Artificial Cathode-Electrolyte Interphase towards High-Performance Lithium-Ion Batteries: A Case Study of β-AgVO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SVO Nanowires
2.2. Al2O3 ALD Coating on SVO Electrode
2.3. Sample Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Wu, L.; Xiong, F.; Pei, C.; An, Q.; Mai, L. Vanadium-Based Cathode Materials for Rechargeable Multivalent Batteries: Challenges and Opportunities. Electrochem. Energy Rev. 2018, 1, 169–199. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent Progress of Surface Coating on Cathode Materials for High-Performance Lithium-Ion Batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef] [Green Version]
- Ran, R.; Meng, X.; Zhang, Z. Facile Preparation of Novel Graphene Oxide-Modified Ag2O/Ag3VO4/AgVO3 Composites with High Photocatalytic Activities under Visible Light Irradiation. Appl. Catal. B 2016, 196, 1–15. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Wang, S.; He, H.; Sun, C.; Yang, S. A Novel Ternary Plasmonic Photocatalyst: Ultrathin G-C3N4 Nanosheet Hybrided by Ag/AgVO3 Nanoribbons with Enhanced Visible-Light Photocatalytic Performance. Appl. Catal. B 2015, 165, 335–343. [Google Scholar] [CrossRef]
- Zhang, S.W.; Li, J.X.; Wang, X.K.; Huang, Y.S.; Zeng, M.Y.; Xu, J.Z. Rationally Designed 1D Ag@AgVO3 Nanowire/Graphene/Protonated G-C3N4 Nanosheet Heterojunctions for Enhanced Photocatalysis Via Electrostatic Self-Assembly and Photochemical Reduction Methods. J. Mater. Chem. A 2015, 3, 10119–10126. [Google Scholar] [CrossRef]
- Bao, S.J.; Bao, Q.L.; Li, C.M.; Chen, T.P.; Sun, C.Q.; Dong, Z.L.; Gan, Y.; Zhang, J. Synthesis and Electrical Transport of Novel Channel-Structured Beta-AgVO3. Small 2007, 3, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Zhu, H.O.; Dai, J.; Yan, W.S.; Yang, J.L.; Tian, Y.C.; Wei, S.Q.; Xie, Y. Room-Temperature Ferromagnetic Silver Vanadium Oxide (Ag1.2V3O8): A Magnetic Semiconductor Nanoring Structure. Adv. Funct. Mater. 2010, 20, 3666–3672. [Google Scholar] [CrossRef]
- Singh, A.; Dutta, D.P.; Ballal, A.; Tyagi, A.K.; Fulekar, M.H. Visible Light Driven Photocatalysis and Antibacterial Activity of AgVO3 and Ag/AgVO3 Nanowires. Mater. Res. Bull. 2014, 51, 447–454. [Google Scholar] [CrossRef]
- Shao, M.W.; Lu, L.; Wang, H.; Wang, S.; Zhang, M.L.; Ma, D.D.; Lee, S.T. An Ultrasensitive Method: Surface-Enhanced Raman Scattering of Ag Nanoparticles from Beta-Silver Vanadate and Copper. Chem. Commun. 2008, 28, 2310–2312. [Google Scholar] [CrossRef]
- Scholtens, B.B.; Polder, R.; Broers, G.H.J. Some Thermodynamic, Electrical and Electrochemical Properties of Silver Vanadium Bronzes. Electrochim. Acta 1978, 23, 483–488. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Transition Metal Vanadium Oxides and Vanadate Materials for Lithium Batteries. J. Mater. Chem. 2011, 21, 9841. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Li, C.; Chen, J. Synthesis, Characterization, and Electrochemical Properties of Ag2V4O11 and AgVO3 1-D Nano/Microstructures. J. Phys. Chem. B 2006, 110, 24855–24863. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, D. Self-Coiling of Ag2V4O11 Nanobelts into Perfect Nanorings and Microloops. J. Am. Chem. Soc. 2006, 128, 11762–11763. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Zhou, L.; Zhang, L. Silver Vanadium Oxides Nanobelts and Their Chemical Reduction to Silver Nanobelts. J. Cryst. Growth 2006, 293, 404–408. [Google Scholar] [CrossRef]
- Feng, M.; Luo, L.B.; Nie, B.; Yu, S.H. P-Type Beta-Silver Vanadate Nanoribbons for Nanoelectronic Devices with Tunable Electrical Properties. Adv. Funct. Mater. 2013, 23, 5116–5122. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, J.; Pan, A.; Li, Y.; Chen, T.; Tian, Z.; Ding, H. Facile Synthesis of β-AgVO3 Nanorods as Cathode for Primary Lithium Batteries. Mater. Lett. 2012, 74, 176–179. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wu, S.; Liao, C.; Zhou, Z.; Liu, X.; Djurišić, A.B.; Xie, M.; Tang, C.; Shih, K. Facile Synthesis, Characterization, and Electrochemical Performance of Multi-Scale AgVO3 Particles. J. Alloys Compd. 2016, 674, 56–62. [Google Scholar] [CrossRef]
- Kim, T.; Shin, J.; You, T.-S.; Lee, H.; Kim, J. Thermally Controlled V2O5 Nanoparticles as Cathode Materials for Lithium-Ion Batteries with Enhanced Rate Capability. Electrochim. Acta 2015, 164, 227–234. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Zheng, L.; Yan, W.; Xie, Y. Pillar Effect on Cyclability Enhancement for Aqueous Lithium Ion Batteries: A New Material of β-Vanadium Bronze M0.33V2O5 (M = Ag, Na) Nanowires. J. Mater. Chem. 2011, 21, 14466. [Google Scholar] [CrossRef]
- Song, Y.; Liu, T.-Y.; Yao, B.; Kou, T.-Y.; Feng, D.-Y.; Liu, X.-X.; Li, Y. Amorphous Mixed-Valence Vanadium Oxide/Exfoliated Carbon Cloth Structure Shows a Record High Cycling Stability. Small 2017, 13, 1700067. [Google Scholar] [CrossRef]
- Yu, M.; Zeng, Y.; Han, Y.; Cheng, X.; Zhao, W.; Liang, C.; Tong, Y.; Tang, H.; Lu, X. Valence-Optimized Vanadium Oxide Supercapacitor Electrodes Exhibit Ultrahigh Capacitance and Super-Long Cyclic Durability of 100000 Cycles. Adv. Funct. Mater. 2015, 25, 3534–3540. [Google Scholar] [CrossRef]
- Sudant, G.; Baudrin, E.; Dunn, B.; Tarascon, J.-M. Synthesis and Electrochemical Properties of Vanadium Oxide Aerogels Prepared by a Freeze-Drying Process. J. Electrochem. Soc. 2004, 151, A666. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Q.-Y.; Jing, M.-X.; Chen, F.; Yuan, W.-Y.; Ju, B.-W.; Tu, F.-Y.; Shen, X.-Q.; Qin, S.-B. Improved Interface Stability and Room-Temperature Performance of Solid-State Lithium Batteries by Integrating Cathode/Electrolyte and Graphite Coating. ACS Appl. Mater. Interfaces 2020, 12, 15120–15127. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of Inorganic Films Grown by Atomic Layer Deposition: Overview and General Trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.A.; Cavanagh, A.S.; George, S.M.; Lee, S.-H.; Dillon, A.C. Improved Mechanical Integrity of ALD-Coated Composite Electrodes for Li-Ion Batteries. Electrochem. Solid-State Lett. 2011, 14, A29. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Lu, P.; Ahn, D. Ultrathin Multifunctional Oxide Coatings for Lithium Ion Batteries. Adv. Mater. 2011, 23, 3911–3915. [Google Scholar] [CrossRef]
- He, Y.; Yu, X.; Wang, Y.; Li, H.; Huang, X. Alumina-Coated Patterned Amorphous Silicon as the Anode for a Lithium-Ion Battery with High Coulombic Efficiency. Adv. Mater. 2011, 23, 4938–4941. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Pi, Y.; An, Q.; Mai, L.; Xie, J.; Xu, X.; Xu, L.; Zhao, Y.; Niu, C.; Khan, A.M.; et al. Substrate-Assisted Self-Organization of Radial Beta-AgVO3 Nanowire Clusters for High Rate Rechargeable Lithium Batteries. Nano Lett. 2012, 12, 4668–4673. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Xu, Y.; Zhang, Z.; Du, X.; Sun, X.; Xiong, L.; Rodriguez, R.; Holze, R. Low-Cost Al2O3 Coating Layer as a Preformed Sei on Natural Graphite Powder to Improve Coulombic Efficiency and High-Rate Cycling Stability of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6512–6519. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K.; Kwon, S.H.; Park, H.S.; Kang, S.W. Peald of a Ruthenium Adhesion Layer for Copper Interconnects. J. Electrochem. Soc. 2004, 151, C753–C756. [Google Scholar] [CrossRef]
- Liang, L.; Liu, H.; Yang, W. Synthesis and Characterization of Self-Bridged Silver Vanadium Oxide/Cnts Composite and Its Enhanced Lithium Storage Performance. Nanoscale 2013, 5, 1026–1033. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Y.; Lei, Y.; Liu, H. 1-Dimensional AgVO3 Nanowires Hybrid with 2-Dimensional Graphene Nanosheets to Create 3-Dimensional Composite Aerogels and Their Improved Electrochemical Properties. Nanoscale 2014, 6, 3536–3539. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Yuan, N.; Hu, B.; Ding, J.; Ge, S. Graphite-Based Lithium Ion Battery with Ultrafast Charging and Discharging and Excellent Low Temperature Performance. J. Power Sources 2019, 430, 74–79. [Google Scholar] [CrossRef]
- Tang, D.; Ben, L.; Sun, Y.; Chen, B.; Yang, Z.; Gu, L.; Huang, X. Electrochemical Behavior and Surface Structural Change of LiMn2O4 charged to 5.1 V. J. Mater. Chem. A 2014, 2, 14519–14527. [Google Scholar] [CrossRef]
- Patel, R.L.; Xie, H.; Park, J.; Asl, H.Y.; Choudhury, A.; Liang, X. Significant Capacity and Cycle-Life Improvement of Lithium-Ion Batteries through Ultrathin Conductive Film Stabilized Cathode Particles. Adv. Mater. Interfaces 2015, 2, 1500046. [Google Scholar] [CrossRef]
- Lu, J.; Zhan, C.; Wu, T.; Wen, J.; Lei, Y.; Kropf, A.; Wu, H.; Miller, D.; Elam, J.; Sun, Y.; et al. Effectively Suppressing Dissolution of Manganese from Spinel Lithium Manganate Via a Nanoscale Surface-Doping Approach. Nat. Commun. 2014, 5, 5693. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-K.; Han, D.-W.; Ryu, W.-H.; Lim, S.-J.; Kwon, H.-S. Al2O3 Coating on LiMn2O4 by Electrostatic Attraction Forces and Its Effects on the High Temperature Cyclic Performance. Electrochim. Acta 2012, 71, 17–21. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Peng, H.; Yang, Z.; Martin, D.J.; Bund, A.; Nanjundan, A.K.; Yamauchi, Y. Electrochemical Characteristics of Cobaltosic Oxide in Organic Electrolyte According to Bode Plots: Double-Layer Capacitance and Pseudocapacitance. ChemElectroChem 2019, 6, 2456–2463. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R.; Gileadi, E. Physical Interpretation of the Warburg Impedance. Corrosion 1995, 51, 664–671. [Google Scholar] [CrossRef]


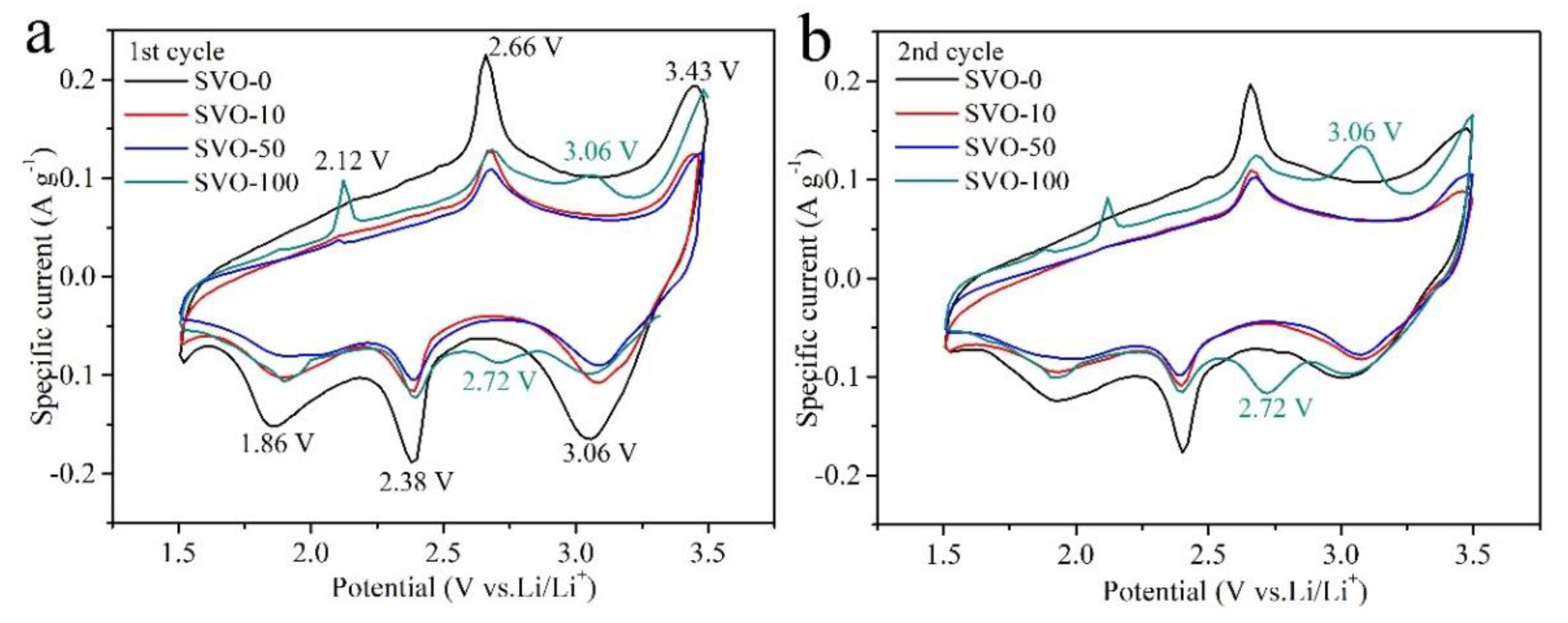
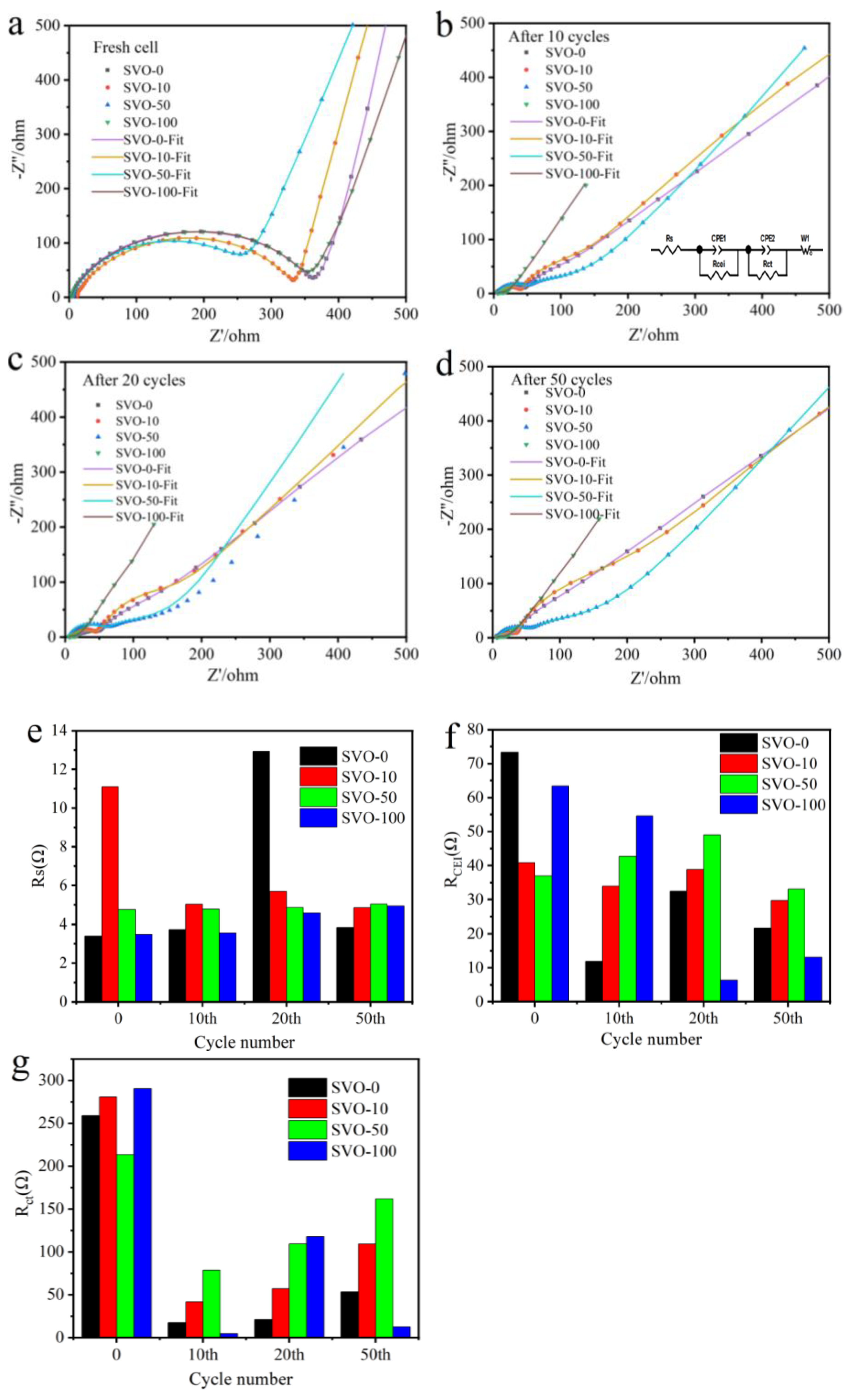
| Al2O3 ALD Cycles | 1st Scan | 2nd Scan | 3rd Scan | ||||||
|---|---|---|---|---|---|---|---|---|---|
| O | R | ΔV | O | R | ΔV | O | R | ΔV | |
| 0 | 2.660 | 2.379 | 0.281 | 2.657 | 2.402 | 0.255 | 2.653 | 2.406 | 0.247 |
| 10 | 2.666 | 2.392 | 0.274 | 2.66 | 2.398 | 0.262 | 2.655 | 2.404 | 0.251 |
| 50 | 2.683 | 2.397 | 0.286 | 2.679 | 2.402 | 0.277 | 2.675 | 2.406 | 0.269 |
| 100 | 2.682 | 2.398 | 0.284 | 2.678 | 2.403 | 0.275 | 2.674 | 2.407 | 0.267 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Dai, W.; Zhu, H.; Gu, Y.; Wang, K.; Li, C.; Pan, C.; Zhou, M.; Liu, J. Artificial Cathode-Electrolyte Interphase towards High-Performance Lithium-Ion Batteries: A Case Study of β-AgVO3. Nanomaterials 2021, 11, 569. https://doi.org/10.3390/nano11030569
Liu L, Dai W, Zhu H, Gu Y, Wang K, Li C, Pan C, Zhou M, Liu J. Artificial Cathode-Electrolyte Interphase towards High-Performance Lithium-Ion Batteries: A Case Study of β-AgVO3. Nanomaterials. 2021; 11(3):569. https://doi.org/10.3390/nano11030569
Chicago/Turabian StyleLiu, Liang, Wei Dai, Hongzheng Zhu, Yanguang Gu, Kangkang Wang, Chao Li, Chaofeng Pan, Min Zhou, and Jian Liu. 2021. "Artificial Cathode-Electrolyte Interphase towards High-Performance Lithium-Ion Batteries: A Case Study of β-AgVO3" Nanomaterials 11, no. 3: 569. https://doi.org/10.3390/nano11030569
APA StyleLiu, L., Dai, W., Zhu, H., Gu, Y., Wang, K., Li, C., Pan, C., Zhou, M., & Liu, J. (2021). Artificial Cathode-Electrolyte Interphase towards High-Performance Lithium-Ion Batteries: A Case Study of β-AgVO3. Nanomaterials, 11(3), 569. https://doi.org/10.3390/nano11030569






