Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers
Abstract
1. Introduction
- ✓
- formation of organic polymers in presence of preformed inorganic materials;
- ✓
- formation of organic polymers in presence of preformed inorganic materials;
- ✓
- simultaneous formation of both components; and,
- ✓
- building block approach: inorganic and organic building blocks.
- ✓
- To obtain polymer nanocomposites, various processes are used:
- ✓
- mixing of dispersed particles with polymers in liquids;
- ✓
- mixing of particles with monomers followed by polymerization;
- ✓
- nanocomposite formation by means of molten or solid polymers; and,
- ✓
- concomitant formation of particles and polymers.
2. Results
- ➢
- transportation: facilitate replacement of gasoline powered passenger, military, and mass transit vehicles with Hybrid electric vehicles (HEVs), Plug-in hybrid electric vehicles (PHEVs), and, ultimately, all-electric vehicles; and,
- ➢
- utilities: safe and reliable stationary energy storage.
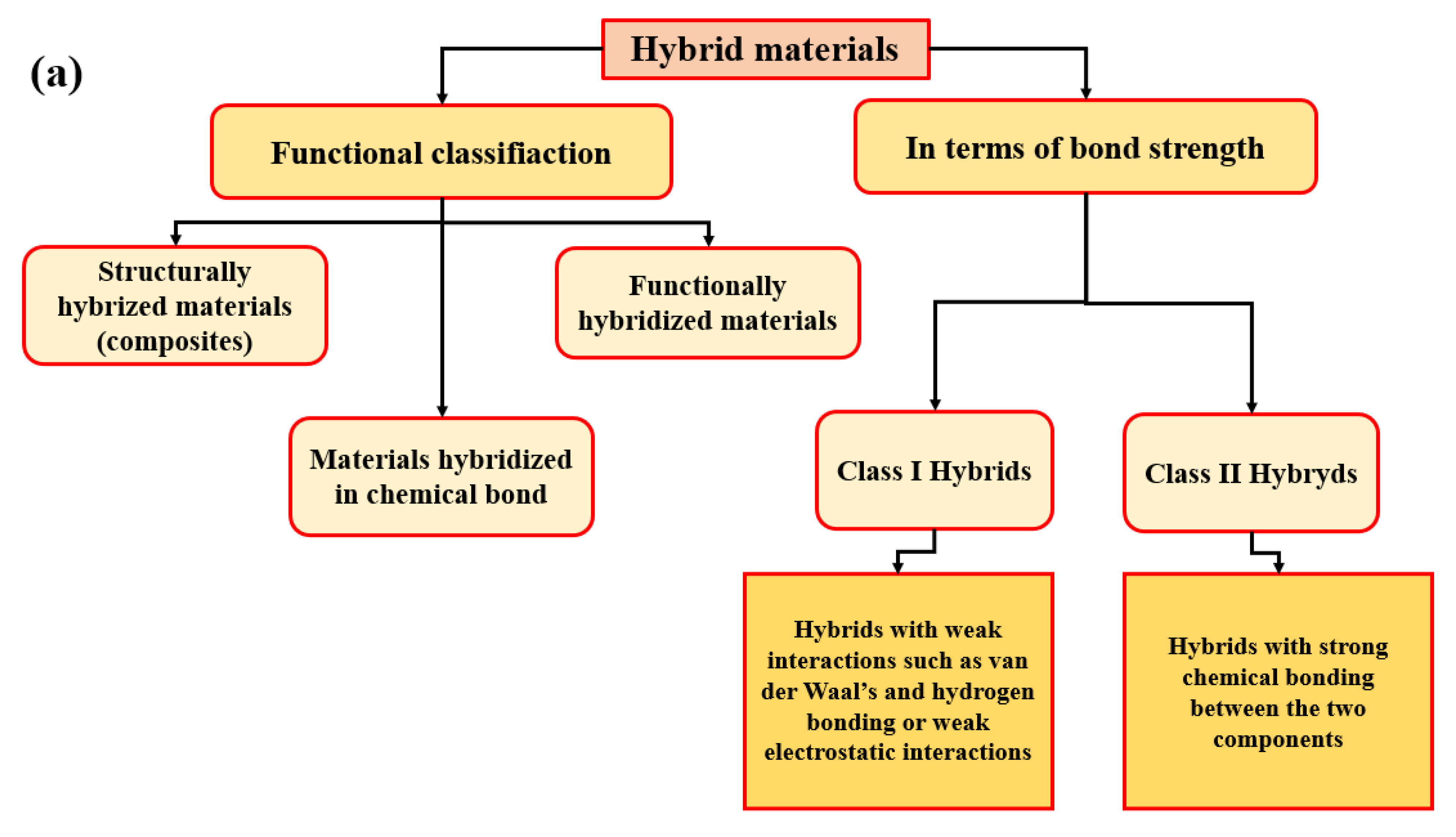
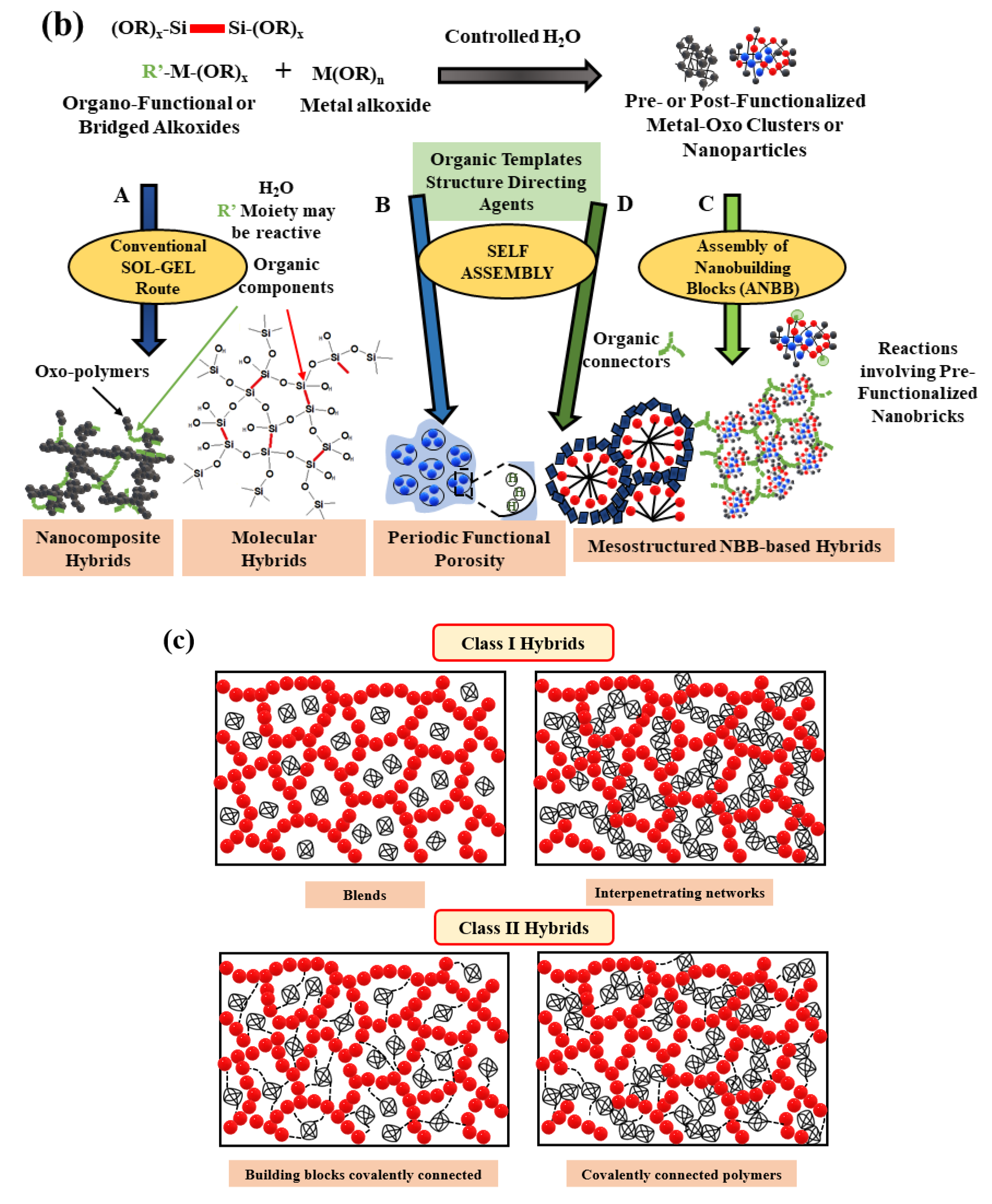
2.1. Hybrid Materials
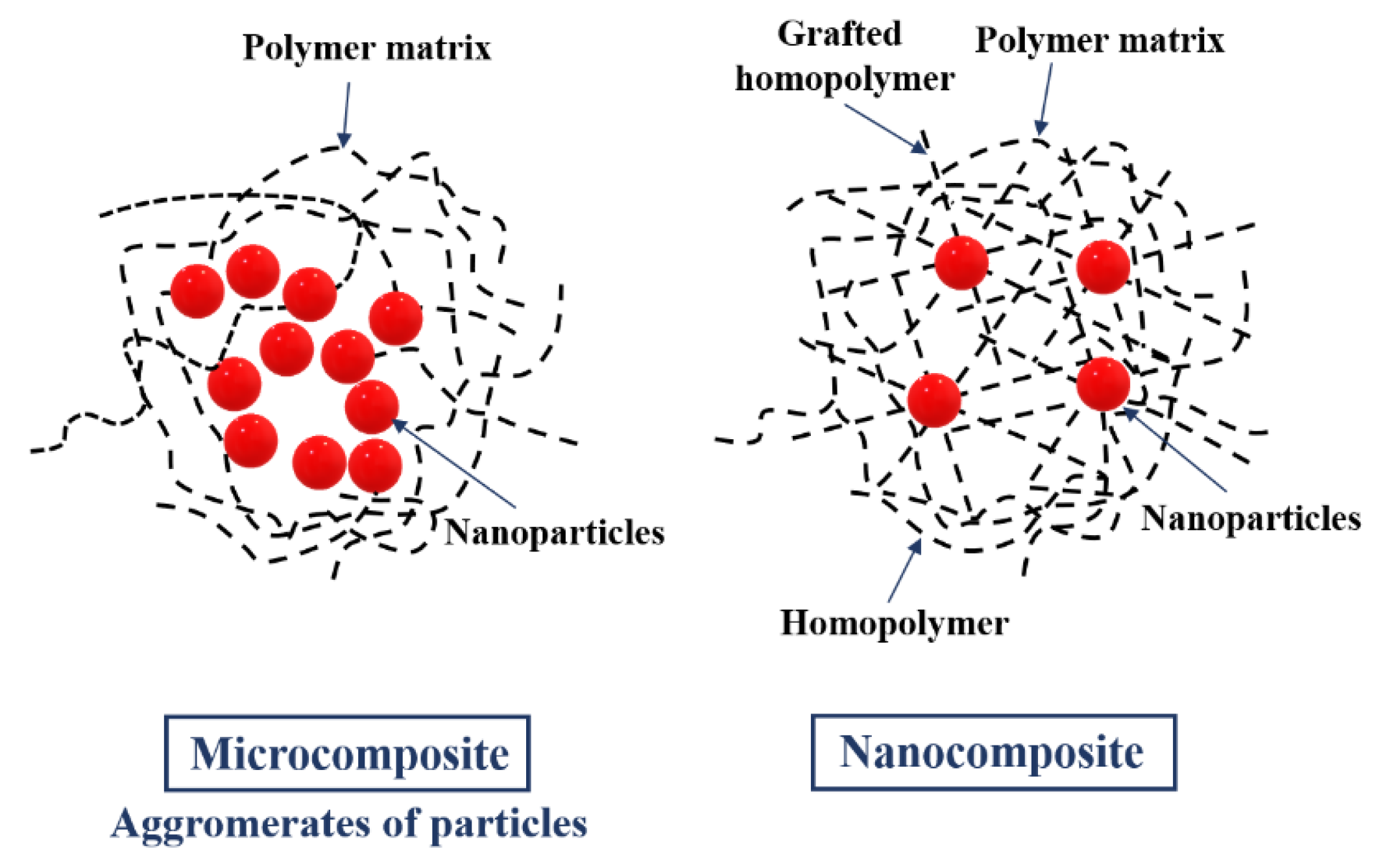
2.2. Polymeric Nanocomposites
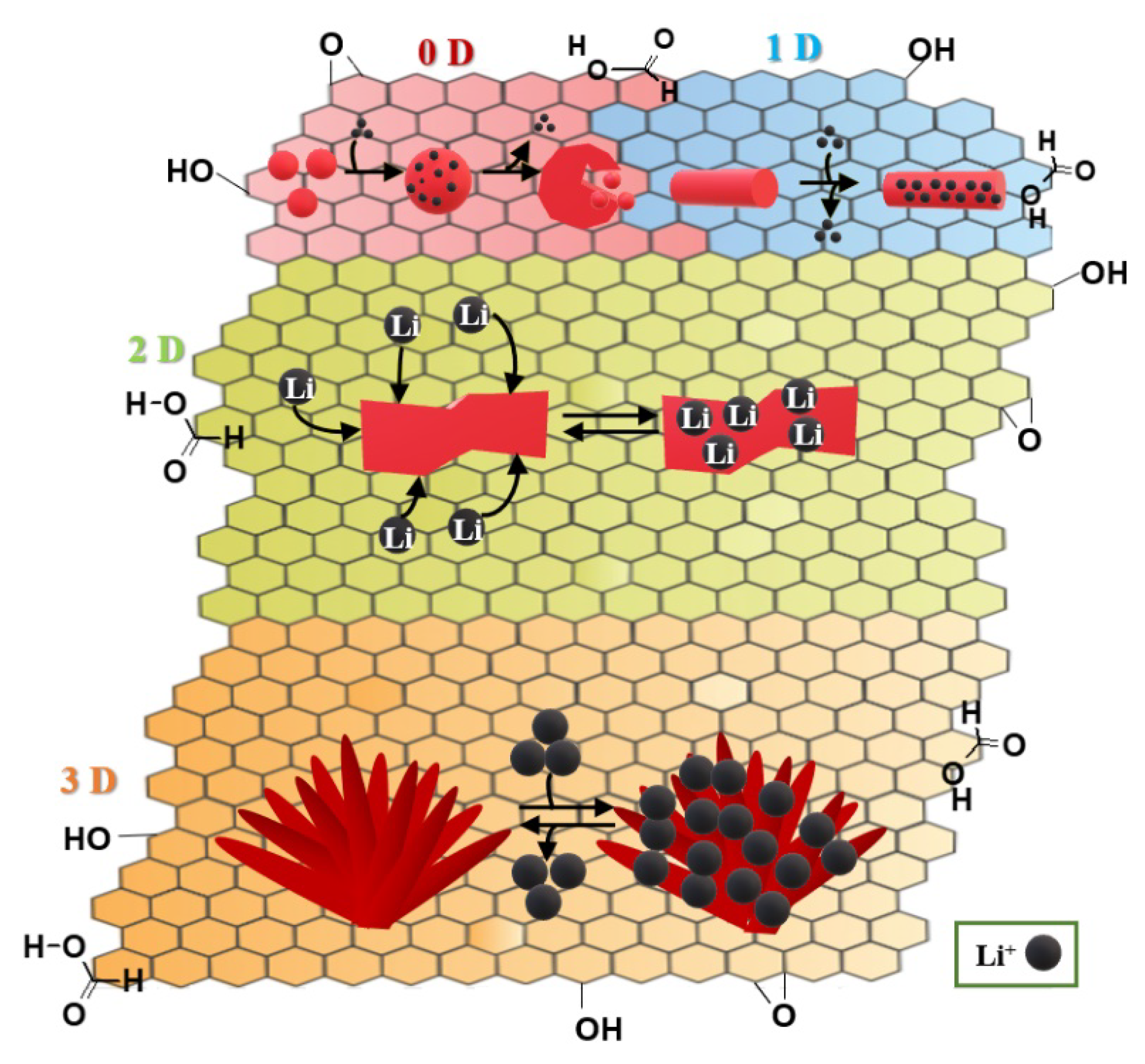
2.3. Nanocomposites for Lithium-Ion Cells
2.3.1. Anode Materials
Graphene-Based Nanocomposites
Si-Based Nanocomposites
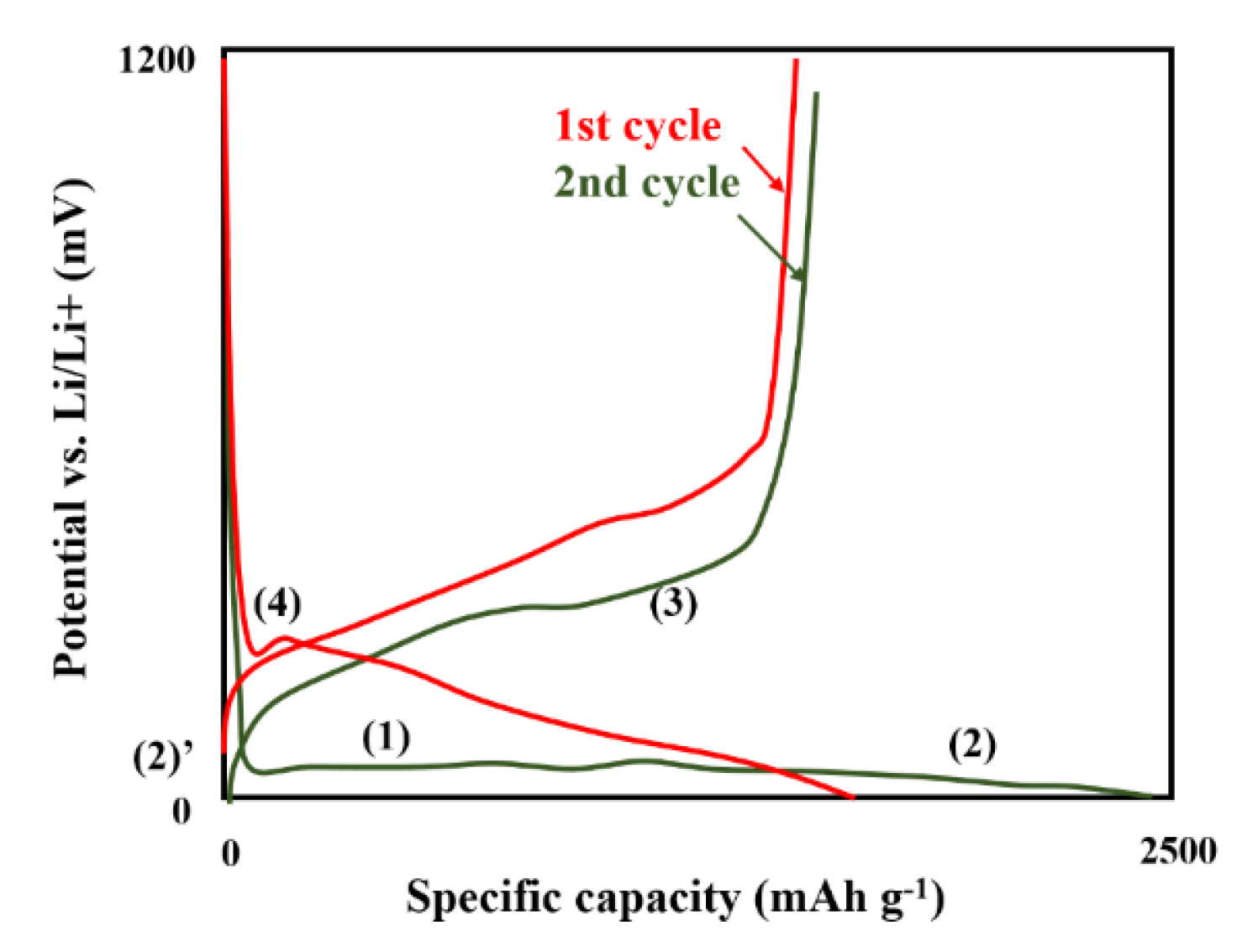
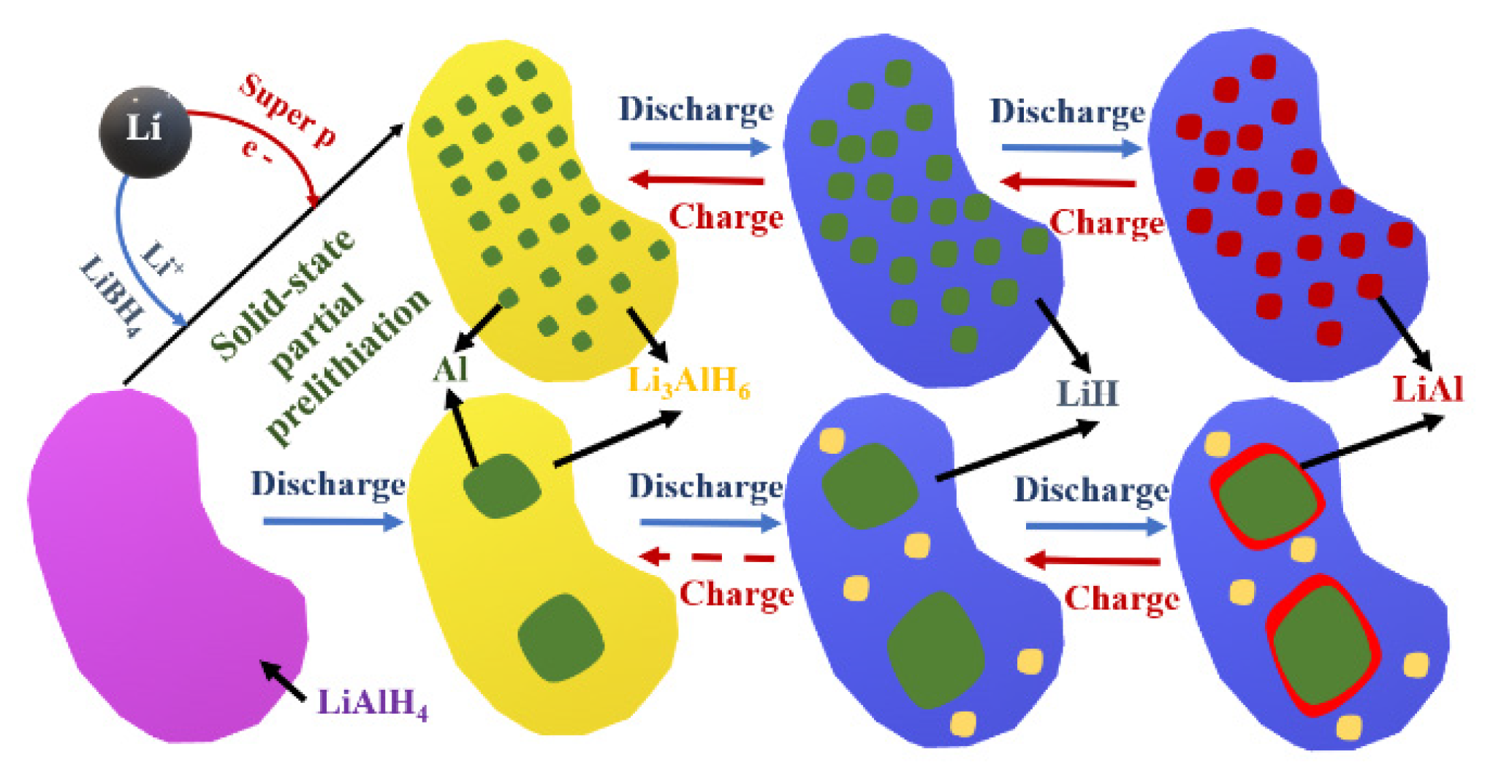
Li3AlH6-Al-Based Nanocomposites
Cobalt-Based Mesoporous Nanocomposites
SnO2-Based Nanocomposites
Lignocellulosic Biomass-Based Nanocomposites
Polymer-Based Nanocomposites
2.3.2. Cathode Materials
- ❖
- contain an ion easily undergoing redox reaction, e.g., a transition metal ion;
- ❖
- have a high redox potential of the intercalated compound with respect to lithium. To achieve high voltage, the transition metal should have a high degree of oxidation;
- ❖
- capable of a high speed and reversible lithium intercalation/deintercalation process to ensure long cell life;
- ❖
- be able to reversibly incorporate a large amount of lithium (at least one atom per metal atom) into available places in the material structure to maximize the cell capacity;
- ❖
- it is characterized by high electronic and ionic conductivity, which allows achieving minimal polarization losses during the processes of charging and discharging the battery and achieving good efficiency of the cell;
- ❖
- be chemically stable. The electrode compound should not decompose under the cell’s operating conditions or react with the electrolyte; and,
- ❖
- in addition, the cathode material should not be expensive, difficult to synthesize, toxic, and harmful to the environment.
Graphene-Based Nanocomposites
LiF-Fe Nanocomposites
VOxNTs-Polyaniline Nanocomposites
Carbon-Polymer Composites
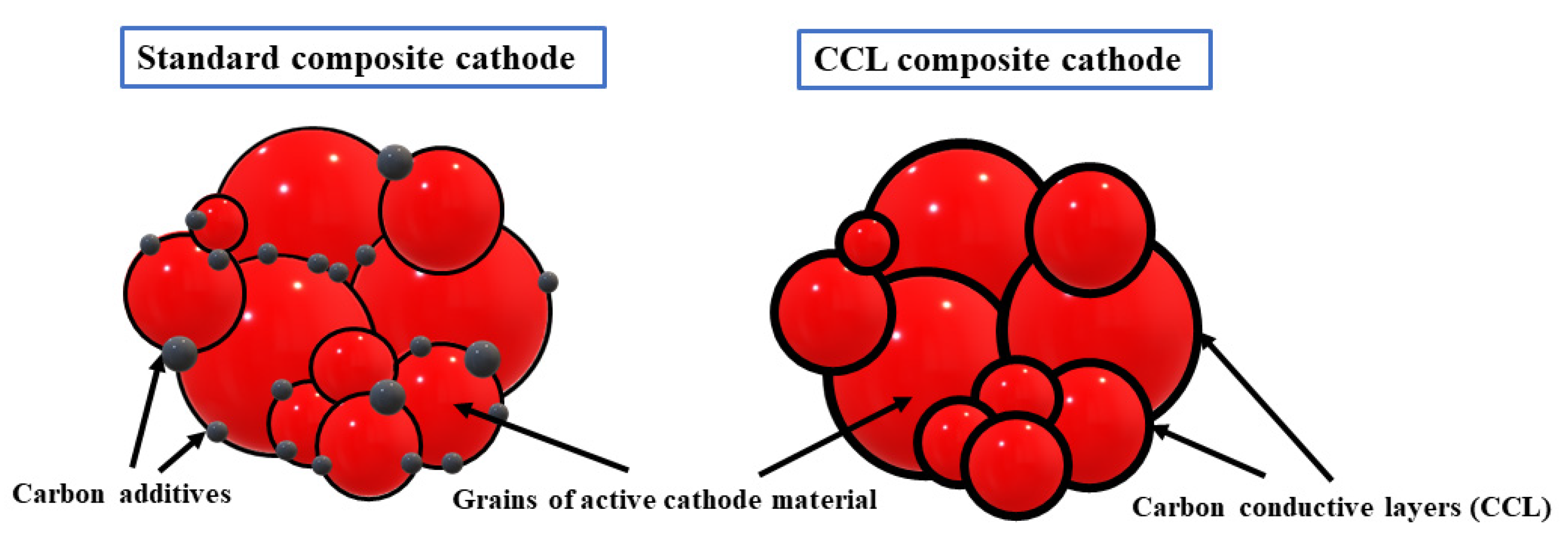
Nanocomposites with Self-Assembled Conductive Carbon Layers (CCL)
2.4. Sodium-Ion Cells
2.5. Supercapacitors
2.5.1. NiO-TiO2 Nanocomposites
2.5.2. Bi2O3-MnO2 Nanocomposites
2.5.3. Fe3O4@FeS2 Nanocomposites
2.5.4. RuO2-Based Nanocomposites
- ❖
- RuO2-based mixed metal oxide nanocomposites are used to reduce the loading of expensive RuO2 resulting in smaller capacitance (NiO/RuO2 nanocomposite—specific capacitance of 210 F g−1 at 5 mA cm−2 [110]; TiO2/RuO2 nanocomposites—good electrochemical results ~990 F g−1 at a scan rate of 100 mV s−1 [111]; RuO2-Mn3O4 composite nanofiber-mats exhibited gravimetric capacitance of 293 F g−1 at 10 mV s−1; RuO2/TiO2 nano-tubular composite achieved a capacitance as high as 1263 F g−1 [112]);
- ❖
- RuO2-based conducting polymer nanocomposites used because of tunable electronic properties (RuO2/polyaniline exhibited specific capacitance of 708 F g−1 at 5 mV s−1 [113]; porous PANI–763 RuO2 composite with a capacitance 664 F g−1 at the scan rate of 5 mVs−1 [114]; RuO2 based PEDOT-PSS (poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonic acid)) that achieved a maximum gravimetric capacitance of 653 F g−1 [115]);
- ❖
- RuO2-based activated porous carbon nanocomposites used to achieve an improved conductivity and charge-storage efficiencies (hydrous-RuO2 with activated carbon nanocomposite exhibited a specific capacitance of 319.3 F g−1 at current density of 1 A g−1 [116]; carbon nano-onion-based RuO2 composites with the capacitance of 570 F g−1 [117]);
- ❖
- RuO2-based CNT nanocomposites to improve a chemical stability and mechanical strength and decrease the weight (RuO2 nanoparticles/MWCNT with capacitance of 450 F g−1 at 10 mV s−1 synthesized via the microwavepolyol process and via electrodeposition-sinthesized nanocomposite achieved even 1652 F g−1 at 10 mV s−1 [118,119]);
- ❖
- RuO2-based functionalized graphene binary composites (RuO2/reduced graphene oxide nanoribbon composite achieved a gravimetric capacitance of 677 F g−1 at current density of 1 A g−1 [120]; RuO2/graphene monolith attained a really huge volumetric capacitance of 1485 F cm−3 recorded at 0.1 A g−1 [121]); and,
- ❖
- RuO2-based ternary composites (Graphene/RuO2/Co3O4 nanocomposites with a specific capacitance of 715 F g−1 at current of 1 A g−1 [122]).
2.5.5. Graphene-Gold Nanoparticle-Based Nanocomposites
2.5.6. Graphene Sheets-Cotton Cloth Nanocomposites
2.5.7. Graphene-NiFe2O4 Nanocomposites
2.5.8. Graphene-Mn-MoO4 Nanocomposites
2.5.9. Titanium Dioxide/Graphene Oxide
2.5.10. SnO2-Carbon Nanocomposites
2.5.11. Polymer Nanocomposites
2.6. Nanocomposites for Fuel Cells
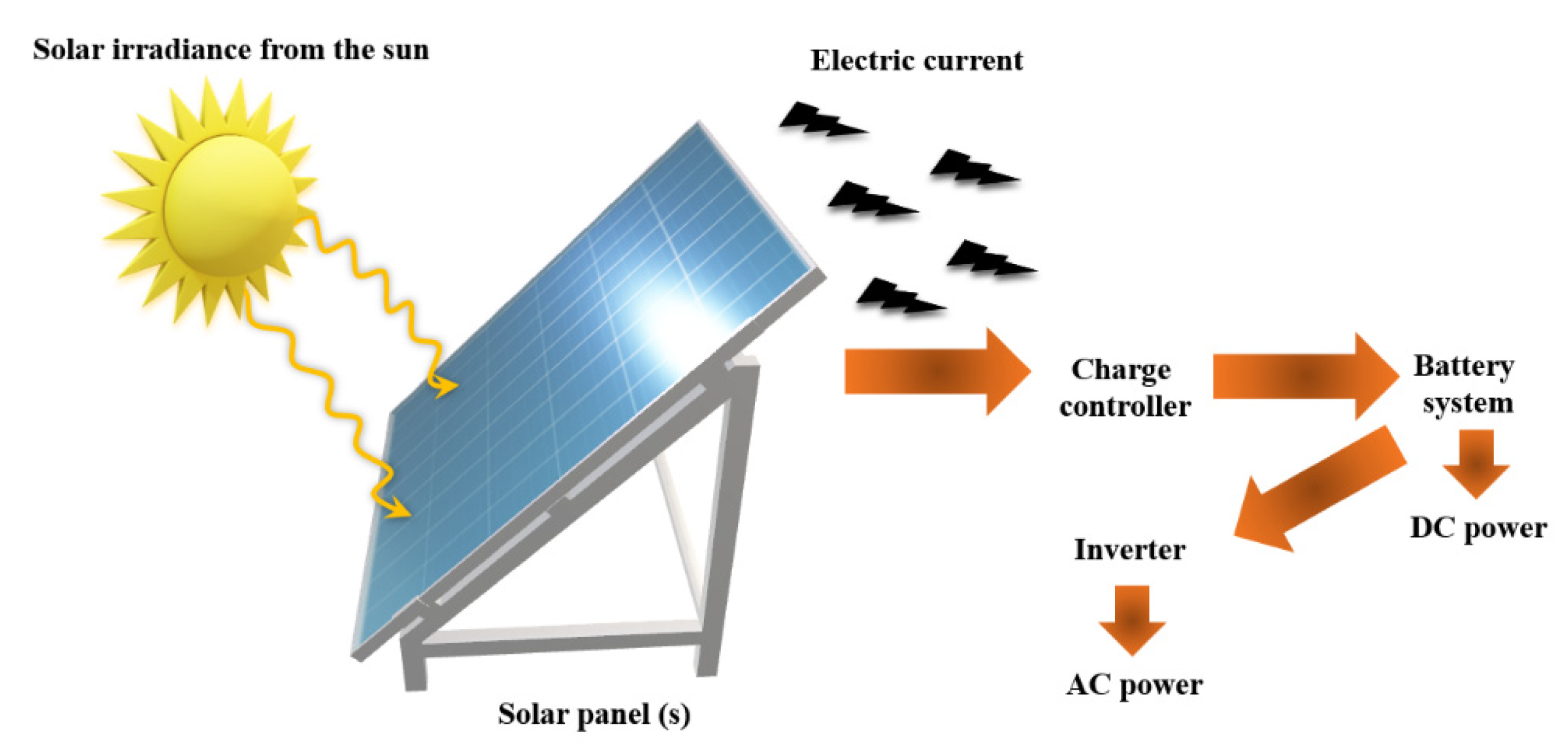
2.7. Solar Cells
2.8. Nanocomposite Application in Flexible Energy Storage and Generation Device Application
2.9. Safety of Energy Carriers
- ➢
- measurement of system voltage,
- ➢
- current and temperature,
- ➢
- cell charge level,
- ➢
- cell protection, temperature management,
- ➢
- controlling the loading/unloading procedure,
- ➢
- data acquisition,
- ➢
- communication with internal and external modules, and
- ➢
- monitoring and storage of past data.
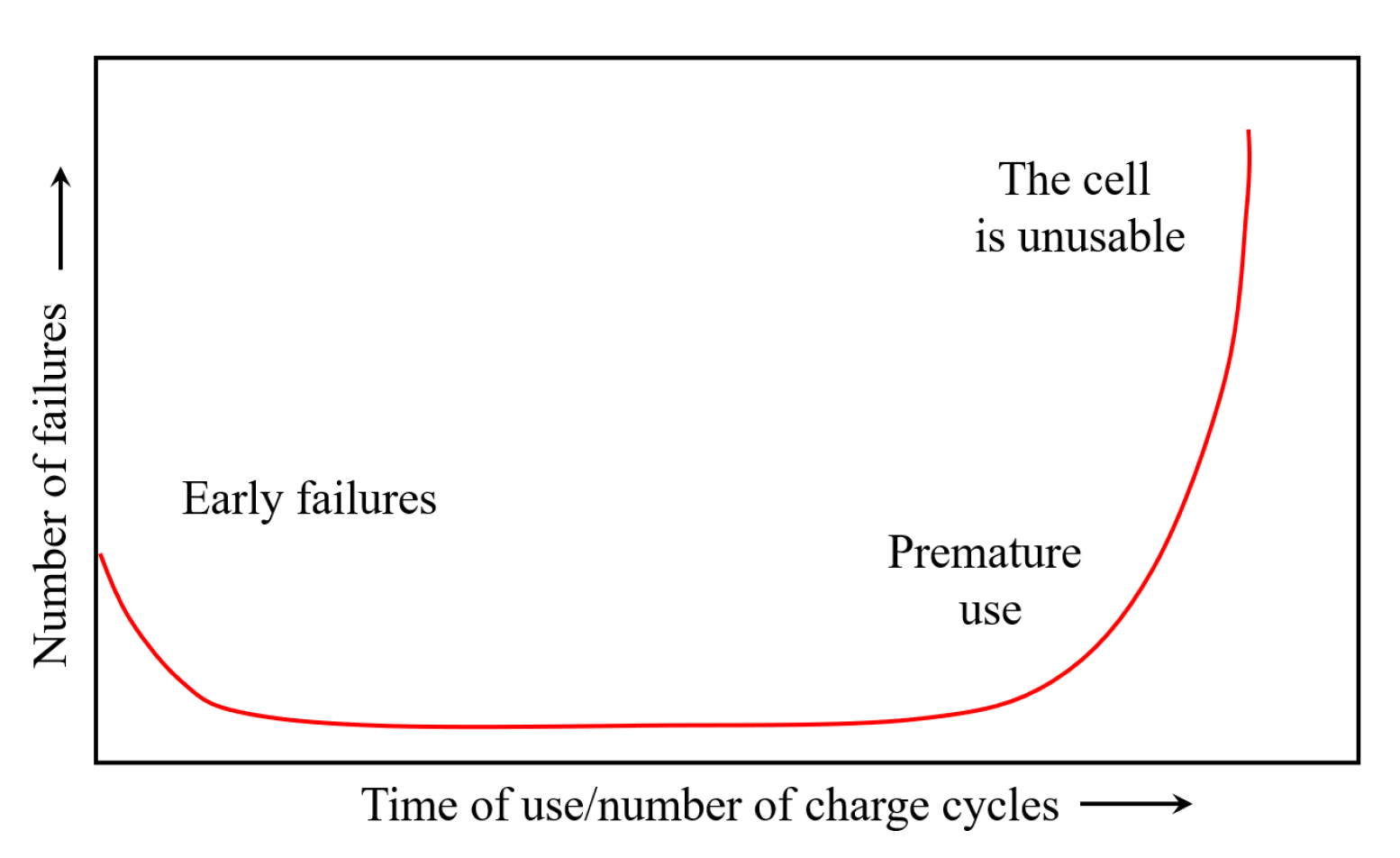
2.10. Electrode Degradation
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kickelbick, G. Introduction to Hybrid Materials, Hybrid Materials: Synthesis, Characterization, and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007. [Google Scholar]
- Currie, H.A.; Patwardhan, S.V.; Perry, C.; Roach, P.; Shirtcliff, N.J. Natural and Artificial Hybrid Biomaterials, Characterization, and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007; pp. 285–290. [Google Scholar]
- Hossain, S.K.S.; Hoque, M.E. Polymer Nanocomposite Materials in Energy Storage: Properties and Applications Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 239–282. [Google Scholar]
- Iro, Z.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Wang, T.; Su, D.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. Electrode Materials for Sodium-Ion Batteries: Considerations on Crystal Structures and Sodium Storage Mechanisms. Electrochem. Energy Rev. 2018, 1, 200–237. [Google Scholar] [CrossRef]
- Liu, R. Hybrid Organic/Inorganic Nanocomposites for Photovoltaic Cells. Materials 2014, 7, 2747–2771. [Google Scholar] [CrossRef]
- Sarasini, F. 7—Low-velocity impact behaviour of hybrid composites, Hybrid Polymer Composite Materials, Properties and Characterization. Elsevier 2017, 151–168. [Google Scholar] [CrossRef]
- Singh, A.; Verma, N.; Kumar, K. Chapter 2—Hybrid composites: A revolutionary trend in biomedical engineering: Bioactive Materials for Biomedical Engineering, Bioactive Materials, Properties, and Applications. Elsevier 2019, 33–46. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Sanchez, C. Hybrid Materials, Functional Applications. An Introduction; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2004; pp. 1–14. [Google Scholar] [CrossRef]
- Iurzhenko, M. Electrical, Thermomechanical and Sorption Properties of Hybrid Organic-Inorganic Systems Based on Urethane Oligomers and Silicates. Ph.D. Thesis, Université ClaudeBernard-Lyon I, Villeurbanne, France, 2009; pp. 1–149. [Google Scholar]
- Admassie, S.; Ajjan, F.N.; Elfwinga, A.; Inganäs, O. Biopolymer hybrid electrodes for scalable electricity storage. Mater. Horiz. 2016, 3, 174–185. [Google Scholar] [CrossRef]
- Chen, G.Z. Understanding supercapacitors based on nano-hybrid materials with interfacial conjugation. Prog. Nat. Sci. Mater. Int. 2013, 23, 245–255. [Google Scholar] [CrossRef]
- Choi, E.; Chae, S.J.; Kim, A.; Kang, K.W.; Oh, M.S.; Kwon, S.H.; Yoon, S.P.; Pyo, S.G. Hybrid Electrodes of Carbon Nanotube and Reduced Graphene Oxide for Energy Storage Applications. J. Nanosci. Nanotechnol. 2015, 15, 9104–9109. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, K.; Lira-Cantú, M.; Casañ-Pastor, N.; Asensio, J.A. Hybrid Materials Approach in the Design of Electrodes and Electrolytes for Energy Storage and Conversion. MRS Online Proc. Library Arch. 2004, 847. [Google Scholar] [CrossRef]
- Malak, A.; Fic, K.; Lota, G. Hybrid materials for supercapacitor application. J. Solid State Electrochem. 2010, 14, 811–816. [Google Scholar] [CrossRef]
- Schmidt, S.; Sheptyakov, D.; Jumas, J.C.; Medarde, M.; Benedek, P.; Novák, P.; Villevieille, C. Lithium iron methylenediphosphonate: A model material for new organic-inorganic hybrid positive electrode materials for Li ion batteries. Chem. Mater. 2015, 27, 7889–7895. [Google Scholar] [CrossRef]
- Wei, C.; He, W.; Zhang, X.; Shen, J.; Ma, J. Recent progress in hybrid cathode materials for lithium ion batteries. New J. Chem. 2016, 40, 2984–2999. [Google Scholar] [CrossRef]
- Fleischmann, S.; Tolosa, A.; Zeiger, M.; Krüner, B.; Peter, N.J.; Grobelsek, I.; Quade, A.; Kruthd, A.; Presser, V. Vanadia–titania multilayer nanodecoration of carbon onions via atomic layer deposition for high performance electrochemical energy storage. J. Mater. Chem. A 2017, 5, 2792–2801. [Google Scholar] [CrossRef]
- Hu, X.; Yan, Z.; Li, Q.; Yang, Q.; Kang, L.; Lei, Z.; Liu, Z.-H. Graphene/vanadium oxide hybrid electrodes for electrochemical capacitor. Coll. Surf. A Physicochem. Eng. Asp. 2014, 461, 105–112. [Google Scholar] [CrossRef]
- Wang, J.; Ran, R.; Tade, M.O.; Shao, Z. Self-assembled mesoporous TiO2/carbon nanotube composite with a three-dimensional conducting nanonetwork as a high-rate anode material for lithium-ion battery. J. Power Sour. 2014, 254, 18–28. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Szalaty, T.J.; Kurc, B.; Stanisz, M.; Skrzypczak, A.; Jesionowski, T. Functional Hybrid Materials Based on Manganese Dioxide and Lignin Activated by Ionic Liquids and Their Application in the Production of Lithium Ion Batteries. Int. J. Mol. Sci. 2017, 18, 1509. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Singh, G.; Cha, W.; Kim, S.; Yi, J.B.; Hwang, S.-J.; Vinu, A. Recent Advances in Developing Hybrid Materials for Sodium Ion Battery Anodes. ACS Energy Lett. 2020, 5, 1939–1966. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.; Pasta, M. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Ashish, A.G.; Arunkumar, P.; Babu, B.; Manikandan, P.; Sarang, S.; Shaijumon, M.M. TiNb2O7/Graphene hybrid material as high performance anode for lithium-ion batteries. Electrochim. Acta 2015, 176, 285–292. [Google Scholar] [CrossRef]
- Hao, J.; Yang, F.; Zhang, S.; He, H.; Xia, G.; Liu, Y.; Guo, Z. Designing a hybrid electrode toward high energy density with a staged Li+ and PF6− deintercalation/intercalation mechanism. Proc. Natl. Acad. Sci. USA 2020, 117, 2815–2823. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Aguzzi, C.; Viseras, C.; Caramella, C. Clay minerals for tissue regeneration, repair, and engineering. Wound Heal. Biomater. 2016, 385–402. [Google Scholar] [CrossRef]
- Said, R.A.M.; Hasan, M.A.; Abdelzaher, A.M.; Abdel-Raoof, A.M. Review—Insights into the Developments of Nanocomposites for Its Processing and Application as Sensing Materials. J. Electrochem. Soc. 2020, 167, 037549. [Google Scholar] [CrossRef]
- Duz, I.; Guner, S.B.; Erdem, O.; Demir, I.; Kapucu, V.; Çelik, Ş.; Kemal, O.; Hossain, S.; Gencer, A.; Yanmaz, E. Comparison of Levitation Forces of Bulk MgB2 Superconductors Produced by Nano Boron and Carbon-Doped Nano Boron. J. Supercond. Novel Magn. 2014, 27, 2241–2247. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, A.; Liu, B.; Xing, S.; Wu, X.; Xu, J. High cyclic performance of V2O5@PPy composite as cathode of recharged lithium batteries. J. Appl. Electrochem. 2012, 42, 139–144. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.F.; Lin, J. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 5794–5800. [Google Scholar] [CrossRef]
- Han, X.; Chang, C.; Yuan, L.; Sun, T.; Sun, J. Aromatic carbonyl derivative polymers as high performance Li-ion storage materials. Adv. Mater. 2007, 19, 1616–1621. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.H.; Li, Z.P. A reduced graphene oxide/SnO2/polyaniline nanocomposite for the anode material of Li-ion batteries. Solid State Ionics 2016, 294, 6–14. [Google Scholar] [CrossRef]
- Zheng, H.; Ncube, N.M.; Raju, K.; Mphahlele, N.; Mathe, M. The effect of polyaniline on TiO2 nanoparticles as anode materials for lithium ion batteries. SpringerPlus 2016, 5, 630. [Google Scholar] [CrossRef]
- Kummer, M.; Badillo, J.P.; Schmitz, A.; Bremes, H.-G.; Winter, M.; Schulz, C. Silicon/polyaniline nanocomposites as anode material for lithium ion batteries. J. Electrochem. Soc. 2014, 161, A40–A45. [Google Scholar] [CrossRef]
- Huang, F.; Lou, F.; Chen, D. Exploring aligned-carbon-nanotubes@polyaniline arrays on household Al as supercapacitors. ChemSusChem 2012, 5, 888–895. [Google Scholar] [CrossRef]
- Jurewicz, K.; Delpeux, S.; Bertagna, V.; Beguin, F.; Frąckowiak, E. Supercapacitors from nanotubes/polypyrrole composites. Chem. Phys. Lett. 2001, 347, 36–40. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically synthesized graphene/ polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Sun, X.; Gan, M.; Ma, L.; Wang, H.; Zhou, T.; Wang, S. Fabrication of PANI-coated honeycomb-like MnO2 nanospheres with enhanced electrochemical performance for energy storage. Electrochim. Acta 2015, 180, 977–982. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2020, 1–64. [Google Scholar] [CrossRef]
- Kurc, B.; Wysokowski, M.; Rymaniak, L.; Lijewski, P.; Piasecki, A.; Fuć, P. The impact of the vanadium oxide addition on the physicochemical performance stability and intercalation of lithium ions of the TiO2-rGO-electrode in lithium ion batteries. Materials 2020, 13, 1018. [Google Scholar] [CrossRef]
- Zhu, J.; Duan, R.; Zhang, S.; Jiang, N.; Zhang, Y.; Zhu, J. The application of graphene in lithium ion battery electrode materials. SpringerPlus 2014, 3, 585. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y. Graphene-based nanocomposite anodes for lithium-ion batteries. Nanoscale 2014, 6, 11528–11552. [Google Scholar] [CrossRef] [PubMed]
- Siwińska-Ciesielczyk, K.; Kurc, B.; Rymarowicz, D.; Kubiak, A.; Piasecki, A.; Moszyński, D.; Jesionowski, T. Crystallization of TiO2-MoS2 hybrid material under hydrothermal treatment and its electrochemical performance. Materials 2020, 13, 1–21. [Google Scholar]
- Cen, Y.; Sisson, R.D.; Qin, Q. Current Progress of Si/Graphene Nanocomposites for Lithium-Ion Batteries. J. Carbon Res. 2018, 4, 18. [Google Scholar] [CrossRef]
- Kim, H.; Lee, E.-J.; Sun, Y.-K. Recent advances in the Si-based nanocomposite materials as high capacity anode materials for lithium ion batteries. Mater. Today 2014, 17, 285–297. [Google Scholar] [CrossRef]
- Munaò, D.; Valvo, M.; Van Erven, J.; Kelder, E.M.; Hassoun, J.; Panero, S. Silicon-based nanocomposite for advanced thin film anodes in lithium-ion batteries. J. Mater. Chem. 2012, 22, 1556. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Sun, S.; Yang, L. The preparation and characterization of SnO2/rGO nanocomposites electrode materials for supercapacitor. Adv. Compos. Lett. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, X.; Shi, X.; Xu, F.; Sun, L.; Yang, J.; Zheng, S. Solid-State Prelithiation Enables High-Performance Li-Al-H Anode for Solid-State Batteries. Adv. Energy Mater. 2020, 10, 1902795. [Google Scholar] [CrossRef]
- Saad, A.; Cheng, Z.; Shen, H. Recent Advances in Nanocasting Cobalt-Based Mesoporous Materials for Energy Storage and Conversion. Electrocatalysis 2021, 11, 465–484. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, X.; Yang, M.; Wu, L.; Wen, Z.; Shen, X. Hierarchically ordered mesoporous Co3O4 materials for high performance Li-ion batteries. Sci. Rep. 2016, 19, 19564. [Google Scholar] [CrossRef]
- Shijiao, S.; Zhaoyin, W.; Jun, J.; Yanming, C.; Yan, L. Synthesis of ordered mesoporous CuCo2O4 with different textures as anode material for lithium ion battery. Micropor. Mesopor. Mat. 2013, 169, 242–247. [Google Scholar]
- Zhao, R.; Li, Q.; Wang, C.; Yin, L. Highly ordered mesoporous spinel ZnCo2O4 as a high-performance anode material for lithium-ion batteries. Electrochim. Acta 2016, 197, 58–67. [Google Scholar] [CrossRef]
- Ette, P.M.; Selvakumar, K.; Kumar, S.M.S.; Ramesha, K. Ordered 1D and 3D mesoporous Co3O4 structures: Effect of morphology on Li-ion storage and high rate performance. Electrochim. Acta 2019, 310, 184–194. [Google Scholar] [CrossRef]
- Kebede, M.A. Tin oxide–based anodes for both lithium-ion and sodium-ion batteries. Curr. Opin. Electrochem. 2020, 21, 182–187. [Google Scholar] [CrossRef]
- Wei, D.; Zhong, S.; Zhang, H.; Zhang, X.; Zhu, C.; Duan, J.; Li, L.; Chen, Z.; Liu, P.; Zhang, G.; et al. In situ construction of interconnected SnO2/nitrogen-doped Carbon@TiO2 networks for lithium-ion half/full cells. Electrochim. Acta 2018, 290, 312–321. [Google Scholar] [CrossRef]
- Liang, G.; Sun, X.; Lai, J.; Wei, C.; Huang, Y.; Hu, H. Adding lithium fluoride to improve the electrochemical properties SnO2@C/MWCNTs composite anode for lithium-ion batteries. J. Electroanal. Chem. 2019, 853, 113401–113407. [Google Scholar] [CrossRef]
- Ye, H.; Li, H.; Jiang, F.; Yin, J.; Zhu, H. In situ fabrication of nitrogendoped carbon-coated SnO2/SnS heterostructures with enhanced performance for lithium storage. Electrochim. Acta 2018, 266, 170–177. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2020, 1–112. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Z.; Li, W.; Chen, C.; Yang, J.; Liu, J.; Gong, F.; Liao, J.; Wu, M. Cellulose-Hydrogel-Derived Self-Activated Carbon/SnO2 Nanocomposites for High-Performance Lithium Storage. ACS Appl. Energy Mater. 2019, 2, 5171–5182. [Google Scholar] [CrossRef]
- Kugler, S.; Spychaj, T. Functionalized CdS quantum dots-based luminescence probe for detection of heavy and transition metal ions in aqueous solution. Spectrochim. Acta 2008, 69, 1044. [Google Scholar]
- Krzyczmonik, P.; Socha, E.; Skrzypek, S.; Soliwoda, K. Celichowski Nanostruktury węglowe i błony lub powłoki polimerowe z ich udziałem. Polimery 2013, 58, 93. [Google Scholar]
- Jiang, H.; Zhang, K.; Li, W.; Cui, Z.; He, S.-A.; Zhao, S.; Li, J.; He, G.; Shearing, P.R.; Brett, D.J.L. MoS2/NiS core-shell structures for improved electrocatalytic process of hydrogen evolution. J. Power Sour. 2020, 472, 228497. [Google Scholar] [CrossRef]
- Krzyczmonik, P.; Socha, E.; Skrzypek, S.; Soliwoda, K.; Celichowski, G.; Grobelny, J. Honeycomb-structured porous poly(3,4-ethylenedio-xythiophene) composite layers on a gold electrode. Thin Solid Films 2014, 565, 54. [Google Scholar] [CrossRef]
- Honda, K.; Rao, T.N.; Tryk, D.; Fujishima, A.; Watanabe, M.; Yasui, K.; Masuda, H. Electrochemical Characterization of the Nanoporous Honeycomb Diamond Electrode as an Electrical Double-Layer Capacitor. J. Electrochem. Soc. 2000, 147, 659–664. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, Y.; Wang, M.; Zhang, F.; Nie, H. A hierarchical porous electrode using a micron-sized honeycomb-like carbon material for high capacity lithium–oxygen batteries. Nanoscale 2013, 5, 4647. [Google Scholar] [CrossRef]
- Rapta, P.; Neudeck, A.; Bartl, A.; Dunsch, L. Microstructured conductive polypyrrole Electrodes. Electrochim. Acta 1999, 44, 3483–3489. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Birkin, P.R.; Ghanem, M.A. Electrochemical deposition of macroporous platinum, palladium and cobalt films using polystyrene latex sphere templates. Chem. Commun. 2000, 17, 1671. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D. Carbon-Based Polymer Nanocomposite for Lithium-Ion Batteries. Carbon-Based Polymer Nanocompos. Environ. Energy Appl. 2018, 537–557. [Google Scholar] [CrossRef]
- Li, J.; Zou, M.; Zhao, Y.; Lin, Y.; Lai, H.; Guan, L.; Huang, Z. Coaxial MWNTs@MnO2 confined in conducting PPy for kinetically efficient and long-term lithium ion storage. Electrochim. Acta 2013, 111, 165–171. [Google Scholar] [CrossRef]
- Kim, D.-W.; Hwang, I.-S.; Kwon, S.J.; Kang, H.-Y.; Park, K.-S.; Choi, Y.-J.; Choi, K.-J.; Park, J.-G. Highly conductive coaxial SnO2-In2O3 heterostructured nanowires for Li ion battery electrodes. Nano Lett. 2007, 7, 3041–3045. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, G.; Yin, L.; Ren, W.; Li, F.; Cheng, H. Graphene/metal oxide composite electrode materials for energy storage. NanoEnergy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Yi, L.; Liu, L.; Guo, G.; Chen, X.; Zhang, Y.; Yu, S. Expanded graphite@SnO2@polyaniline composite with enhanced performance as anode materials for lithium ion batteries. Electrochim. Acta 2017, 240, 63–71. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Xiao, J.; Schwenzer, B.; Engelhard, M.H.; Saraf, L.V. A soft approach to encapsulate sulfur:polyaniline nanotubes for lithium-sulfur batteries with long cycle life. Adv. Mater. 2012, 24, 1176–1181. [Google Scholar] [CrossRef]
- Liu, B.R.; Soares, P.; Checkles, C.; Zhao, Y.; Yu, G.H. Three-dimensional hierarchical ternary nanostructures for high-performance Li-ion battery anodes. Nano Lett. 2013, 13, 3414–3419. [Google Scholar] [CrossRef]
- Li, Z.-F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Hierarchical Nanocomposites of Vanadium Oxide Thin Film Anchored on Graphene as High-Performance Cathodes in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 18894–18900. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, Y.; Liang, S.; Pan, A. Controllable Preparation of V2O5/Graphene Nanocomposites as Cathode Materials for Lithium-Ion Batteries. Nanoscale Res. Lett. 2016, 11, 549. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.Z.; Ai, X.P.; Cao, Y.L.; Yang, H.X. LiF/Fe nanocomposite as a lithium-rich and high capacity conversion cathode material for Li-ion batteries. J. Power Sour. 2012, 217, 54–58. [Google Scholar] [CrossRef]
- Zhou, X.; He, T.; Chen, X.; Zhao, Z.; Guo, X.; Sun, L.; Liu, Z. Synthesis and lithium storage properties of vanadium oxide nanotubes (VOxNTs)-Polyaniline nanocomposite as cathode material for lithium ion batteries. J. Mater. Sci. Mater. Electr. 2017, 28, 11098–11107. [Google Scholar] [CrossRef]
- Mutalib, M.; Rashid, M.; Aziz, F. 22-Carbon-Based Polymer Nanocomposite for Photovoltaic Devices. Carbon-Based Polym. Nanocomposites for Environ. Energy Appl. 2018, 17, 559–584. [Google Scholar]
- He, B.-L.; Dong, B.; Wang, W.; Li, H.-L. Performance of polyaniline/multi-walled carbon nanotubes composites as cathode for rechargeable lithium batteries. Mater. Chem. Physics 2009, 114, 371–375. [Google Scholar] [CrossRef]
- Lian, J.; Wang, X.H.; Zhang, W.X.; Huang, Y.H.; Xia, T.; Lian, Y.F. A ternary polyaniline/active carbon/lithium iron phosphate composite as cathode material for lithium ion battery. J. Nanosci. Nanotechnol. 2016, 16, 6494–6497. [Google Scholar] [CrossRef]
- Van Le, T.; Nguyen, T.A.; Nguyen, N.M.T.; Luu, A.T.; Nguyen, L.-T.; Nguyen, H.T. Synthesis and characterization of nanocomposites based on poly (3-hexylthiophene)-graft-carbon nanotubes with LiNi0.5Mn1.5O4 and its application as potential cathode materials for lithium-ion batteries. Bull. Mater. Sci. 2016, 39, 1177–1184. [Google Scholar] [CrossRef]
- Wang, C.; Wan, W.; Chen, J.T.; Zhou, H.H.; Zhang, X.X.; Yuan, L.X. Dual core-shell structured sulfur cathode composite synthesized by a one-pot route for lithium sulfur batteries. J. Mater. Chem. A 2013, 1, 1716–1723. [Google Scholar] [CrossRef]
- Molenda, M.; Świder, J.; Świętosławski, M.; Kochanowski, A. Li-ion electrode nanocomposites with self-assembled conductive carbon layers. Polimery 2017, 62, 532–538. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Robles Hernandez, F.C.; Deog Yoo, H.; An, Q.; Yao, Y. Enhancing sodium-ion battery performance with interlayer-expanded MoS2–PEO nanocomposites. Nano Energy 2015, 15, 453–461. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in anode materials for sodium ion batteries—A review. Renew. Sustain. Energy Rev. 2019, 109549. [Google Scholar] [CrossRef]
- Liang, Y.; Lai, W.-H.; Miao, Z.; Chou, S.-L. Nanocomposite Materials for the Sodium-Ion Battery: A Review. Small 2017, 14, 1702514. [Google Scholar] [CrossRef] [PubMed]
- Dirican, M.; Lu, Y.; Ge, Y.; Yildiz, O.; Zhang, X. Carbon-Confined SnO2-Electrodeposited Porous Carbon Nanofiber Composite as High-Capacity Sodium-Ion Battery Anode Material. ACS Appl. Mater. Interfaces 2015, 7, 18387–18396. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Y.; Wang, X.; Wang, C.; Zhou, M.; Fu, Q.; Wu, M.; Lei, Y. Intertwined Cu3V2O7(OH)2·2H2O nanowires/carbon fibers composite: A new anode with high rate capability for sodium-ion batteries. J. Power Sour. 2015, 294, 193–200. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Yu, C.; Jiao, L.; Chen, J. MnFe2O4@C Nanofibers as High-Performance Anode for Sodium-Ion Batteries. Nano Lett. 2016, 16, 3321–3328. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Z.; Sun, J. Bi2Se3/C Nanocomposite as a New Sodium-Ion Battery Anode Material. NanoMicro Lett. 2018, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Nam, K.-H.; Park, C.-M. Robust Polyhedral CoTe2-C Nanocomposite as High-Performance Li- and Na-Ion Battery Anodes. ACS Appl. Energy Mater. 2020, 3, 4877–4887. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Bismuth: A new anode for the Na-ion battery. Nano Energy 2015, 12, 88–95. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Chen, C.; Li, Z.; Huang, Y.; Hu, X. Flexible and Binder-Free Electrodes of Sb/rGO and Na3V2(PO4)3/rGO Nanocomposites for Sodium-Ion Batteries. Small 2015, 11, 3822–3829. [Google Scholar] [CrossRef]
- Yan, J.; Li, Q.; Hao, Y.; Dai, C.; Chen, Y. MoS2/SnS2 nanocomposite as stable sodium-ion battery anode. Funct. Mater. Lett. 2019, 13, 1950095. [Google Scholar] [CrossRef]
- Yiang, J.; Zhou, T.; Zhu, R.; Chen, X.; Guo, Z. Highly Ordered Dual Porosity Mesoporous Cobalt Oxide for Sodium-Ion Batteries. Adv. Mater. Interfaces 2016, 3, 1500464. [Google Scholar] [CrossRef]
- Lu, Y.; Su, N.; Cheng, L.; Liu, J.; Yang, L.; Yang, H.; Yang, Q.; Li, S.; Min, J.; Lei, M. Na0.33V2O5 nanosheet@graphene composites: Towards high performance cathode materials for sodium ion batteries. Mater. Lett. 2016, 183, 346. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Q.; Xu, C.; Li, Q.; An, P.; Zhang, J.; Sheng, L.; Zhou, L.M. Layer-by-Layer Na3V2(PO4)3 Embedded in Reduced Graphene Oxide as Superior Rate and Ultralong-Life Sodium-Ion Battery Cathode. Adv. Energy Mater. 2016, 6, 1600389. [Google Scholar] [CrossRef]
- Peng, M.; Li, B.; Yan, H.; Zhang, D.; Wang, X.; Xia, D.; Guo, G. Ruthenium-oxide-coated sodium vanadium fluorophosphate nanowires as high-power cathode materials for sodium-ion batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 6452. [Google Scholar] [CrossRef] [PubMed]
- Longoni, G.; Wang, J.E.; Jung, Y.H.; Kim, D.K.; Mari, C.M.; Ruffo, R. The Na2FeP2O7-carbon nanotubes composite as high rate cathode material for sodium ion batteries. J. Power Sour. 2016, 302, 61. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-based polyanionic compounds as cathode materials for sodium-ion batteries: Toward high-energy and high-power applications. J. Energy Chem. 2020, 55, 361–390. [Google Scholar] [CrossRef]
- Balli, B.; Şavk, A.; Şen, F. Graphene and polymer composites for supercapacitor applications. Nanocarbon Compos. 2019, 123–151. [Google Scholar] [CrossRef]
- Salameh, Z. Chapter 4—Energy Storage. Renewable Energy System Design; Academic Press: Cambridge, MA, USA, 2014; pp. 201–298. [Google Scholar] [CrossRef]
- Anandhi, P.; Kumar, V.J.S.; Harikrishnan, S. Preparation and Improved Capacitive Behavior of NiO/TiO2 Nanocomposites as Electrode Material for Supercapacitor. Curr. Nanosci. 2020, 16, 79–85. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, C.; Zhou, L.; Liu, Y.; Huang, H. Supercapacitor application of nickel oxide–titania nanocomposites. Compos. Sci. Technol. 2009, 69, 2108–2114. [Google Scholar] [CrossRef]
- Singh, S.; Sahoo, R.K.; Shinde, N.M.; Yun, J.M.; Mane, R.S.; Kim, K.H. Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor. Energies 2019, 12, 3320. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Shinde, P.V.; Shaikh, S.F.; Al-Enizi, A.M.; Mane, R.S. Facile Synthesis of Bi2O3@MnO2 Nanocomposite Material: A Promising Electrode for High Performance Supercapacitors. Solid State Sci. 2020, 102, 106158. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Yan, W.; Wang, Y.; Huang, C.; Wang, Z. Hierarchical Fe3O4@FeS2 Nanocomposite as High Specific Capacitance Electrode Materials for Supercapacitor. Energy Technol. 2020, 8, 2000544. [Google Scholar] [CrossRef]
- Majumdara, D.; Thandavarayan, M.; Jiang, Z. A Review on Recent Progress in Ruthenium Oxide-based Composites for Supercapacitor Applications. ChemElectroChem. 2019, 1–85. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, X.G. NiO-based composite, electrode with RuO2 for electrochemical capacitors. Electrochim. Acta 2004, 49, 229–232. [Google Scholar] [CrossRef]
- Hu, C.C.; Yang, Y.L.; Lee, T.C. Microwave-Assisted Hydrothermal Synthesis of RuO2 center dot xH(2)O-TiO2 Nanocomposites for High Power Supercapacitors. Electrochem. Solid State Lett. 2010, 13, A173–A176. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X. Preparation and electrochemical capacitance of RuO2/TiO2 nanotubes composites. Electrochim. Acta 2004, 49, 1957–1962. [Google Scholar]
- Nam, H.S.; Kim, K.M.; Kim, S.H.; Kim, B.C.; Wallace, G.G.; Ko, J.M. Supercapacitive properties of polyaniline/hydrous RuO2 composite electrode. Polym. Bull. 2012, 68, 553–560. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Patil, S.V.; Bulakhe, R.N.; Sartale, S.D.; Lokhande, C.D. Inexpensive synthesis route of porous polyaniline–ruthenium oxide composite for supercapacitor application. Chem. Eng. J. 2014, 257, 82–89. [Google Scholar] [CrossRef]
- Huang, L.M.; Lin, H.Z.; Wen, T.C.; Gopalan, A. Highly dispersed hydrous ruthenium oxide in poly (3, 4-ethylenedioxythiophene)-poly (styrene sulfonic acid) for supercapacitor electrode. Electrochim. Acta 2006, 52, 1058–1062. [Google Scholar]
- Su, Y.-F.; Wu, F.; Bao, L.-Y.; Yang, Z.-H. RuO2/activated carbon composites as a positive electrode in an alkaline electrochemical capacitor. New Carbon Mater. 2007, 22, 53–57. [Google Scholar] [CrossRef]
- Muniraj, V.K.A.; Kamaja, C.K.; Shelke, M.V. RuO2·nH2O Nanoparticles Anchored on Carbon Nano-onions: An Efficient Electrode for Solid State Flexible Electrochemical Supercapacitor. ACS Sustain. Chem. Eng. 2016, 4, 2528–2534. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Park, S.H.; Kimet, K.-B. Microwave-polyol synthesis of nanocrystalline ruthenium oxide nanoparticles on carbon nanotubes for electrochemical capacitors. Electrochim. Acta 2010, 55, 8056–8061. [Google Scholar] [CrossRef]
- Hsieh, T.F.; Chuang, C.C.; Chen, W.J.; Huang, J.H.; Chen, W.T.; Shu, C.M. Hydrous ruthenium dioxide/multi-walled carbon-nanotube/titanium electrodes for supercapacitors. Carbon 2012, 50, 1740–1747. [Google Scholar] [CrossRef]
- Wang, R.; Jia, P.; Yang, Y.; An, N.; Zhang, Y.; Wu, H.; Hu, Z. Ruthenium Oxide/Reduced Graphene Oxide Nanoribbon Composite and Its Excellent Rate Capability in Supercapacitor Application. Chin. J. Chem. 2016, 34, 114–122. [Google Scholar] [CrossRef]
- Ma, H.; Kong, D.; Xu, Y.; Xie, X.; Tao, Y.; Xiao, Z.; Lv, W.; Jang, H.D.; Huang, J.; Yang, Q.-H. Disassembly–Reassembly Approach to RuO2/Graphene Composites for Ultrahigh Volumetric Capacitance Supercapacitor. Small 2017, 13, 1701026. [Google Scholar] [CrossRef]
- Yi, C.; Zou, J.; Yang, H.; Leng, X. A facile hydrothermal synthesis of graphene/RuO2/Co3O4 nanocomposites with high pseudocapacity. N. J. Chem. 2018, 42, 7066–7072. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.; Tamara, M. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-ion Batteries. Chem. Mater. 2013, 25, 17, 3480–3487. [Google Scholar] [CrossRef]
- Thangappan, R.; Dhinesh Kumar, R.; Jayavel, R. Synthesis, structural and electrochemical properties of Mn-MoO4/graphene nanocomposite electrode material with improved performance for supercapacitor application. J. Energy Stor. 2020, 27, 101069. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Li, Y.; Liu, Z.; Hao, Z. Synthesis of graphene–NiFe2O4 nanocomposites and their electrochemical capacitive behavior. J. Mater. Chem. A 2013, 1, 6393. [Google Scholar] [CrossRef]
- Tang, J.; Dysart, A.D.; Pol, V.G. Advancement in sodium-ion rechargeable batteries. Curr. Opin. Chem. Eng. 2015, 9, 34–41. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kurc, B. Preparation and application of a titanium dioxide/graphene oxide anode material for lithium-ion batteries. J. Power Sour. 2015, 299, 286–292. [Google Scholar] [CrossRef]
- Kim, S.-C.; Park, Y.-K.; Kim, B.H.; An, K.-H.; Lee, H.; Lee, S.-J.; Jung, S.-C. Tin Oxide/Carbon Nanocomposites as the Electrode Material for Supercapacitors Using a Liquid Phase Plasma Method. J. Nanosci. Nanotechnol. 2017, 17, 2578–2581. [Google Scholar] [CrossRef]
- Hu, Q.; Li, W.; Abouelamaiem, D.I.; Xu, C.; Jiang, H.; Han, W.; He, G. Hollow Cu-doped NiO microspheres as anode materials with enhanced lithium storage performance. RSC Adv. 2019, 9, 20963–20967. [Google Scholar] [CrossRef]
- Daneshvar, F.; Aziz, A.; Abdelkader, A.; Zhang, T.; Sue, H.-J.; Welland, M.E. Porous SnO2-CuxO nanocomposite thin film on carbon nanotubes as electrodes for high performance supercapacitors. Nanotechnology 2018, 30, 1–22. [Google Scholar] [CrossRef]
- Woo, H.S.; Czerw, R.; Webster, S.; Carroll, D.L. Organic light emitting diodes fabricated with single wall carbon nanotubes dispersed in a hole conducting buffer: The role of carbon nanotubes in a hole conducting polymers. Synth. Met. 2001, 116, 369. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors; Kluwer Academic: New York, NY, USA, 1999; pp. 1–698. [Google Scholar]
- Frąckowiak, E.; Béguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40, 1775. [Google Scholar] [CrossRef]
- Koetz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483. [Google Scholar] [CrossRef]
- Sarangapani, S.; Tilak, B.V.; Chen, C.P. Materials for Electrochemical Capacitors. J. Electrochem. Soc. 1996, 143, 3791. [Google Scholar] [CrossRef]
- Feng, W.; Bai, X.D.; Lian, Y.Q.; Liang, J.; Wang, X.G.; Yoshino, K. Well-aligned polyaniline/carbon-nanotube composite films grown by in-situ aniline polymerization. Carbon 2003, 41, 1551. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Stephan, O.; Colliex, C.; Trauth, D. Aligned Carbon Nanotube Arrays Formed by Cutting a Polymer Resin—Nanotube Composite. Science 1994, 265, 1212. [Google Scholar] [CrossRef]
- Schadler, L.S.; Giannaris, S.C.; Ajayan, P.M. Load transfer in carbon nanotube epoxy composites. Appl. Phys. Lett. 1998, 73, 3842. [Google Scholar] [CrossRef]
- Wagner, H.D.; Lourie, O.; Feldman, Y.; Tenne, R. Stress-induced fragmentation of multiwall carbon nanotubes in a polymer matrix. Appl. Phys. Lett 1998, 72, 188. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Fan, S.; Chapline, M.G.; Franklin, N.R.; Tombler, T.W. Self-Oriented Regular Arrays of Carbon Nanotubes and Their Field Emission Properties. Science 1999, 283, 512. [Google Scholar] [CrossRef]
- Tans, S.J.; Verschueren, A.R.M.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1971, 277, 1971–1975. [Google Scholar] [CrossRef]
- Ago, H.; Petritsch, K.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Composites of carbon nanotubes and conjugated polymers for photovoltaic devices. Adv. Mater. 1999, 11, 1281. [Google Scholar] [CrossRef]
- Kymakis, E.; Amaratunga, G.A. Single-wall carbon nanotube/conjugated polymer photovoltaic devices. J. Appl. Phys. Lett. 2002, 80, 112. [Google Scholar] [CrossRef]
- Frąckowiak, E.; Khomenko, V.; Jurewicz, K.; Lota, K.; Béguin, F. Supercapacitors based on conducting polymers/nanotubes composites. J. Power Sour. 2006, 153, 413. [Google Scholar] [CrossRef]
- Yu, Y.; Che, B.; SI, Z.; Li, L.; Chen, W.; Xue, G. Carbon nanotube/polyaniline core-shell nanowires prepared by in situ inverse microemulsion. Synth. Met. 2005, 150, 271. [Google Scholar] [CrossRef]
- Zhou, Y.; He, B.; Zhou, W.; Huang, J.; Li, X.; Wu, B.; Li, H. Electrochemical capacitance of well-coated single-walled carbon nanotube with polyaniline composites. Electrochim. Acta 2004, 49, 257. [Google Scholar] [CrossRef]
- Cheng, Q.; Pavlinek, V.; He, Y. Facile fabrication and characterization of novel polyaniline/titanate composite nanotubes directed by block copolymer. Eur. Polym. J. 2007, 43, 3760. [Google Scholar] [CrossRef]
- Guo, J.; Kang, L.; Lu, X.; Zhao, S.; Li, J.; Shearing, P.R.; Wang, R.; Brett, D.J.I.; He, G.; Chai, G.; et al. Self-activated cathode substrates in rechargeable zinc–air batteries. Energy Storage Mater. 2021, 35, 530–537. [Google Scholar] [CrossRef]
- Nishio, K.; Fujimoto, M.; Yoshinaga, N. Electrochemical characteristics of polyaniline synthesized by various methods. J. Power Sour. 1995, 56, 189. [Google Scholar] [CrossRef]
- Morgan, H.; Foot, P.J.S.; Brooks, N.W. The effects of composition and processing variables on the properties of thermoplastic polyaniline blends and composites. J. Mater. Sci. 2001, 36, 5369. [Google Scholar] [CrossRef]
- Ryabenko, G.; Fokeeva, L.S.; Dorofeeva, T.V. Spectroscopic study of suspensions of single wall carbon nanotubes in polyaniline solutions in N-methylpyrrolidone in UV—Vis—NIR regions. Rus. Chem. Bull. 2004, 53, 2695. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liu, Z. Conducting polymer/carbon nanotube composite films made by in situ electropolymerization using an ionic surfactant as the supporting electrolyte. Carbon 2005, 43, 2186. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Pachfule, P.; Banerjee, R.; Kurungot, S. Porous Carbons from Nonporous MOFs: Influence of Ligand Characteristics on Intrinsic Properties of End Carbon. Cryst. Growth Des. 2013, 13, 4195–4199. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal-organic framework (MOF) as a template for synthesis of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Liu, X.-W.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zhao, F.; Wang, S.-G. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Gnana, G.; Suk, K. Polymer Nanocomposites—Fuel Cell Applications. Advances in Nanocomposites—Synthesis, Characterization and Industrial Applications. IntechOpen 2011, 640. [Google Scholar] [CrossRef]
- Jafary, T.; Ghasemi, M.; Alam, J.; Aljlil, S.A.; Yusup, S. Carbon-Based Polymer Nanocomposites as Electrodes for Microbial Fuel Cells. Carbon Based Polym. Nanocompos. Environ. Energy Appl. 2018, 361–390. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.; Pati, S.A.; Pant, D. Microbial Fuel Cells: Electrode Materials. Encycl. Interfacial Chem. Surface Sci. Electrochem. 2018, 20, 309–318. [Google Scholar]
- Raza, R.; Liu, Q.; Nisar, J.; Wang, X.; Ma, Y.; Zhu, B. ZnO/NiO nanocomposite electrodes for low-temperature solid oxide fuel cells. Electrochem. Commun. 2011, 13, 917–920. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresource Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Song, H.S.; Hyun, S.H.; Kim, J.; Lee, H.-W.; Moon, J. A nanocomposite material for highly durable solid oxide fuel cell cathodes. J. Mater. Chem. 2008, 18, 1087. [Google Scholar] [CrossRef]
- Raza, R.; Zhu, B. Microwave Sintered Nanocomposite Electrodes for Solid Oxide Fuel Cells. J. Nanosci. Nanotechnol. 2011, 11, 5450–5454. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, D.-B.; Qin, H.; Gao, F.; Inui, H.; Zhu, B. Nanocomposite electrode materials for low temperature solid oxide fuel cells using the ceria-carbonate composite electrolytes. Int. J. Hydrogen Energy 2012, 37, 19351–19356. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Q.; Chen, J.; Wang, B.; Wang, Y.; Liu, K. Hierarchically three-dimensional nanofiber based. Textile with high conductivity and biocompatibility as a microbial fuel cell anode. Environ. Sci. Technol. 2016, 50, 7889–7895. [Google Scholar] [CrossRef]
- Zou, L.; Qiao, Y.; Wu, Z.Y.; Wu, X.S.; Xie, J.L.; Yu, S.H. Tailoring unique mesopores of hierarchically porous structures for fast direct electrochemistry in microbial fuel cells. Adv. Energy Mater. 2016, 6, 1501535. [Google Scholar] [CrossRef]
- Katuri, K.; Ferrer, M.L.; Gutiérrez, M.C.; Jiménez, R.; del Monte, F.; Leech, D. Three-dimensional microchanelled electrodes in flow-through configuration for bioanode formation and current generation. Energy Environ. Sci. 2011, 4, 4201–4210. [Google Scholar] [CrossRef]
- Gnana Kumar, G.; Kirubaharan, C.J.; Udhayakumar, S.; Karthikeyan, C.; Nahm, K.S. Conductive polymer/graphene supported platinum nanoparticles as anode catalysts for the extended power generation of microbial fuel cells. Ind. Eng. Chem. Res. 2014, 53, 16883–16893. [Google Scholar] [CrossRef]
- Hou, Y.; Yuan, H.; Wen, Z.; Cui, S.; Guo, X.; He, Z. Nitrogen-doped graphene/CoNi alloy encased within bamboo-like carbon nanotube hybrids as cathode catalysts in microbial fuel cells. J. Power Sour. 2016, 307, 561–568. [Google Scholar] [CrossRef]
- Modi, A.; Singh, S.; Verma, N. In situ nitrogen-doping of nickel nanoparticle-dispersed carbon nanofiberbased electrodes: Its positive effects on the performance of a microbial fuel cell. Electrochim. Acta 2016, 190, 620–627. [Google Scholar] [CrossRef]
- Modi, A.; Singh, S.; Verma, N. Improved performance of a single chamber microbial fuel cell using nitrogen-doped polymer-metal-carbon nanocomposite-based air-cathode. Int. J. Hydrogen Energy 2017, 42, 3271–3280. [Google Scholar] [CrossRef]
- Merkisz, J.; Gallas, D.; Siedlecki, M.; Szymlet, N.; Sokolnicka, B. Exhaust emissions of an LPG powered vehicle in real operating conditions. In E3S Web of Conferences. EDP Sci. 2019, 100, 53. [Google Scholar]
- Lijewski, P.; Szymlet, N.; Rymaniak, Ł.; Sokolnicka, B.; Domowicz, A. The impact of operating conditions on exhaust emissions from a two-wheeled urban vehicle. In E3S Web of Conferences. EDP Sci. 2019, 100, 00047. [Google Scholar]
- Szymlet, N.; Lijewski, P.; Sokolnicka, B.; Siedlecki, M.; Domowicz, A. Analysis of Research Method, Results and Regulations. Regarding the Exhaust Emissions from Two-Wheeled Vehicles under Actual Operating Conditions. J. Ecol. Eng. 2020, 21, 128–139. [Google Scholar] [CrossRef]
- Burela, R.G.; Kamineni, J.N.; Harursampath, D. Multifunctional polymer composites for 3D and 4D printing. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 231–257. [Google Scholar] [CrossRef]
- Miles, R.W.; Hynes, K.M.; Forbes, I. Photovoltaic solar cells: An overview of state-of-the-art cell development and environmental issues. Progr. Crystal Growth Charact. Mater. 2005, 51, 1. [Google Scholar] [CrossRef]
- Ramachandran, R.; Mani, V.; Chen, S.-M.; Kumar, G.P.G. Recent Progress in Electrode Fabrication Materials and Various Insights in Solar cells: Review. Int. J. Electrochem. Sci. 2015, 10, 3301–3318. [Google Scholar]
- Liu, Z.; Sun, Y.; Yuan, J.; Wei, H.; Huang, X.; Han, L.; Wang, W.; Wang, H.; Ma, W. High-efficiency hybrid solar cells based on polymer/PbSxSe1−x nanocrystals benefiting from vertical phase segregation. Adv. Mater. 2013, 25, 5772–5778. [Google Scholar] [CrossRef]
- Zhou, R.J.; Stalder, R.; Xie, D.P.; Cao, W.R.; Zheng, Y.; Yang, Y.X.; Plaisant, M.; Holloway, P.H.; Schanze, K.S.; Reynolds, J.R.; et al. Enhancing the Efficiency of Solution-Processed Polymer: Colloidal Nanocrystal Hybrid Photovoltaic Cells Using Ethanedithiol Treatment. ACS Nano 2013, 7, 4846–4854. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, H.; Du, X.H.; Cheng, X.; Chen, X.G.; Jiang, Y.Y.; Yang, B. From planar-heterojunction to n-i structure: An efficient strategy to improve short-circuit current and power conversion efficiency of aqueous-solution-processed hybrid solar cells. Energy Environ. Sci. 2013, 6, 1597–1603. [Google Scholar] [CrossRef]
- Ren, S.; Chang, L.Y.; Lim, S.K.; Zhao, J.; Smith, M.; Zhao, N.; Bulovic, V.; Bawendi, M.; Gradecak, S. Inorganic-organic hybrid solar cell: Bridging quantum dots to conjugated polymer nanowires. Nano Lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Mor, G.K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Effect of device geometry on the performance of TiO2 nanotube array-organic semiconductor double heterojunction solar cells. J. Non Cryst. Solids 2008, 354, 2767–2771. [Google Scholar] [CrossRef]
- Mor, G.K.; Kim, S.; Paulose, M.; Varghese, O.K.; Shankar, K.; Basham, J.; Grimes, C.A. Visible to Near-infrared Light Harvesting in TiO2 Nanotube Array-P3HT Based Heterojunction Solar Cells. Nano Lett. 2009, 9, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, D.; Prakasam, A. Preparation of MoS2/graphene Nanocomposite-Based Photoanode for Dye-Sensitized Solar Cells (DSSCs). Inorg. Chem. Commun. 2020, 118, 108016. [Google Scholar] [CrossRef]
- Kazmi, S.A.; Hameed, S.; Ahmed, A.S.; Arshad, M.; Azam, A. Electrical and optical properties of graphene-TiO2 nanocomposite and its applications in dye sensitized solar cells (DSSC). J. Alloys Compd. 2017, 691, 659–665. [Google Scholar] [CrossRef]
- Effendi, N.A.S.; Samsi, N.S.; Zawawi, S.A.; Hassan, O.H.; Zakaria, R.; Yahya, M.Z.A. Studies on graphene zinc-oxide nanocomposites Photoanodes for high-efficient dye-sensitized solar cells. AIP Conf. Proc. Adv. Mater. Nanotechnol. 2017, 1877, 090005. [Google Scholar] [CrossRef]
- Jung, C.-H.; Noh, Y.-J.; Bae, J.-H.; Yu, J.-H.; Hwang, I.-T.; Shin, J.; Shin, K.; Lee, J.-S.; Choi, J.-H.; Na, S.-I. Polyacrylonitrile-grafted reduced graphene oxide hybrid: An all-round and efficient hole-extraction material for organic and inorganic-organic hybrid photovoltaics. Nano Energy 2017, 31, 19–27. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kim, H.S. TiO2/silver/carbon nanotube nanocomposite working electrodes for high-performance dye-sensitized solar cells. J. Compos. Mater. 2013, 48, 1679–1690. [Google Scholar] [CrossRef]
- Suresh Kumar, M.; Balachander, K. Performance analysis of different top metal electrodes in inverted polymer solar cells. Optik 2016, 127, 2725–2731. [Google Scholar] [CrossRef]
- Rivkin, B.; Fassl, P.; Sun, Q.; Taylor, A.D.; Chen, Z.; Vaynzof, Y. Effect of Ion Migration-Induced Electrode Degradation on the Operational Stability of Perovskite Solar Cells. ACS Omega 2018, 3, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Łapkowski, M.; Golba, S.; Żak, J. Conductive polymers containing phenothiazine units in the main chains. Polimery 2009, 54, 255. [Google Scholar] [CrossRef]
- Genies, E.; Bidan, G.; Diaz, A. Spectroelectrochemical study of polypyrrole films. J. Electroanal. Chem. Interfacial Electrochem. 1983, 149, 101. [Google Scholar] [CrossRef]
- Tezuka, Y.; Yamamoto, T.; Kamikado, Y.; Tanaka, H. Partially interpenetrating heterojunction on bilayer photovoltaic devices of electrodeposited polythiophene/methanofullerene. Solar Energy Mater. Solar Cells 2012, 105, 167. [Google Scholar] [CrossRef]
- Atobe, M.; Yoshidab, N.; Sakamotob, K. Preparation of highly aligned arrays of conducting polymer nanowires using templated electropolymerization in supercritical fluids. Electrochim. Acta 2013, 87, 409. [Google Scholar] [CrossRef]
- DiCarmine, P.M.; Fokina, A.; Seferos, D.S. Solvent/Electrolyte Control of the Wall Thickness of Template-Synthesized Nanostructures. Chemistry of Materials. Chem. Mater. 2011, 23, 3787. [Google Scholar] [CrossRef]
- Hajian, A.; Rafati, A.A.; Afraz, A.; Naja, M. Electrosynthesis of Polythiophene Nanowires and Their Application for Sensing of Chlorpromazine. J. Electrochem. Soc. 2014, 161, B196. [Google Scholar] [CrossRef]
- Nam, D.-H.; Kim, M.-J.; Lim, S.-J.; Song, I.-S.; Kwon, H.-S. Single-step synthesis of polypyrrole nanowires by cathodic electropolymerization. J. Mater. Chem. A 2013, 1, 8061. [Google Scholar] [CrossRef]
- Wang, W.; Lei, W.; Yao, T. One-pot synthesis of graphene/SnO2/PEDOT ternary electrode material for supercapacitors. Electrochim. Acta 2013, 108, 118. [Google Scholar] [CrossRef]
- Lagoutte, S.; Aubert, P.-H.; Pinault, M.; Tran-Van, F.; Mayne-L’Hermite, M.; Chevrot, C. Poly(3-methylthiophene)/Vertically Aligned Multi-walled Carbon Nanotubes: Electrochemical Synthesis, Characterizations and Electrochemical Storage Properties in Ionic Liquids. Electrochim. Acta 2014, 130, 754–765. [Google Scholar] [CrossRef]
- Shallcross, R.C.; D’Ambruoso, G.D.; Pyun, J.; Armstrong, N.R. Photoelectrochemical Processes in Polymer-Tethered CdSe Nanocrystals. J. Am. Chem. Soc. 2010, 132, 2622. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yan, X.; Li, Y. Electrochromic Poly(DNTD)/WO3 Nanocomposite Films via Electorpolymerizatin. J. Phys. Chem. C 2012, 116, 286. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, S. Contacts between Conducting Polymers and Noble Metal Nanoparticles Studied by Current-Sensing Atomic Force Microscopy. J. Phys. Chem. B 2006, 110, 656. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (version 42). Progr. Photovolt. Res. Appl. 2013, 21, 827–837. [Google Scholar] [CrossRef]
- Jayawardena, K.D.G.I.; Rozanski, L.J.; Mills, C.A.; Beliatis, M.J.; Nismy, N.A.; Silva, S.R.P. Inorganics-in-Organics”: Recent developments and outlook for 4G polymer solar cells. Nanoscale 2013, 5, 8411. [Google Scholar] [CrossRef] [PubMed]
- Palewicz, M.; Iwan, A. Polimerowe ogniwa słoneczne. Polimery 2011, 56, 99. [Google Scholar] [CrossRef]
- Palewicz, M.; Iwan, A. Photovoltaic phenomenon in polymeric thin layer solar cells. Curr. Phys. Chem. 2011, 1, 27. [Google Scholar] [CrossRef]
- Iwan, A.; Chuchmała, A. Perspectives of applied graphene: Polymer solar cells. Progr. Polym. Sci. 2012, 37, 1805. [Google Scholar] [CrossRef]
- Iwan, A.; Palewicz, M.; Chuchmała, A. Opto(electrical) properties of new aromatic polyazomethines with fluorene moieties in the main chain for polymeric photovoltaic devices. Synth. Metals 2012, 162, 143. [Google Scholar] [CrossRef]
- Iwan, A.; Palewicz, M.; Ozimek, M. Influence of aluminium electrode preparation on PCE values of polymeric solar cells based on P3HT and PCBM. Org. Electr. 2012, 13, 2525. [Google Scholar] [CrossRef]
- Chuchmała, A.; Palewicz, M.; Sikora, A.; Iwan, A. Influence of graphene oxide interlayer on PCE value of polymer solar cells. Synth. Metals 2013, 169, 33. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I. Structural and electrical properties of mixture based on P3HT:PCBM and low band gap naphthalene diimide-imines. Synth. Metals 2014, 189, 183. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Filapek, M. Enhanced power conversion efficiency in bulk heterojunction solar cell based on new polyazomethine with vinylene moieties and [6,6]-phenyl C61 butyric acid methyl ester by adding 10-camphorsulfonic acid. Electrochim. Acta 2015, 159, 81. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I. Silver Nanoparticles in PEDOT:PSS Layer for Polymer Solar Cell Application. Int. J. Photoenergy 2015, 764938, 1–9. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I. Effect of chiral photosensitive liquid crystalline dopants on the performance of organic solar cells. Solid State Electr. 2015, 104, 53. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Hreniak, A. Polymer solar cells with a TiO2:Ag layer. J. Modern Opt. Spec. Issue Org. Photovolt. 2014, 61, 1767. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K. New air-stable aromatic polyazomethines with triphenylamine or phenylenevinylene moieties towards photovoltaic application. Synth. Metals 2014, 195, 341. [Google Scholar] [CrossRef]
- Iwan, A. XIII Krajowa Konferencja Elektroniki. Mat. Konf. 2014, 9, 317. [Google Scholar]
- Parafiniuk, K.; Iwan, A.; Tazbir, I. Synthesis of new polyazomethines for application in polymer photovoltaics. Elektronika 2014, 55, 89. [Google Scholar]
- Iwan, A.; Tazbir, I.; Sibiński, M. Optical, electrical and mechanical properties of indium tin oxide on polyethylene terephthalate substrates: Application in bulk-heterojunction polymer solar cells. Mater. Sci. Semicond. Process. 2014, 24, 110. [Google Scholar] [CrossRef]
- Macedo, A.G.; Silva, D.C.; Yamamotoc, N.A.D. Bilayer and bulk heterojunction solar cells with functional poly(2,2′-bithiophene) films electrochemically deposited from aqueous emulsion. Synth. Metals 2013, 170, 63. [Google Scholar] [CrossRef]
- Pushparaj, V.L.; Shaijumon, M.M.; Kumar, A.; Murugesan, S.; Ci, L.; Vajtai, R. Flexible energy storage devices based on nanocomposite paper. Proc. Natl. Acad. Sci. USA 2007, 104, 13574–13577. [Google Scholar] [CrossRef]
- Yang, Y. A mini-review: Emerging All-Solid-State Energy Storage Electrode Materials for Flexible Devices. Nanoscale 2020, 12, 3560–3573. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Gowda, S.R.; Shaijumon, M.M.; Ajayan, P.M. Hybrid Nanostructures for Energy Storage Applications. Adv. Mater. 2012, 24, 5045–5064. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Jin, A.; Huang, X.; Yang, Y.; Huang, R.; Brock, J.D.; Abruña, H.D. SnS/C nanocomposites for high-performance sodium ion battery anodes. RSC Adv. 2018, 8, 23847–23853. [Google Scholar] [CrossRef]
- Youn, D.-Y.; Tuller, H.L.; Hyun, T.-S.; Choi, D.K.; Kim, I.-D. Facile Synthesis of Highly Conductive RuO2-Mn3O4 Composite Nanofibers via Electrospinning and Their Electrochemical Properties. Electrochem. Soc. 2011, 158, A970–A975. [Google Scholar] [CrossRef]
- Górecki, P. Akumulatory litowe. Elektr. Prakt. 2015, 3, 1–8. [Google Scholar]
- Kurpiel, W.; Polnik, B.; Miedziński, B. System nadzorujący pracę baterii akumulatorów (BMS) w celu zwiększenia bezpieczeństwa ich funkcjonowania i żywotności stosowanych ogniw. Mech. Autom. Gór 2014, 5, 1–7. [Google Scholar]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sour. 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J.L. Cathode Design for Aqueous Rechargeable Multivalent Ion Batteries: Challenges and Opportunities. Adv. Funct. Mater. 2021, 2010445, 1–35. [Google Scholar] [CrossRef]
- Cao, J.; Schofield, N.; Emadi, A. Battery Balancing Methods: A Comprehensive Review. IEEE Vehicle Power Propuls. Conf. 2008, 1–6. [Google Scholar] [CrossRef]
- Daowd, M.; Omar, N.; Van Den Bossche, P.; Van Mierlo, J. Passive and Active Battery Balancing comparison based on MATLAB Simulation. In Proceedings of the 7th IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 6–9 September 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: Review. Energy Environ. Sci. 2011, 4, 3243. [Google Scholar] [CrossRef]
- Behabtu, H.; Messagie, M.; Coosemans, T. A Review of Energy Storage Technologies’Application Potentials in Renewable Energy SourcesGrid Integration. Sustainability 2020, 12, 511. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332. [Google Scholar] [CrossRef]
- Hirayama, M.; Ido, H.; Kim, K.S.; Cho, W.; Tamura, K.; Mizuki, J.; Kanno, R. Dynamic Structural Changes at LiMn2O4/Electrolyte Interface during Lithium Battery Reaction. J. Am. Chem. Soc. 2010, 132, 15268. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sour. 2006, 155, 401. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 320–323. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sour. 2014, 257, 421. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Z.; Zhang, W.; Hu, X.; Chen, J.; Huang, Y.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sour. 2010, 195, 7904. [Google Scholar] [CrossRef]
- Kurc, B.; Siwińska-Stefańska, K.; Jesionowski, T. Bismuth-titanium-silicon-based ternary oxide system: A comprehensive analysis and electrochemical utility. Solid State Ionics 2018, 324, 92–102. [Google Scholar] [CrossRef]
- Balke, N.; Jesse, S.; Morozovska, A.N.; Eliseev, E.; Chung, D.W.; Kim, Y.; Adamczyk, L.; García, R.E. Nanoscale mapping of ion diffusion in a lithium-ion battery cathode. Nat. Nanotechnol. 2010, 5, 749. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sour. 2010, 195, 939. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O. Ageing mechanisms in lithium-ion batteries. J. Power Sour. 2005, 147, 269. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359. [Google Scholar] [CrossRef]




| Hybrid System | Description | Specific Capacity of Battery/SC | Limitations | Function of the Hybrid | Electrochemical Device | References |
|---|---|---|---|---|---|---|
| Polypyrrole-lignin | Conjugated polymer/lignin hybrid for scalable energy storage. Stable supercabatteries (combining the merits of battery and supercapacitor). In stationary storage low cost of lignin biopolymer | Stability/lifetime and self-discharge | Electrode | Supercabattery | [11] | |
| CNT-TMO CNT-ECP CNT-polyaniline CNT-PPy | Nano-hybrid material with interfacial conjugation (Π-Π interactions) for supercapacitors.Electronically conducting polymers (ECPs) and TMOs are semiconductors. CNT (carbon nanotube) hybrids create thick electrode films and enable high energy capacity devices | Low conductivity | Electrode | Supercapacitor | [12] | |
| CNT-reduced graphene oxide | Highly stable, electrical conductive, with high mechanical strength hybrids. The use of hybrid system in enhancing the performance of LIBs and supercapacitors to support the large-energy storage devices (electric vehicles) | Structural flaws, defects (lower capacity) | Cathode/Anode | Supercapacitor/LIB | [13] | |
| Polyaniline/PMo12 ABPBI/PMo12 | Polyoxometalates in PEM fuel cells, electrochemical capacitors, catalysis, sensors, photoelectrochemical conversion for electrodes and electrolytes. They have high good protonic conductivity. Limitations: lack of adequate cyclability. | Cathode | Supercapacitor | [14] | ||
| Lithiated λ-MnO2 | Hybrid material for supercapacitor’s application: manganese oxide (MnO2) from spinel as promising material. The system consists of: lithiated λ-MnO2 (cathode) and activated carbon (anode) | 60 F g−1 | At higher current densities and voltage scan range reduction of specific capacitance | Cathode | Supercapacitor | [15] |
| Li1.4Fe6.8[CH2 (PO3)2]3[CH2(PO3)(PO3H)·4H2O | Hydrothermally synthesized lithium iron methylene diposphonate (transition metal) as a new organic-inorganic hybrid cathode material for LIBs. Coulombic efficiency of 97.6% | 128 mAh g−1 after 200 cycles at 20 mA g−1 | Low initial coulombic efficiency | Cathode | LIB | [16] |
| LiFePO4– Li3V2(PO4)3 LiFePO4–LiCoO2 LiFePO4–LiMn2O4 LiFePO4–LiVPO4F LiFePO4– LiMnPO4 Li3V2(PO4)3– LiMnPO4 Li3V2(PO4)3– LiVPO4F Li3V2(PO4)3– LiVOPO4 LiCoO2–LiMn2O4 | Hybrid cathode materials for LIBs in electric vehicles, hybrid electric vehicles. F. e. LFP-LVP hybrid has max. initial discharge specific capacity in equal to 166 mAh g−1 at 0.1 C | LFP-LVPF 160 mAh g−1 at 0.2 C; LMP-LVP 154 mAh g−1 at C/50 | Synthesis has many internal and external influential factors, mechanism of mixing process, how to ensure uniformity | Cathode | LIB | [17] |
| Vanadia–titania | Vanadia–titania multilayer nanodecoration of carbon onions via atomic layer deposition for high performance anode for fuel cell | 382 mAh g−1 of the composite electrode (554 mAh g−1 per metal oxide) with an impressive capacity retention of 82 mAh g−1 (120 mAh g−1 per metal oxide) at a high discharge rate of 20 A g−1 | Vanadium dissolution at low voltages | Anode | Fuel cell | [18] |
| Graphene-vanadium oxide | Hybrid electrodes for supercapacitors. RG (0.5)/VOx·nH2O electrode with RG content of 10 wt% (60% capacity retention) | 384 F g−1 at a scan rate of 5 mV s−1 after 1000 cycles | Graphene amount | Electrode | Supercapacitor | [19] |
| TiO2-CNTs | 3D conductive network hybrid nanostructures as anode materials in LIBs. Mesoporous TiO2/CNTs stable capacity retention, high Li storage capacity, superior rate performance | 203 mAh g−1 at 100 mA g−1 | Relatively small specific capacity | Anode | LIB | [20] |
| Manganese dioxide-lignin | Manganese dioxide and lignin activated by ionic liquids-based anode material for LIBs | 610 mAh g−1 at 50 mA g−1 570 mAh g−1 at 1000 mA g−1 | Non-farradaic reactions MnO2/KL+A|Li, MnO2/KL+B|Li systems (absence of oxidation and reduction peaks – KL-kraft lignin) | Anode | LIB | [21] |
| TMOs-carbon Ti-based TMO Nb-based TMO Fe-based TMO Co-based TMO Ni-based TMO Cu-based TMO Mo-based TMO | TMO-based hybrid material as NIB anode is highly electroactive. Nb-based transition metal oxiede(TMO) has high chemical stability Fe-based TMO exhibit high theoretical capacity, are non-toxic Co-based TMO exhibit high theoretical capacity and small volume expansion during charging-discharging Ni-based TMO has high specific capacity Cu-based TMO exhibits stable capacity. Mo-based TMO shows high cyclability TMOs hybridized with carbonaceous materials have the high specific capacity over long cycles | Low electrical conductivity, poor ion diffusivity (TMOs-carbon) Concomitant severe pulverization phenomenon (Fe-based TMO) Low electrical conductivity, poor cycling stability (Co-based TMO) Sluggish kinetics (Ni-based TMO) | Anode | NIB | [22] | |
| TMSs-carbon Mo-based TMSs Fe-based TMSs Co-based TMSs Ni-based TMSs Sn-based TMSs | TMSs-carbon hybrid structures for NIB anode. Despite the recent significant progress made in the synthesis of TMSs | There is still a lot of areas that could be explored for achieving better results for NIB negative electrode. | Anode | NIB | [22] | |
| Phosphorene– graphene | A sandwiched phosphorene–graphene hybrid material as a high-capacity anode for NIB shows an 83% capacity retention. The presence of graphene layers in the hybrid material works as a mechanical backbone and an electrical highway | 2440 mAh g−1 at a current density of 0.05 A g−1 after 100 cycles | Relatively low first-cycle coulombic efficiency of 80% | Anode | NIB | [23] |
| TiNb2O7- graphene | TiNb2O7-graphene (TNO-TG) hybrid nanomaterial as an anode for LIBs with high rate capability (Coulombic efficiency of 80% at 16 C), high safety | 230 mAh g−1 after 50 cycles at 0.1 C | Relatively high resistances | Anode | LIB | [24] |
| LiFePO4(LFP)- graphite | LFP/graphite-20% cathode electrode delivered 51.0% of the capacity retention, and the capacity returned back to its initial value when the current density was reduced to 1 C, suggesting the excellent reversibility of both Li+ and PF6- storage in the hybrid material. | 78.7 mAh g−1 at 20 C | Low capacity retention | Cathode | LIB | [25] |
| Electrochemical System | Description of PNC | Reference | |
|---|---|---|---|
| Lithium-ion cell | Specific capacity (mAh g−1) | ||
| Cathode | |||
| V2O5/PPy | The PPy layer on the surface of V2O5 plays a role of plastic protecting shell, and the collapse of V2O5 due to volume expansion during the charge/discharge process can be prevented | [29] | |
| UGF(ultrathin graphite foam)-V2O5/PEDOT core-shell | A coating of PEDOT(poly(3,4-ethylenedioxythiophene) thin shell is the key to the high performance. An excellent high-rate capability and ultrastable cycling up to 1000 cycles are demonstrated | 297 at 1 C. | [30] |
| PTCDA/CNT | PTCDA/CNT exhibited an enhanced rate capability. Polymerization increased the cycling stability of organic cathode materials | 115 at 2 C | [31] |
| Anode | |||
| rGO/SnO2/PANI | rGO/SnO2/PANI composite accommodate for the volume expansion during the insertion/extraction | 397 at 10 A g−1 | [32] |
| PANI/TiO2 | In case of PANI/TiO2 coating polymer helps the particles to remain electronically connected and also creates an electrically conductive route for the electrons transfer | 281 at 20 mA g−1 | [33] |
| PANI/Si | In n-Si/PANI polymer can accommodate volume changes (buffers stress structure) increase the electric conductivity | 561 at 0.1 C | [34] |
| Supercapacitor | Specific capacitance (F g−1) | ||
| PANI@ACNT(aligned small carbon nanotube) | PANI@AACNT showed high specific energy of 18.9 Wh kg−1 high maximum specific power of 11.3 kW kg−1 in an aqueous electrolyte at 1.0 A g−1, excellent rate performance and cycling stability | 163 (only 50 for pristine CNT) | [35] |
| PPy/CNT | The open network of CNT-polypyrrole favors the formation of 3D double layer | [36] | |
| PPy/RGO | The RGO provide large accessible surface area for charge separation at the electrode/electrolyte interface and PPy contribute pseudocapacitance to the energy storage | 424 | [37] |
| PANI-coated honeycomb-like MnO2 nanosphere | The nanocomposite cathode has a Coulombic efficiency of 77% after 1000 cycles at 8 A g−1 | 565 at 0.8 A g−1 | [38] |
| Nanocomposite System | Intercalation-Deintercalation Mechanism | Description |
|---|---|---|
| Graphene-supported transitional metal oxides | When M is a transitional metal such as Ni, Co, Cu, Fe or Mn, the final product would be a homogeneous distribution of metal nanoparticles embedded in a Li2O matrix. | General |
| Graphene–Sn/Si/Ge-based nanocomposites | General | |
| Graphene-supported metal sulfides | Molybdenum sulfide | |
| Graphene-supported metal sulfides | Tin sulfide | |
| Graphene-supported metal sulfides | Cobalt/nickel sulfide |
| Type | Nanocomposite | Specific Capacity (mAh g−1) | Coulombic Efficiency (%) | Reference |
|---|---|---|---|---|
| Anodes | ||||
| Carbon-based | PCNF@SnO2@C | 374 mAh g−1 after 100 cycles | 98.9% | [88] |
| CuVOH-NWs | 287.4 mAh g−1 after 50 cycles at a current density of 0.5 A g−1 | 90% | [89] | |
| MnFe2O4 (MFO)@C | 305 mAh g−1 at 10 A g−1 after 4200 cycles | [90] | ||
| Bi2Se3/C | 527 mAh g−1 at 0.1 A g−1 over 100 cycles | 89% | [91] | |
| Robust Polyhedral CoTe2–C | 323 mAh g–1 stable capacity retentions over 200 cycles, and fast C-rate behavior (240 mAh g–1 at 2 C rate) | [92] | ||
| Graphene-based | Bi@graphene | 561 mAh g−1 at the current density of 40 mA g−1 | [93] | |
| Sb/rGO | 500 mAh g−1 at density current of 1 A g−1 after 100 cycles | [94] | ||
| Sulfide-based | MoS2/SnS2 | 750 mAh g−1 and 600 mAh g−1 after 100 cycles at the current density of of 0.1 A g−1 | 89% | [86] |
| MoS2/PEO | 225 mAh g−1 under a current density of 50 mA g−1, twice as high as that of commercial MoS2 (com-MoS2), improved rate performance due to enhanced Na-ion diffusivity | 90% | [85] | |
| SnS/C | 400 mAh g−1 at 800 mA g−1 | [94] | ||
| Black phosphorus-based | Black phosphorus (BP)/Ti3C2 MXene | 774.4 mAh g−1 was achieved in the 2nd cycle at a current density of 0.1 A g−1 | [95] | |
| Cobalt-based | Dual-meso Co3O4 | 267–416 mAh g−1 at (2430–90 mA g−1 respectively) after 100 cycles | [96] | |
| Cathodes | ||||
| Metal oxides | Na0.33V2O5 nanosheet@graphene | 213 mAh g−1 at 20 mA g−1, good cycling stability, at 50 mA g−1 after 100 cycles | 83.3% | [97] |
| Polyanionic compounds | NVP@rGO | 118 mAh g−1 at 0.5 C, superior rate capability of 73 mAh g−1 at 100 C | 70.0% | [98] |
| RuO2-coated Na3V2O2(PO4)2F | 120 mAh g−1 at 1 C and 95 mAh g−1 at 20 C after 1000 cycles | [99] | ||
| Na2FeP2O7-CNTs | 86 mAh g−1 after 140 cycles at 1 C and 68 mAh g−1 at 10 C | [100] | ||
| Vanadium-based polyanionic compounds | Na3V2O2(PO4)3/C-Ag | 114.9 mAh g−1 at 0.2 C | [101] | |
| Electrode System | Application | Fuel Cell Performance (W cm−2) | Reference |
|---|---|---|---|
| ZnO-NiO | Low-temperature solid oxide fuel cells (LTSOFC) | 1107 | [162] |
| Two-chamber (microbial fuel cells) MFC N-doped graphene/CoNi alloy within bamboo-like CNT hybrid | MFC | 2000 | [163] |
| LSM–YSZ | Highly durable solid oxide fuel cell (SOFC) cathodes | 0.65 0.55 | [164] |
| Cu0.15Ni0.85-GDC (gadolinium doped cerium) | LTSOFC | 0.82 | [165] |
| Metal oxides Ni–Cu–Zn-oxide and samarium doped ceria-carbonate nanocomposite | LTSOFC, 300–600 °C | 0.73 | [166] |
| Hierarchically structured textile polypyrrole/poly(vinyl alcohol-co-polyethylene)nanofibers/poly(ethylene terephthalate) | Two-chamber MFC | 0.42 | [167] |
| Tailored unique mesopores, carbon nanofiber aerogel | Two-chamber MFC | 0.18 | [168] |
| Chitosan-dispersed multiwalled carbon nanotubes | Two-chamber MFC | 0.29 | [169] |
| PANI/reduced graphene oxide (rGO)/Pt | Two-chamber MFC | 0.21 | [170] |
| N-doped graphene/CoNi alloy within bamboo-likeCNT hybrid | Two-chamber MFC | 0.20 | [171] |
| N-Ni-Carbon nanofiber (CNF)/activated carbon fiber | Two-chamber MFC | 0.19 | [172] |
| N-Ni-CNF coated with poly(dimethylsiloxane) | Single-chamber MFC | 0.17 | [173] |
| Components | Basic Structure | Photovoltaic Device | PCE (%) | Reference |
|---|---|---|---|---|
| Dithienol [3,2-b:20,3d]pyrrole)-alt -4,7-(2,1,3-benzothiadiazole-PDTPBT:PbSxSe1−x | Nanocrystals | Hybrid solar cell | 5.5 | [180] |
| PCPDTBT:CdSe | Nanorods | Hybrid photovoltaic cell | 5.2; 4.7 | [181] |
| PPV:CdTe | Nanocrystals | Aqueous-solution-processed hybrid solar cell | 4.76 | [182] |
| P3HT:CdS | Quantum dots: QDs + nanowire of P3HT | Inorganic-organic hybrid solar cell | 4.1 | [183] |
| Fluorine tin oxide-FTO/PEDOT:PSS/P3HT:PCBM/TiO2 | Nanotube array of TiO2 | Double heterojunction solar cell | 4.18 | [184] |
| FTO/PEDOT:PSS/P3HT:SQ-1/TiO2 | Nanotube array of TiO2 | Heterojunction solar cell | 3.8 | [185] |
| MoS2/graphene | Uniform spherical shaped nanoparticles | Dye-sensitized solar cell | 8.92 | [186] |
| TiO2-2%G | Nanocomposite | Dye-sensitized solar cell | 7.68 | [187] |
| G-ZnO | Graphene layer and ZnO nanosheets | Dye-sensitized solar cell | 7.01 | [188] |
| PRGO-PTB7-th (thieno[3,4-b]thiophene.benzodithiophene) | Covalently aliphatic polymer-grafted reduced graphene oxide hybrids | Inorganic-organic hybrid solar cell | 7.24 | [189] |
| TiO2/silver/carbon nanotube | Nanocomposite with Ag nanoparticles | Dye-sensitized solar cell | 3.76 | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurc, B.; Pigłowska, M.; Rymaniak, Ł.; Fuć, P. Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers. Nanomaterials 2021, 11, 538. https://doi.org/10.3390/nano11020538
Kurc B, Pigłowska M, Rymaniak Ł, Fuć P. Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers. Nanomaterials. 2021; 11(2):538. https://doi.org/10.3390/nano11020538
Chicago/Turabian StyleKurc, Beata, Marita Pigłowska, Łukasz Rymaniak, and Paweł Fuć. 2021. "Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers" Nanomaterials 11, no. 2: 538. https://doi.org/10.3390/nano11020538
APA StyleKurc, B., Pigłowska, M., Rymaniak, Ł., & Fuć, P. (2021). Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers. Nanomaterials, 11(2), 538. https://doi.org/10.3390/nano11020538









