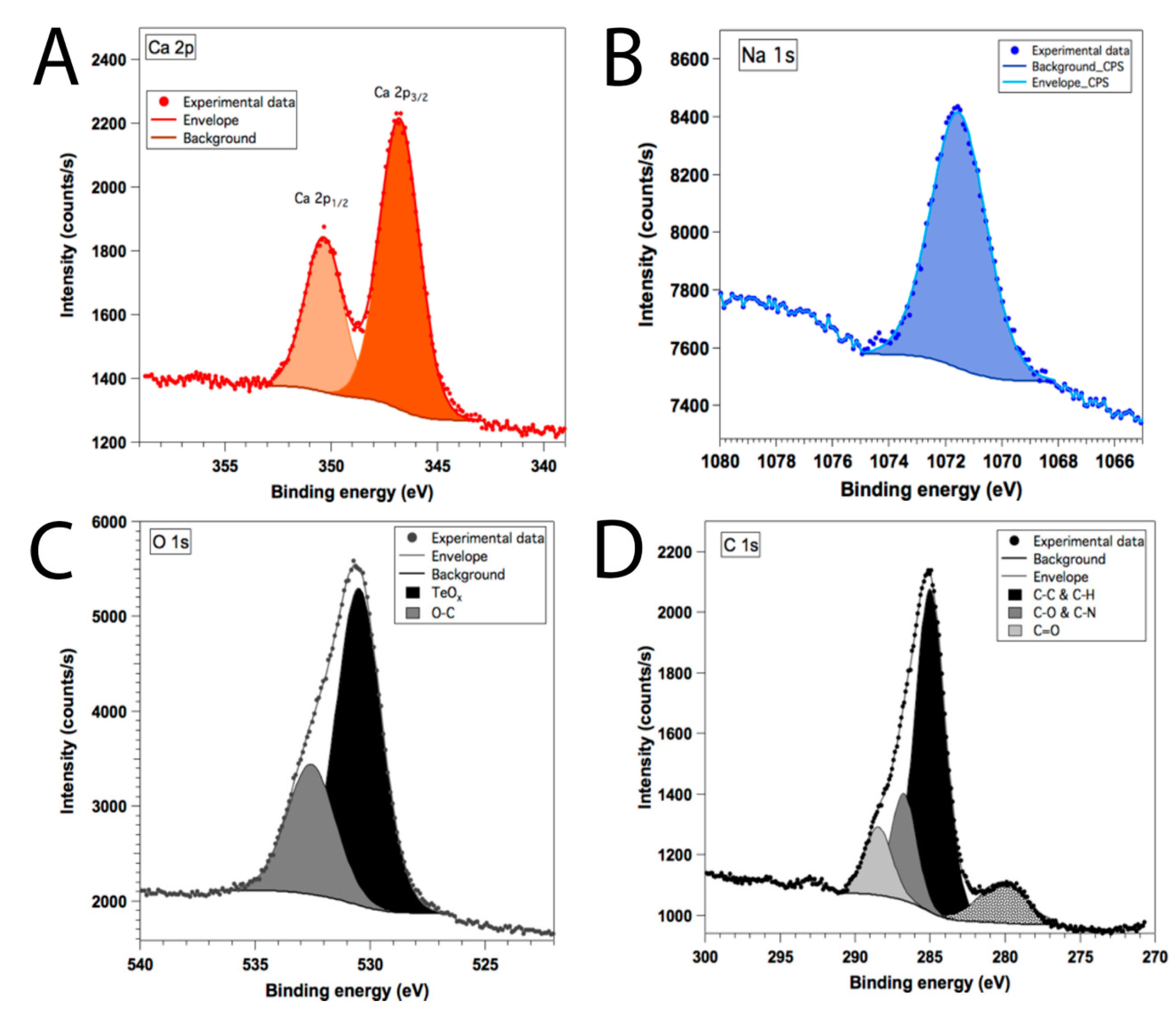
3.4. Chemical Composition Studies of the AV-TeNPs
3.5. Determining the Antimicrobial Activity of AV-TeNPs
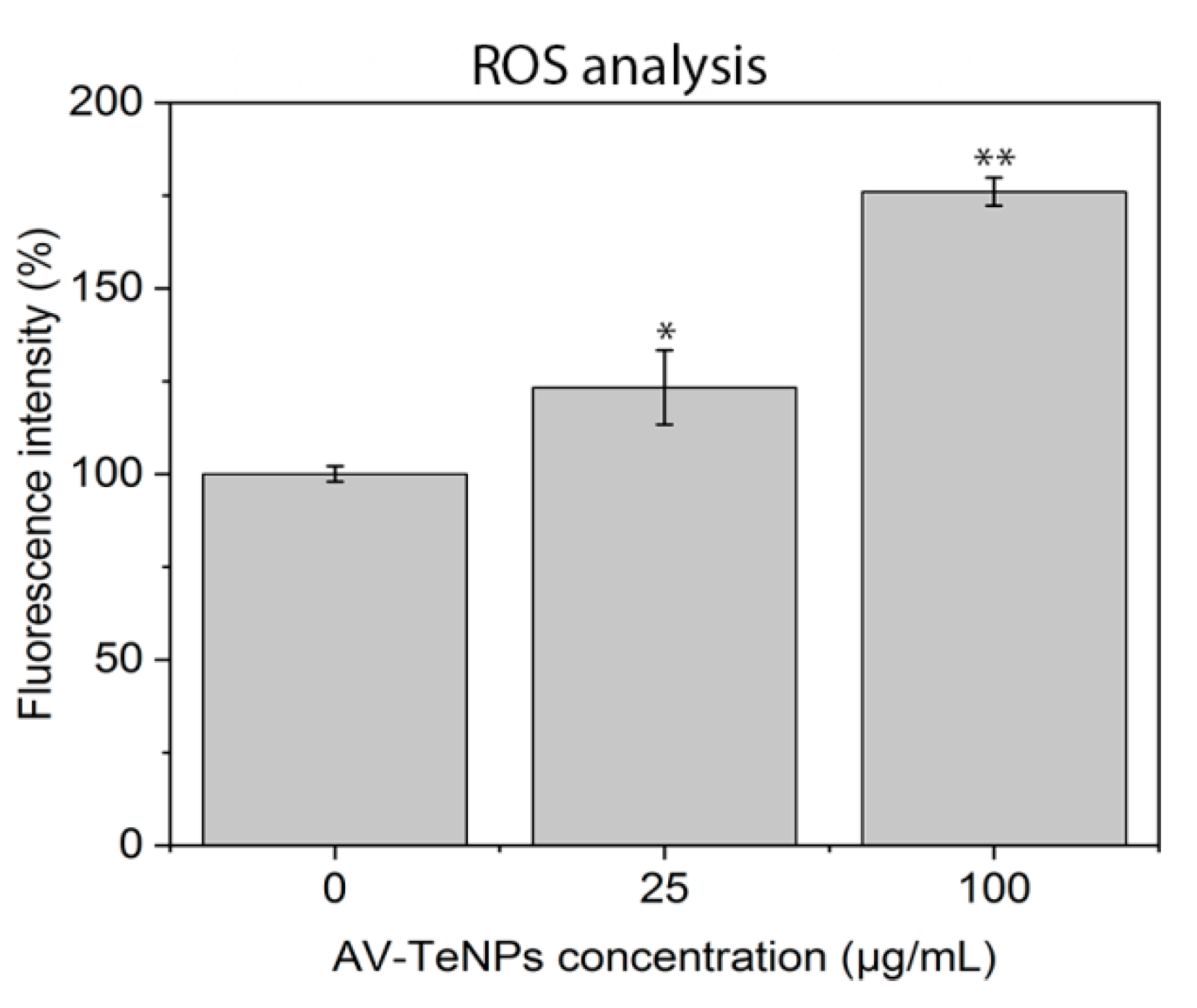
3.6. Reactive Oxygen Species (ROS) Analysis
3.7. In vitro Cytotoxicity Assay with AV-TeNPs
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Schematic Modeling of the AV-TeNPs
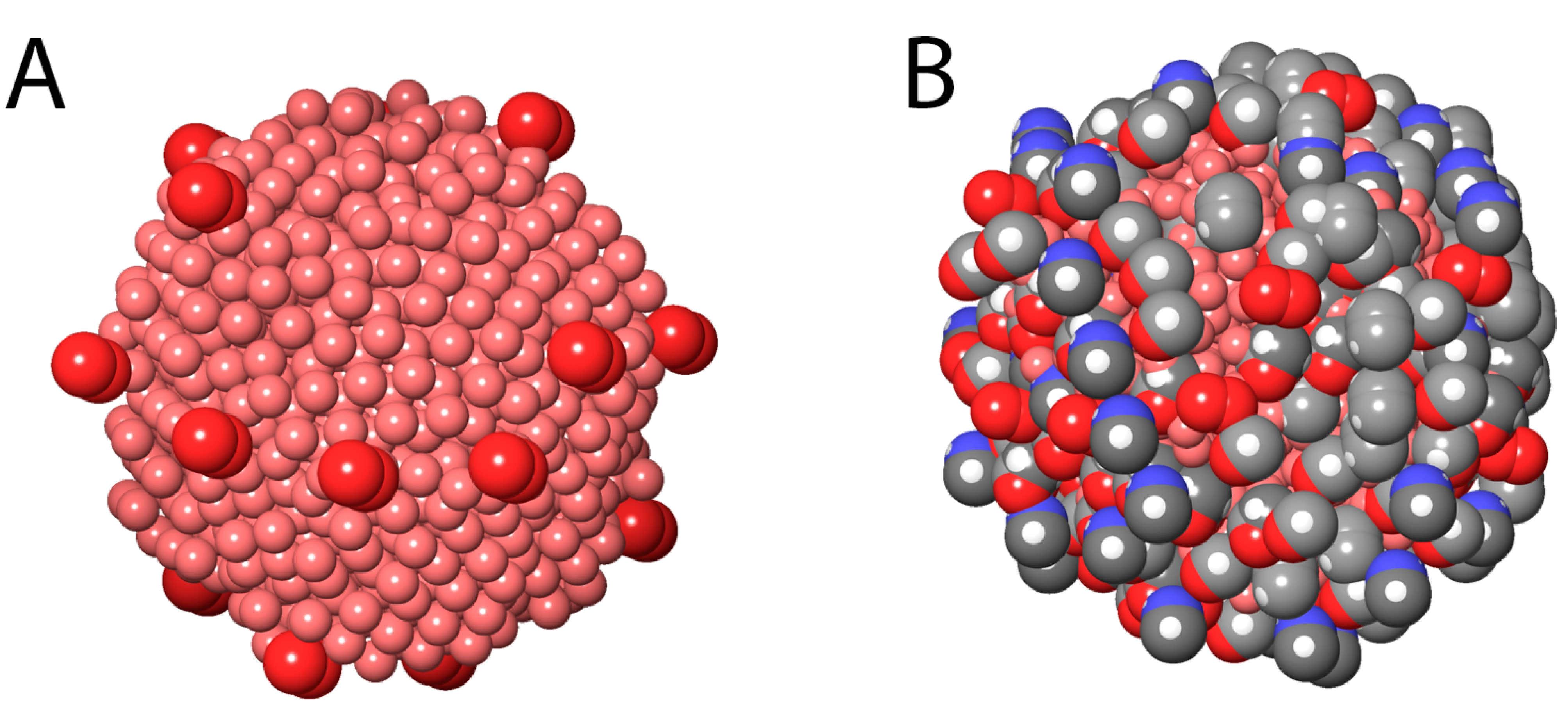
Appendix A.2. Stability Analysis
| Z-potential (mV) | ||
|---|---|---|
| Nanostructure | As-synthesized | 60 days old |
| AV-TeNPs | −24 ± 2 | −17 ± 3 |
Appendix A.3. X-ray Diffraction Analysis

Appendix A.4. Scanning Electron Microscopy Analysis


Appendix A.5. X-ray Photoelectron Microscopy Analysis
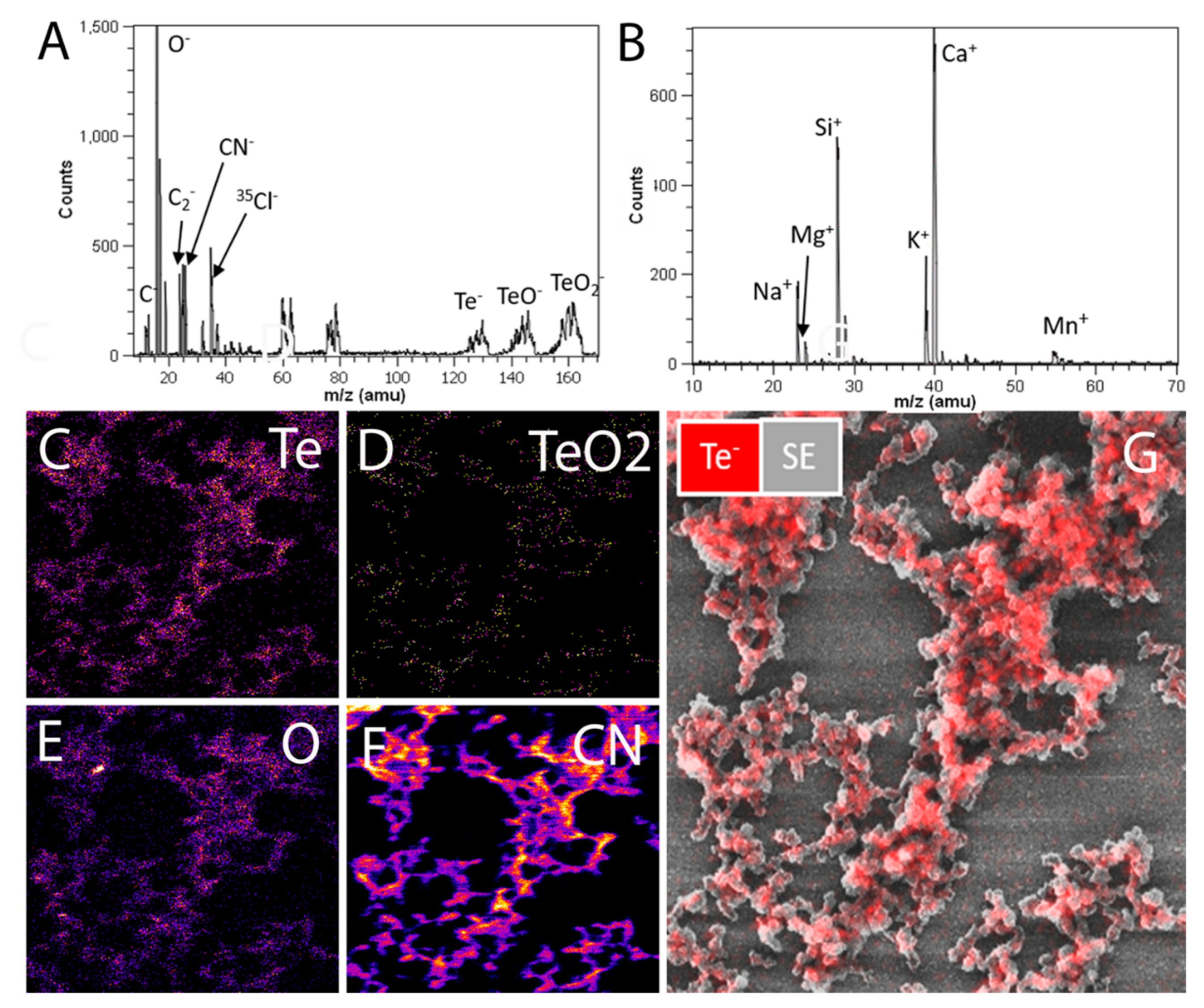
Appendix A.6. Secondary Ion Mass Spectrometry Imaging
Appendix A.7. Determining the Antimicrobial Activity of AV-TeNPs




Appendix A.8. Cell Fixation and SEM Imaging for Bacteria and Human Cells


References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. BioMedicine 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Halyard, M.Y.; Brown, L.C.; Mutter, R.W. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int. J. Women Health 2015, 7, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The nanomedicine revolution: Part 1: Emerging concepts. Pharm. Ther. 2012, 37, 512–525. [Google Scholar]
- Khan, H.; Sakharkar, M.; Nayak, A.; Kishore, U.; Khan, A. Nanoparticles for biomedical applications: An overview. Nanobiomaterials 2018, 357–384. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Lomelí-Marroquín, D.; Medina-Cruz, D.; Nieto-Argüello, A.; Crua, A.V.; Cheng, J.; Torres-Castro, A.; Webster, T.J.; Cholula-Díaz, J.L. Starch-mediated synthesis of mono- and bimetallic silver/gold nanoparticles as antimicrobial and anticancer agents. Int. J. Nanomed. 2019, 14, 2171–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium Dioxide in the Service of the Biomedical Revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Arellano-Jiménez, M.J.; Webster, T.J.; Larese-Casanova, P.; Nash, K.L. Anti-bacterial selenium nanoparticles produced by UV/VIS/NIR pulsed nanosecond laser ablation in liquids. Laser Phys. Lett. 2014, 12, 16003. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisbiers, G.; Mimun, L.C.; Mendoza-Cruz, R.; Nash, K.L. Synthesis of tunable tellurium nanoparticles. Semicond. Sci. Technol. 2017, 32, 04LT01. [Google Scholar] [CrossRef]
- Cruz, D.M.; Tien-Street, W.; Zhang, B.; Huang, X.; Crua, A.V.; Nieto-Argüello, A.; Cholula-Díaz, J.L.; Martínez, L.; Huttel, Y.; González, M.U.; et al. Citric juice-mediated synthesis of tellurium nanoparticles with antimicrobial and anticancer properties. Green Chem. 2019, 21, 1982–1998. [Google Scholar] [CrossRef]
- Vernet-Crua, A.; Medina, D.; Zhang, B.; González, M.U.; Huttel, Y.; García-Martín, J.M.; Cholula-Díaz, J.L.; Webster, T.J. Comparison of cytocompatibility and anticancer properties of traditional and green chemistry-synthesized tellurium nanowires. Int. J. Nanomed. 2019, 14, 3155–3176. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Yang, Y.; Liu, J.-W.; Yu, S.-H. Emerging tellurium nanostructures: Controllable synthesis and their applications. Chem. Soc. Rev. 2017, 46, 2732–2753. [Google Scholar] [CrossRef]
- Battersby, S. News Feature: The solar cell of the future. Proc. Natl. Acad. Sci. USA 2019, 116, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmid, H.J. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candelise, C.; Winskel, M.; Gross, R. Implications for CdTe and CIGS technologies production costs of indium and tellurium scarcity. Prog. Photovolt. Res. Appl. 2012, 20, 816–831. [Google Scholar] [CrossRef]
- Green, M.A. Rare materials for photovoltaics: Recent tellurium price fluctuations and availability from copper refining. Sol. Energy Mater. Sol. Cells 2013, 119, 256–260. [Google Scholar] [CrossRef]
- Shakibaie, M.; Adeli-Sardou, M.; Mohammadi-Khorsand, T.; Zeydabadinejad, M.; Amirafzali, E.; Amirpour-Rostami, S.; Ameri, A.; Forootanfar, H. Antimicrobial and Antioxidant Activity of the Biologically Synthesized Tellurium Nanorods; A Preliminary In vitro Study. Iran. J. Biotechnol. 2017, 15, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef] [Green Version]
- Ba, L.A.; Döring, M.; Jamier, V.; Jacob, C. Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, C.; Zhu, X.; Lin, Y.; Zhang, H.; Wu, S. Understanding the Solvent Molecules Induced Spontaneous Growth of Uncapped Tellurium Nanoparticles. Sci. Rep. 2016, 6, 32631. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Yan, J.; Huang, Y.; Wang, C.; Yang, G. The optical duality of tellurium nanoparticles for broadband solar energy harvesting and efficient photothermal conversion. Sci. Adv. 2018, 4, eaas9894. [Google Scholar] [CrossRef] [Green Version]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.C.-W.; Yamauchi, Y.; Hong, C.-Y.; Yang, Y.-H.; Liang, Y.-H.; Funatsu, T.; Tsunoda, M. Biocompatible, surface functionalized mesoporous titania nanoparticles for intracellular imaging and anticancer drug delivery. Chem. Commun. 2011, 47, 5232–5234. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Sun, H.; Baker, J.R. Improved biocompatibility of surface functionalized dendrimer-entrapped gold nanoparticles. Soft Matter 2007, 3, 71–74. [Google Scholar] [CrossRef]
- Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green Nanotechnology: Advancement in Phytoformulation Research. Medicines 2019, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; Al-Hagar, O.E.; Abdelkhalek, E.S.; Sidkey, N. Synthesis and investigations on tellurium myconanoparticles. Biotechnol. Rep. 2018, 18, e00247. [Google Scholar]
- Medina-Cruz, D.; Tien-Street, W.; Vernet-Crua, A.; Zhang, B.; Huang, X.; Murali, A.; Chen, J.; Liu, Y.; Garcia-Martin, J.M.; Cholula-Díaz, J.L.; et al. Tellurium, the Forgotten Element: A Review of the Properties, Processes, and Biomedical Applications of the Bulk and Nanoscale Metalloid. In Racing for the Surface; Springer International Publishing: Cham, Switzerland, 2020; pp. 723–783. [Google Scholar]
- Rahmani, A.H.; Aldebasi, Y.H.; Srikar, S.; Khan, A.A.; Aly, S.M. Aloe vera: Potential candidate in health management via modulation of biological activities. Pharmacogn. Rev. 2015, 9, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.; Hunter, D.; Samman, S. Evaluation of the Nutritional and Metabolic Effects of Aloe Vera; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 37–54. [Google Scholar]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The Review on Properties of Aloe Vera in Healing of Cutaneous Wounds. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bao, Z.; Ye, X.; Xie, Z.; He, K.; Mergens, B.; Li, W.; Yatcilla, M.; Zheng, Q. Chemical Investigation of Major Constituents in Aloe vera Leaves and Several Commercial Aloe Juice Powders. J. AOAC Int. 2018, 101, 1741–1751. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Logaranjan, K.; Raiza, A.J.; Gopinath, S.C.B.; Chen, Y.; Pandian, K. Shape- and Size-Controlled Synthesis of Silver Nanoparticles Using Aloe vera Plant Extract and Their Antimicrobial Activity. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vélez, E.; Campillo, G.; Morales, G.; Hincapie, C.; Osorio, J.; Arnache, O. Silver Nanoparticles Obtained by Aqueous or Ethanolic Aloe vera Extracts: An Assessment of the Antibacterial Activity and Mercury Removal Capability. J. Nanomater. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef] [PubMed]
- Beamson, D.; Briggs, G. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1992, 70, A25. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. (Eds.) Practical Surface Analysis, 2nd ed.; Auger and Photoelectron Spectroscopy; John Wiley & Sons: New York, NY, USA, 1990; Volume 1. [Google Scholar]
- Medda, S.; Hajra, A.; Dey, U.; Bose, P.; Mondal, N.K. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl. Nanosci. 2015, 5, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Beattie, I.R.; Haverkamp, R.G. Silver and gold nanoparticles in plants: Sites for the reduction to metal. Metallomics 2011, 3, 628–632. [Google Scholar] [CrossRef]
- Gentry, S.T.; Kendra, S.F.; Bezpalko, M.W. Ostwald Ripening in Metallic Nanoparticles: Stochastic Kinetics. J. Phys. Chem. C 2011, 115, 12736–12741. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Extracellular biosynthesis of gold nanoparticles using sugar beet pulp. Chem. Eng. J. 2010, 164, 92–97. [Google Scholar] [CrossRef]
- Rao, C.R.; Trivedi, D. Biphasic synthesis of fatty acids stabilized silver nanoparticles: Role of experimental conditions on particle size. Mater. Chem. Phys. 2006, 99, 354–360. [Google Scholar] [CrossRef]
- Kuponiyi, A.; Kassama, L.; Kukhtareva, T. Physicochemical characterization of silver nanoparticles synthesize using Aloe Vera (Aloe barbadensis). In Nanobiosystems: Processing, Characterization, and Applications VII; International Society for Optics and Photonics: Bellingham, WA, USA, 2014; Volume 9171, p. 91710V. [Google Scholar] [CrossRef]
- Ali, K.; Ahmed, B.; Khan, M.S.; Musarrat, J. Differential surface contact killing of pristine and low EPS Pseudomonas aeruginosa with Aloe vera capped hematite (α-Fe2O3) nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 188, 146–158. [Google Scholar] [CrossRef]
- Lim, Z.X.; Cheong, K.Y. Effects of drying temperature and ethanol concentration on bipolar switching characteristics of natural Aloe vera-based memory devices. Phys. Chem. Chem. Phys. 2015, 17, 26833–26853. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.P.; Kumar, S.; Budhwar, L.; Yadav, A.; Yadav, M. Characterization and Antibacterial Activity of Synthesized Silver and Iron Nanoparticles using Aloe vera. J. Nanomed. Nanotechnol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- El-Mallawany, R. Theoretical and experimental IR spectra of binary rare earth tellurite glasses—1. Infrared Phys. 1989, 29, 781–785. [Google Scholar] [CrossRef]
- Carotenuto, G.; Palomba, M.; De Nicola, S.; Ambrosone, G.; Coscia, U. Structural and Photoconductivity Properties of Tellurium/PMMA Films. Nanoscale Res. Lett. 2015, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The RRUFF, Tellurium—RRUFF Database: Raman, X-ray, Infrared, and Chemistry. 2019. Available online: http://rruff.info/Tellurium/R070376 (accessed on 20 November 2019).
- Champarnaud-Mesjard, J.; Blanchandin, S.; Thomas, P.; Mirgorodsky, A.P.; Merlemejean, T.; Frit, B. Crystal structure, Raman spectrum and lattice dynamics of a new metastable form of tellurium dioxide: γ-TeO2. J. Phys. Chem. Solids 2000, 61, 1499–1507. [Google Scholar] [CrossRef]
- Sal, B. Manifestation of metastable γ-TeO 2 phase in the Raman spectrum of crystals grown in synthetic opal pores. Ukr. J. Phys. Optics 2013, 14, 119–124. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; González, M.U.; Tien-Street, W.; Castro, M.F.; Vernet-Crua, A.; Fernández-Martínez, I.; Martínez, L.; Huttel, Y.; Webster, T.J.; García-Martín, J.M.; et al. Synergic antibacterial coatings combining titanium nanocolumns and tellurium nanorods. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 36–46. [Google Scholar] [CrossRef]
- John, F.; Moulder, W.F.; Stickle, P.E.; Sobol, K.D.B. (Eds.) Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data, 1st ed.; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MI, USA, 1992. [Google Scholar]
- Da Silva, R.R.; Mejia, H.A.G.; Ribeiro, S.J.L.; Shrestha, L.K.; Ariga, K.; Oliveira, O.N., Jr.; Camargo, V.R.; Maia, L.; Araújo, C.B. Facile Synthesis of Tellurium Nanowires and Study of Their Third-Order Nonlinear Optical Properties. J. Braz. Chem. Soc. 2016, 28, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.E. Bacterial tellurite resistance. Trends Microbiol. 1999, 7, 111–115. [Google Scholar] [CrossRef]
- Taylor, D.E.; Rooker, M.; Keelan, M.; Ng, L.-K.; Martin, I.; Perna, N.T.; Burland, N.T.V.; Blattner, F.R. Genomic Variability of O Islands Encoding Tellurite Resistance in Enterohemorrhagic Escherichia coli O157:H7 Isolates. J. Bacteriol. 2002, 184, 4690–4698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puzyn, T.; Rasulev, B.; Gajewicz, A.; Hu, X.; Dasari, T.P.; Michalkova, A.; Hwang, H.-M.; Toropov, A.A.; Leszczynska, D.; Leszczynski, J. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat. Nanotechnol. 2011, 6, 175–178. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Genet. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.-S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Valencia, A.; Morán, J. Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free. Radic. Biol. Med. 2004, 36, 1112–1125. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Ke, H.; Wang, Q.; Tang, Y.; Deng, Y.; Yang, H.; Yang, X.; Yang, P.; Ling, D.; Chen, C.; et al. Bifunctional Tellurium Nanodots for Photo-Induced Synergistic Cancer Therapy. ACS Nano 2017, 11, 10012–10024. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Rivolta, I.; Panariti, A.; Miserocchi, G. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralj, S.; Rojnik, M.; Romih, R.; Jagodič, M.; Kos, J.; Makovec, D. Effect of surface charge on the cellular uptake of fluorescent magnetic nanoparticles. J. Nanoparticle Res. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Singh, R.P.; Poduri, R. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef]
- Treuel, L.; Jiang, X.; Nienhaus, G.U. New views on cellular uptake and trafficking of manufactured nanoparticles. J. R. Soc. Interface 2013, 10, 20120939. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Andriesh, A.; Bertolotti, M. (Eds.) Physics and Applications of Non-Crystalline Semiconductors in Optoelectronics; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Adenis, C.; Langer, V.; Lindqvist, O. Reinvestigation of the structure of tellurium. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 941–942. [Google Scholar] [CrossRef]
- Ahmed, M.; Hussain, F. Chemical Composition and Biochemical Activity of Aloe vera (Aloe barbadensis Miller) Leaves. Available online: www.iscientific.org/Journal.html (accessed on 6 November 2019).
- Shelton, R.M. Aloe Vera: Its Chemical and Therapeutic Properties. Int. J. Dermatol. 1991, 30, 679–683. [Google Scholar] [CrossRef]
- Kong, H.; Yeo, J.-B.; Lee, H.-Y. A Study on the properties of tellurium-oxide thin films based on the variable sputtering gas ratio. J. Korean Phys. Soc. 2015, 66, 1744–1749. [Google Scholar] [CrossRef]
- Garcia, R.; Losilla, N.S.; Martínez, J.; Martinez, R.V.; Palomares, F.J.; Huttel, Y.; Calvaresi, M.; Zerbetto, F. Nanopatterning of carbonaceous structures by field-induced carbon dioxide splitting with a force microscope. Appl. Phys. Lett. 2010, 96, 143110. [Google Scholar] [CrossRef] [Green Version]
- Nevshupa, R.; Martínez, L.; Álvarez, L.; López, M.F.; Huttel, Y.; Méndez, J.; Román, E. Influence of thermal ageing on surface degradation of ethylene-propylene-diene elastomer. J. Appl. Polym. Sci. 2010, 119, 242–251. [Google Scholar] [CrossRef]
- Huang, L. Optimization of a new mathematical model for bacterial growth. Food Control. 2013, 32, 283–288. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Wang, S.; Meredith, H.R.; Zhuang, B.; Dai, Z.; You, L. Robust, linear correlations between growth rates and β-lactam–mediated lysis rates. Proc. Natl. Acad. Sci. USA 2018, 115, 4069–4074. [Google Scholar] [CrossRef] [Green Version]







| Wavenumber (cm−1) | Vibrational Modes | References |
|---|---|---|
| 3280 | -OH (phenols and alcohols) | [58] |
| 2920 | -CH & -CH2 (aliphatic groups) | [57] |
| 2160–2030 | alkynyl C=C stretch | [58,59] |
| 1580 | C=C (aromatic rings) | [58] |
| 1416 | -COO- (carboxylate groups) | [57] |
| 1060 | C-O (rhamnogalacturonan) | [57] |
| 1030 | C-N (aliphatic amines) | [58] |
| 870 | C-H (monosaccharides) | [57] |
| 687–610 | Te-O | [60,61] |
| Technique | C %at | O %at | Te %at | Ca %at | Na %at |
|---|---|---|---|---|---|
| XPS | 36.4 | 35.8 | 20 | 4.3 | 3.5 |
| Drug-Resistant Bacterium | EC50 (µg/mL) | MIC (µg/mL) |
|---|---|---|
| MDR E. coli | 1.9 | 3.53 |
| MRSA | 2.4 | 11.61 |
| Exposed Cells | 24 h | 48 h |
|---|---|---|
| HDF | 70.89 | 71.35 |
| Melanoma | 2.761 | 4.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Cruz, D.; Vernet-Crua, A.; Mostafavi, E.; González, M.U.; Martínez, L.; III, A.-A.D.J.; Kusper, M.; Sotelo, E.; Gao, M.; Geoffrion, L.D.; et al. Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects. Nanomaterials 2021, 11, 514. https://doi.org/10.3390/nano11020514
Medina-Cruz D, Vernet-Crua A, Mostafavi E, González MU, Martínez L, III A-ADJ, Kusper M, Sotelo E, Gao M, Geoffrion LD, et al. Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects. Nanomaterials. 2021; 11(2):514. https://doi.org/10.3390/nano11020514
Chicago/Turabian StyleMedina-Cruz, David, Ada Vernet-Crua, Ebrahim Mostafavi, María Ujué González, Lidia Martínez, A-Andrew D. Jones III, Matthew Kusper, Eduardo Sotelo, Ming Gao, Luke D. Geoffrion, and et al. 2021. "Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects" Nanomaterials 11, no. 2: 514. https://doi.org/10.3390/nano11020514
APA StyleMedina-Cruz, D., Vernet-Crua, A., Mostafavi, E., González, M. U., Martínez, L., III, A.-A. D. J., Kusper, M., Sotelo, E., Gao, M., Geoffrion, L. D., Shah, V., Guisbiers, G., Cholula-Díaz, J. L., Guillermier, C., Khanom, F., Huttel, Y., García-Martín, J. M., & Webster, T. J. (2021). Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects. Nanomaterials, 11(2), 514. https://doi.org/10.3390/nano11020514











