Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation Procedure
2.3. Emulsion Droplet Size
2.4. Encapsulation Process
2.5. Particle Morphology Analysis
2.6. Evaluation of the Extractable Oil
2.7. Accelerated Stability Test
2.8. Peroxide Value (PV) Evaluation
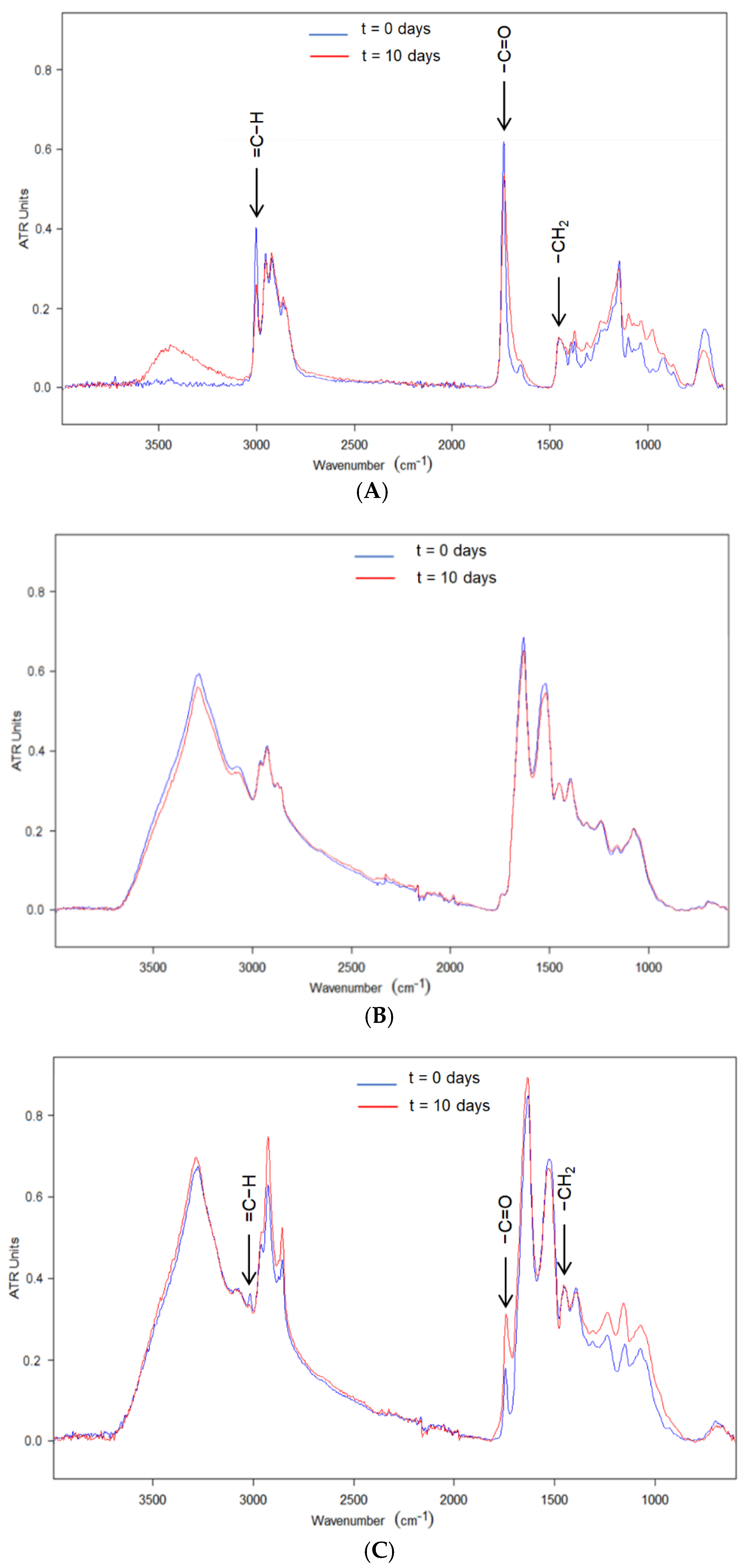
2.9. Attenuated Total Reflection—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.10. Headspace Oxygen Volume Depletion
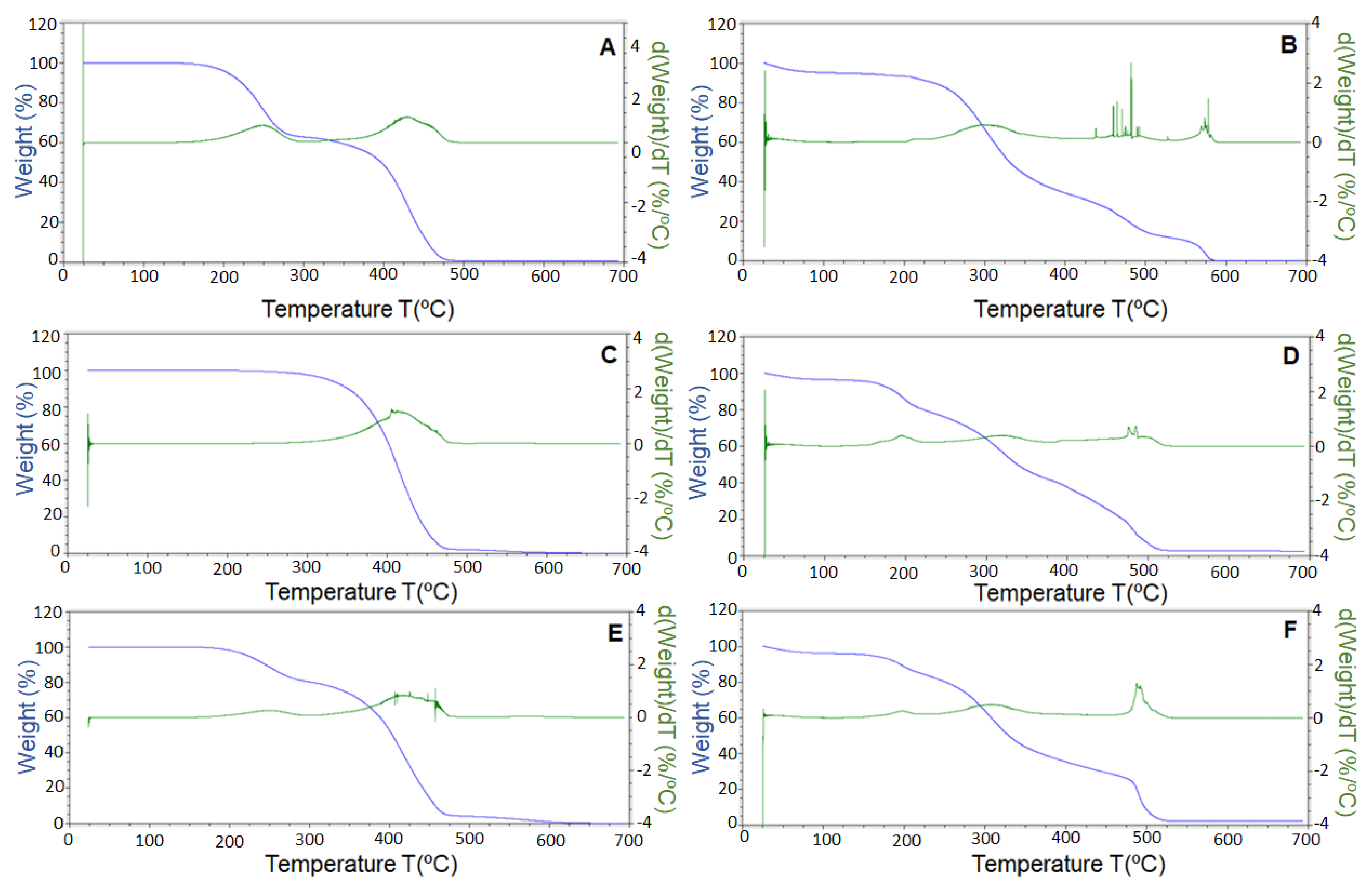
2.11. Thermogravimetric Analysis (TGA)
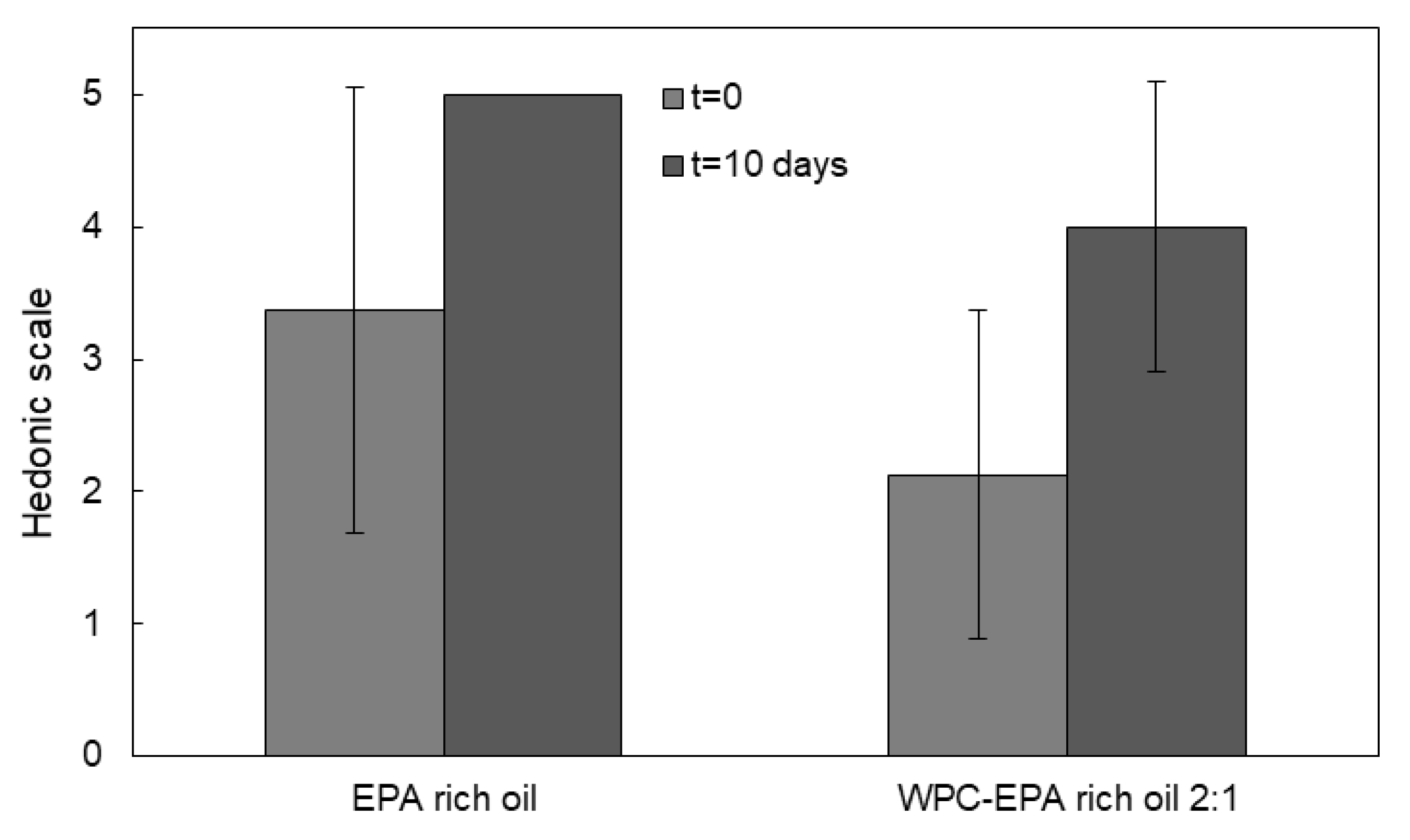
2.12. Organoleptic Test
2.13. Statistical Analysis
3. Results and Discussion
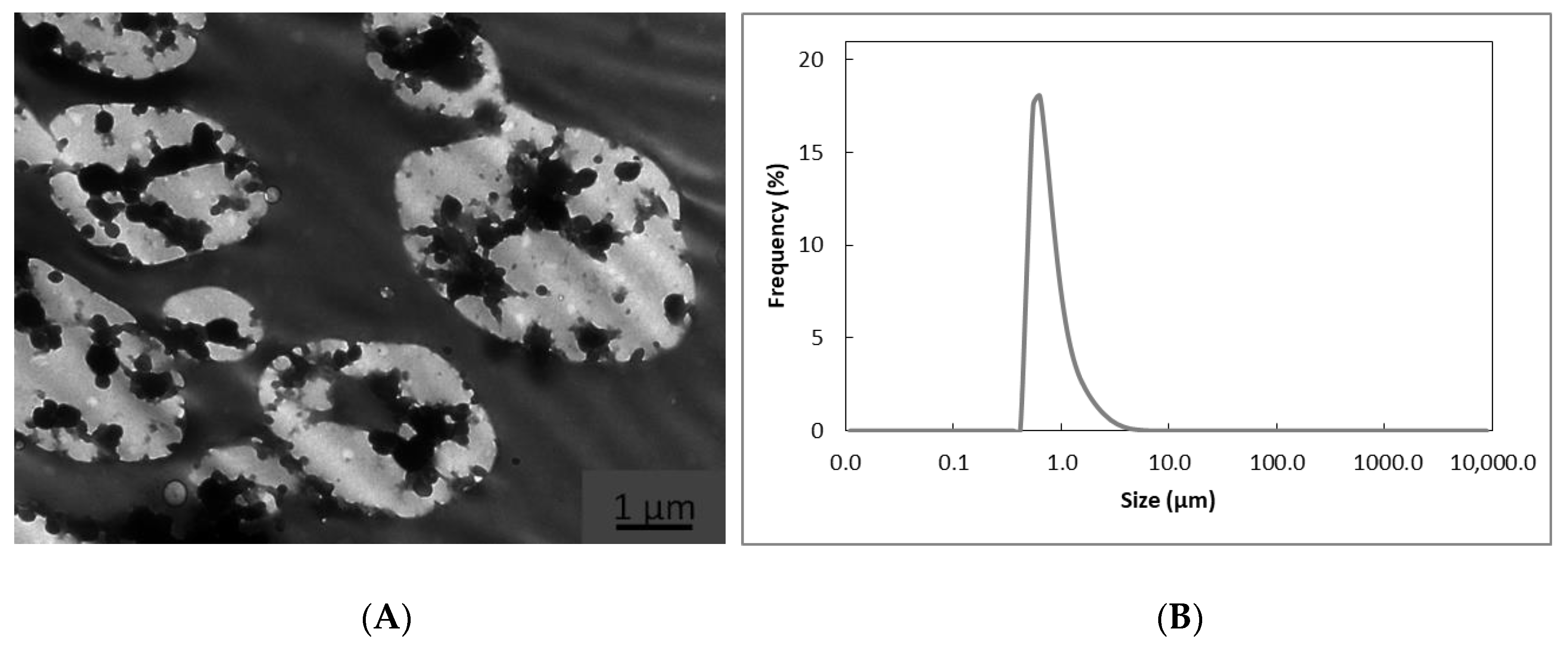
3.1. Morphology
3.2. Extractable Oil
3.3. Accelerated Stability Test
3.4. Headspace Oxygen Volume Depletion Test
3.5. Thermogravimetric Analysis
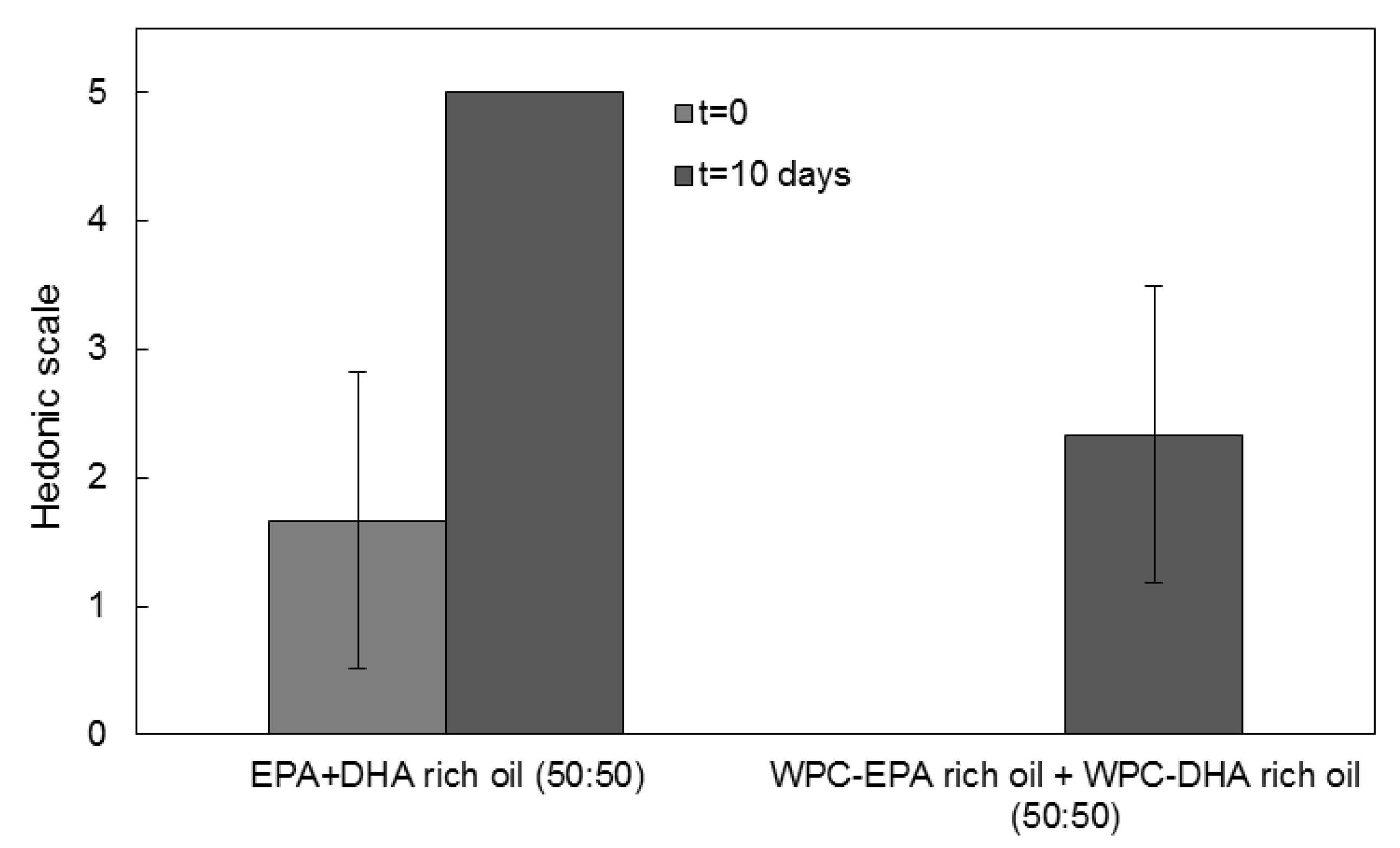
3.6. Organoleptic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panse, M.L.; Phalke, S.D. World Market of Omega-3 Fatty Acids. In Omega-3 Fatty Acids; Springer: Berlin/Heidelberg, Germany, 2016; pp. 79–88. [Google Scholar]
- GOED Omega 3, Council for Responsible Nutrition. Oxidation in Omega-3 Oils: An Overview. 2015. Available online: https://goedomega3.com/storage/app/media/scientific-reports/Oxidation%20in%20Omega-3%20Oils_%20An%20Overview.pdf (accessed on 24 February 2021).
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), maintenance of normal vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), maintenance of normal cardiac function (ID 510, 688, 1360), “maternal health; pregnancy and nursing” (ID 514), “to fulfil increased omega-3 fatty acids need during pregnancy” (ID 539), “skin and digestive tract epithelial cells maintenance” (ID 525), enhancement of mood (ID 536), “membranes cell structure” (ID 4295), “anti-inflammatory action” (ID 4688) and maintenance of normal blood LDL-cholesterol concentrations (ID 4719) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2224. [Google Scholar] [CrossRef] [Green Version]
- Russell, F.D.; Bürgin-Maunder, C.S. Distinguishing Health Benefits of Eicosapentaenoic and Docosahexaenoic Acids. Mar. Drugs 2012, 10, 2535–2559. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Chen, F. Production of Eicosapentaenoic Acid Using Heterotrophically Grown Microalgae. In Single Cell Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Urbana, IL, USA, 2010; pp. 151–177. [Google Scholar]
- Albracht-Schulte, K.; Gonzalez, S.; Jackson, A.; Wilson, S.; Ramalingam, L.; Kalupahana, N.S.; Moustaid-Moussa, N. Eicosapentaenoic Acid Improves Hepatic Metabolism and Reduces Inflammation Independent of Obesity in High-Fat-Fed Mice and in HepG2 Cells. Nutrients 2019, 11, 599. [Google Scholar] [CrossRef] [Green Version]
- Sawada, T.; Tsubata, H.; Hashimoto, N.; Takabe, M.; Miyata, T.; Aoki, K.; Yamashita, S.; Oishi, S.; Osue, T.; Yokoi, K.; et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc. Diabetol. 2016, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Sasaki, J.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Itakura, H.; Hishida, H.; Kita, T.; et al. Relationship between coronary artery disease and non-HDL-C, and effect of highly purified EPA on the risk of coronary artery disease in hypercholesterolemic patients treated with statins: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). J. Atheroscler. Thromb. 2012, 19, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.; Wani, O.; May, H.; Budoff, M. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vasc. Pharmacol. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.-T.; Chiang, Y.-J.; Chen, H.-T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; López-Rubio, A. Protein-based emulsion electrosprayed micro- and submicroparticles for the encapsulation and stabilization of thermosensitive hydrophobic bioactives. J. Colloid Interface Sci. 2016, 465, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to Microencapsulation and Controlled Delivery in Foods. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 3–12. [Google Scholar]
- Miranda-Tavares, G.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk proteins as encapsulation devices and delivery vehicles: Applications and trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Chen, J.; Guo, Z.; Zheng, Y.; Rea, M.C.; Su, H.; Zheng, X.; Zheng, B.; Miao, S. Using polysaccharides for the enhancement of functionality of foods: A review. Trends Food Sci. Technol. 2019, 86, 311–327. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Ocio, M.J.; Lagaron, J.M. Stabilization of a Nutraceutical Omega-3 Fatty Acid by Encapsulation in Ultrathin Electrosprayed Zein Prolamine. J. Food Sci. 2010, 75, N69–N79. [Google Scholar] [CrossRef] [PubMed]
- Busolo, M.; Torres-Giner, S.; Prieto, C.; Lagaron, J. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innov. Food Sci. Emerg. Technol. 2019, 51, 12–19. [Google Scholar] [CrossRef]
- Miguel, G.A.; Jacobsen, C.; Prieto, C.; Kempen, P.J.; Lagaron, J.M.; Chronakis, I.S.; García-Moreno, P.J. Oxidative stability and physical properties of mayonnaise fortified with zein electrosprayed capsules loaded with fish oil. J. Food Eng. 2019, 263, 348–358. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Pelayo, A.; Yu, S.; Busolo, M.; Lagaron, J.M.; Chronakis, I.S.; Jacobsen, C. Physicochemical characterization and oxidative stability of fish oil-loaded electrosprayed capsules: Combined use of whey protein and carbohydrates as wall materials. J. Food Eng. 2018, 231, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Prieto, C.; Lagaron, J.M. Nanodroplets of Docosahexaenoic Acid-Enriched Algae Oil Encapsulated within Microparticles of Hydrocolloids by Emulsion Electrospraying Assisted by Pressurized Gas. Nanomaterials 2020, 10, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, M.A.; Oliver, C.M. Use of Milk Proteins for Encapsulation of Food Ingredients. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 211–226. [Google Scholar]
- Ayache, J.; Beaunier, L.; Boumendil, J.; Ehret, G.; Laub, D. Sample Preparation Handbook for Transmission Electron Microscopy; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- OSRAM. ULTRA-VITALUX 300 W 230 V E27. Available online: https://www.osram.com/pia/ecat/ULTRA-VITALUX-Ultraviolet%20lamps-Specialty%20lamps/com/en/GPS01_1028569/PP_EUROPE_Europe_eCat/ZMP_60829/ (accessed on 24 February 2021).
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present, and future. Expert. Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- Joye, I.; Corradini, M.; Duizer, L.; Bohrer, B.; Lapointe, G.; Farber, J.; Spagnuolo, P.; Rogers, M. A comprehensive perspective of food nanomaterials. In Marine Enzymes Biotechnology: Production and Industrial Applications, Part III—Application of Marine Enzymes; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 88, pp. 1–45. [Google Scholar]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef]
- Olloqui, E.J.; Castañeda-Ovando, A.; Contreras-López, E.; Hernandez-Sanchez, D.; Tapia-Maruri, D.; Piloni-Martini, J.; Añorve-Morga, J. Encapsulation of Fish Oil Into Low-Cost Alginate Beads and EPA-DHA Release in a Rumino-Intestinal In Vitro Digestion Model. Eur. J. Lipid Sci. Technol. 2018, 120, 1800036. [Google Scholar] [CrossRef]
- Aragao, G.M.F.; Corradini, M.G.; Peleg, M. A Phenomenological Model of the Peroxide Value’s Rise and Fall During Lipid Oxidation. J. Am. Oil Chem. Soc. 2008, 85, 1143–1153. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Tong, L.M.; Sasaki, S.; McClements, D.J.; Decker, E.A. Mechanisms of the Antioxidant Activity of a High Molecular Weight Fraction of Whey. J. Agric. Food Chem. 2000, 48, 1473–1478. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Usefulness of the Frequency Data of the Fourier Transform Infrared Spectra To Evaluate the Degree of Oxidation of Edible Oils. J. Agric. Food Chem. 1999, 47, 709–719. [Google Scholar] [CrossRef]
- Moomand, K.; Lim, L.-T. Oxidative stability of encapsulated fish oil in electrospun zein fibres. Food Res. Int. 2014, 62, 523–532. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Characterization of edible oils and lard by fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J. Am. Oil Chem. Soc. 1997, 74, 1281–1286. [Google Scholar] [CrossRef]
- Kiefer, J.; Noack, K.; Bartelmess, J.; Walter, C.; Dörnenburg, H.; Leipertz, A. Vibrational structure of the polyunsaturated fatty acids eicosapentaenoic acid and arachidonic acid studied by infrared spectroscopy. J. Mol. Struct. 2010, 965, 121–124. [Google Scholar] [CrossRef]
- Mizutani, Y.; Matsumura, Y.; Imamura, K.; Nakanishi, K.; Mori, T. Effects of Water Activity and Lipid Addition on Secondary Structure of Zein in Powder Systems. J. Agric. Food Chem. 2003, 51, 229–235. [Google Scholar] [CrossRef]
- Karunathilaka, S.R.; Choi, S.H.; Mossoba, M.M.; Yakes, B.J.; Brückner, L.; Ellsworth, Z.; Srigley, C.T. Rapid classification and quantification of marine oil omega-3 supplements using ATR-FTIR, FT-NIR and chemometrics. J. Food Compos. Anal. 2019, 77, 9–19. [Google Scholar] [CrossRef]
- Safar, M.; Bertrand, D.; Robert, P.; Devaux, M.F.; Genot, C. Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance. J. Am. Oil Chem. Soc. 1994, 71, 371–377. [Google Scholar] [CrossRef]
- Petzold, C.; Lyngstadaas, S.P.; Rubert, M.; Monjo, M. UV-induced chemical coating of titanium surfaces with eicosapentaenoic acid. J. Mater. Chem. 2008, 18, 5502–5510. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.G.; Junior, J.C.D.A.; Viana, C.C.R.; Neves, L.N.D.O.; da Silva, P.H.F.; Bell, M.J.V.; Anjos, V.D.C.D. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Ha, H.-K.; Rankin, S.A.; Lee, M.-R.; Lee, W.-J. Development and Characterization of Whey Protein-Based Nano-Delivery Systems: A Review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.-I.; Krochta, J. Oxygen Barrier Properties of Whey Protein Isolate Coatings on Polypropylene Films. J. Food Sci. 2003, 68, 224–228. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-García, J.D.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles. Nanomaterials 2021, 11, 575. https://doi.org/10.3390/nano11030575
Escobar-García JD, Prieto C, Pardo-Figuerez M, Lagaron JM. Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles. Nanomaterials. 2021; 11(3):575. https://doi.org/10.3390/nano11030575
Chicago/Turabian StyleEscobar-García, Juan David, Cristina Prieto, Maria Pardo-Figuerez, and Jose M. Lagaron. 2021. "Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles" Nanomaterials 11, no. 3: 575. https://doi.org/10.3390/nano11030575
APA StyleEscobar-García, J. D., Prieto, C., Pardo-Figuerez, M., & Lagaron, J. M. (2021). Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles. Nanomaterials, 11(3), 575. https://doi.org/10.3390/nano11030575








