Functionalized Silica Star-Shaped Nanoparticles and Human Mesenchymal Stem Cells: An In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. s-MSN, s-MSN-NH2, and s-MSN-Au Production and Characterization
2.2.1. Star-Shaped Mesoporous Silica Nanoparticles (s-MSN) Synthesis
2.2.2. Amino Functionalization of Silica Colloids (s-MSN-NH2)
2.2.3. Gold Seeds Preparation
2.2.4. Gold Nanoseeds Growth on Silica Surface (s-MSN-Au)
2.2.5. Characterization of Nanomaterials
2.3. Human Adult Mesenchymal Stem Cells: Isolation and Culture
2.4. Culture of Human Adult Mesenchymal Stem Cells on s-MSN, s-MSN-NH2, and s-MSN-Au
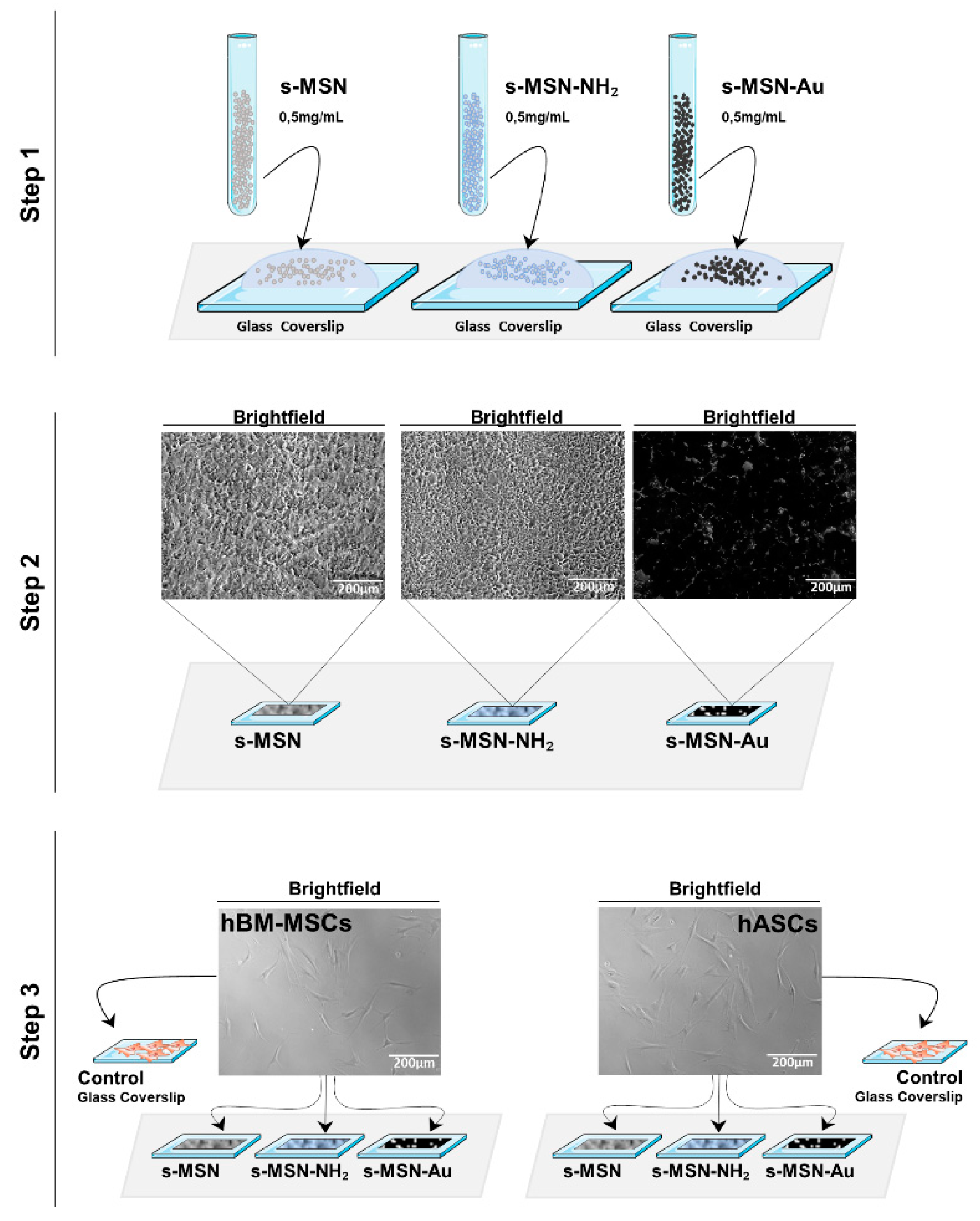
2.4.1. Preparation of a Homogeneous Deposition of NPs on Glass Coverslips
2.4.2. Stem Cells Seeding on NPs
2.5. Stem Cell Proliferation on s-MSN, s-MSN-NH2, and s-MSN-Au
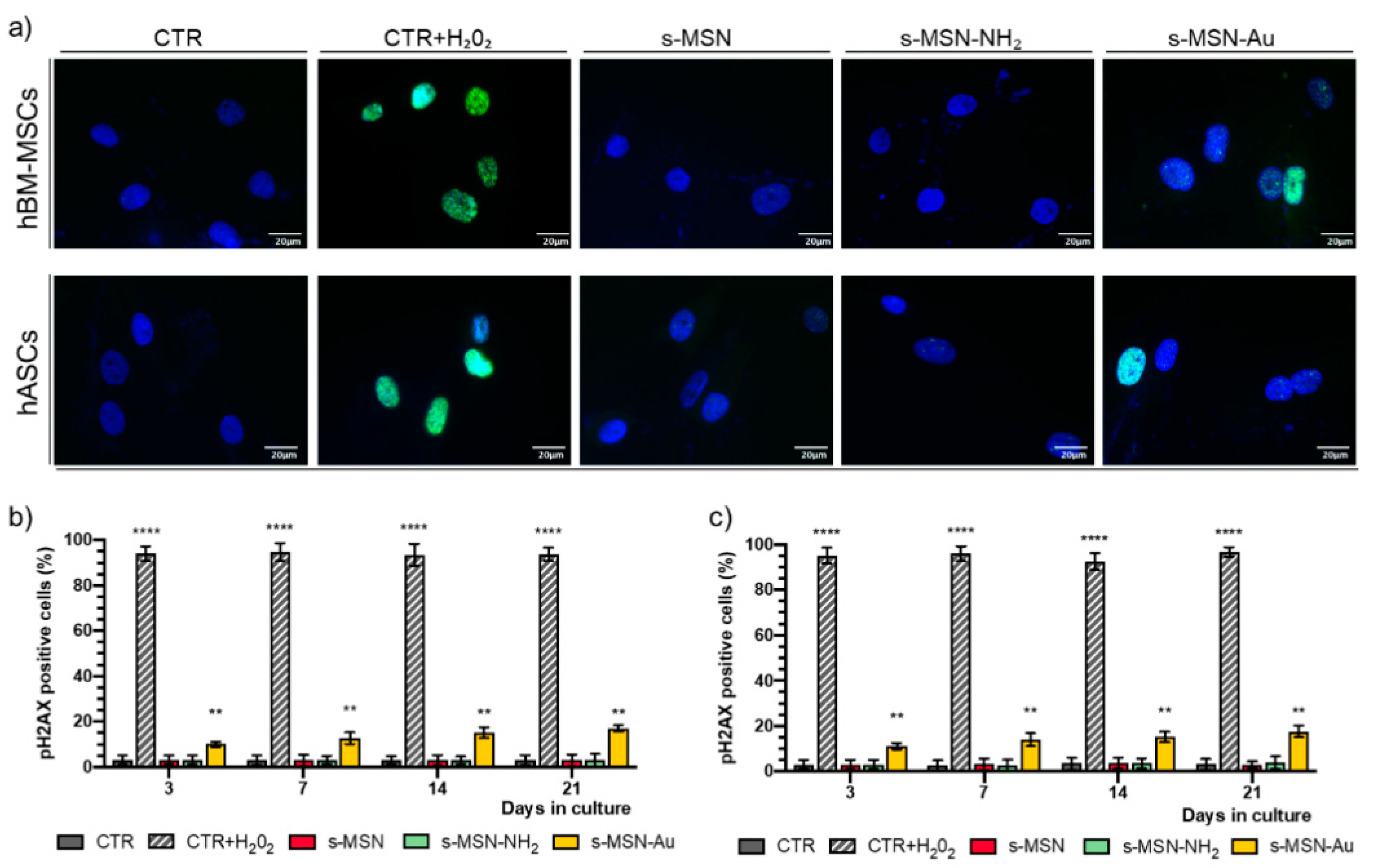
2.6. Genotoxic Effects of s-MSN, s-MSN-NH2, and s-MSN-Au on Human Adult Mesenchymal Stem Cells
2.7. Immunofluorescence
2.8. Acridine Orange Staining
2.9. Images Quantification of Fluorescence
- CTCF = corrected total cell fluorescence
- cFID = cell fluorescence integrated density
- Ac = area of masked cell
- MAb = mean area of background (mean of five different ROI) and
- MFB = mean fluorescence of the background (mean of five different ROI)
2.10. Stem Cell Differentiation
2.10.1. Alizarin Red
2.10.2. Oil Red O
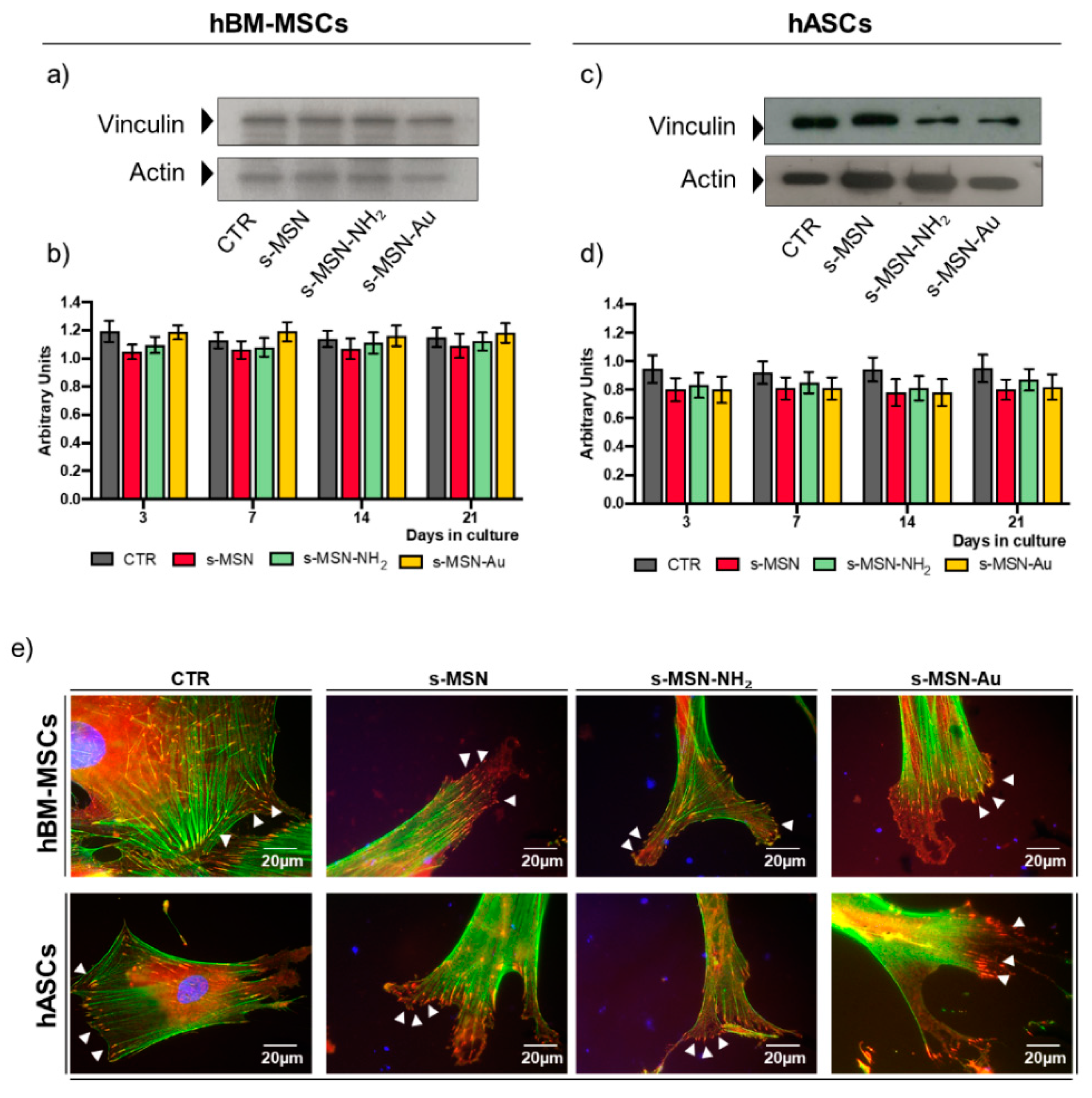
2.11. Protein Extract and Western Blotting
2.12. Cytomorphometric Measurements
2.13. Statistical Analysis
3. Results
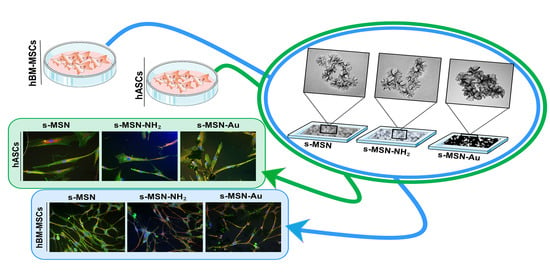
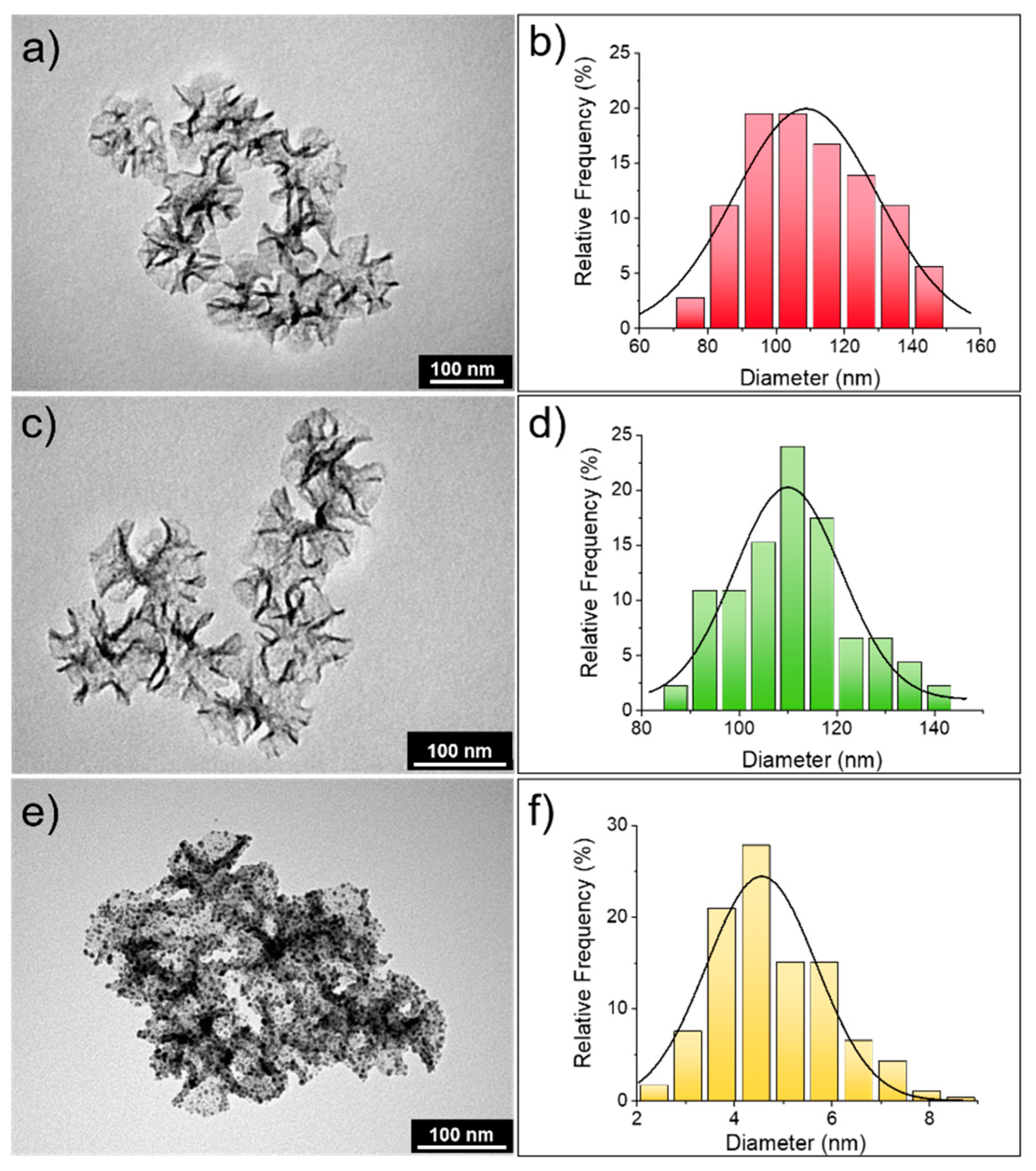
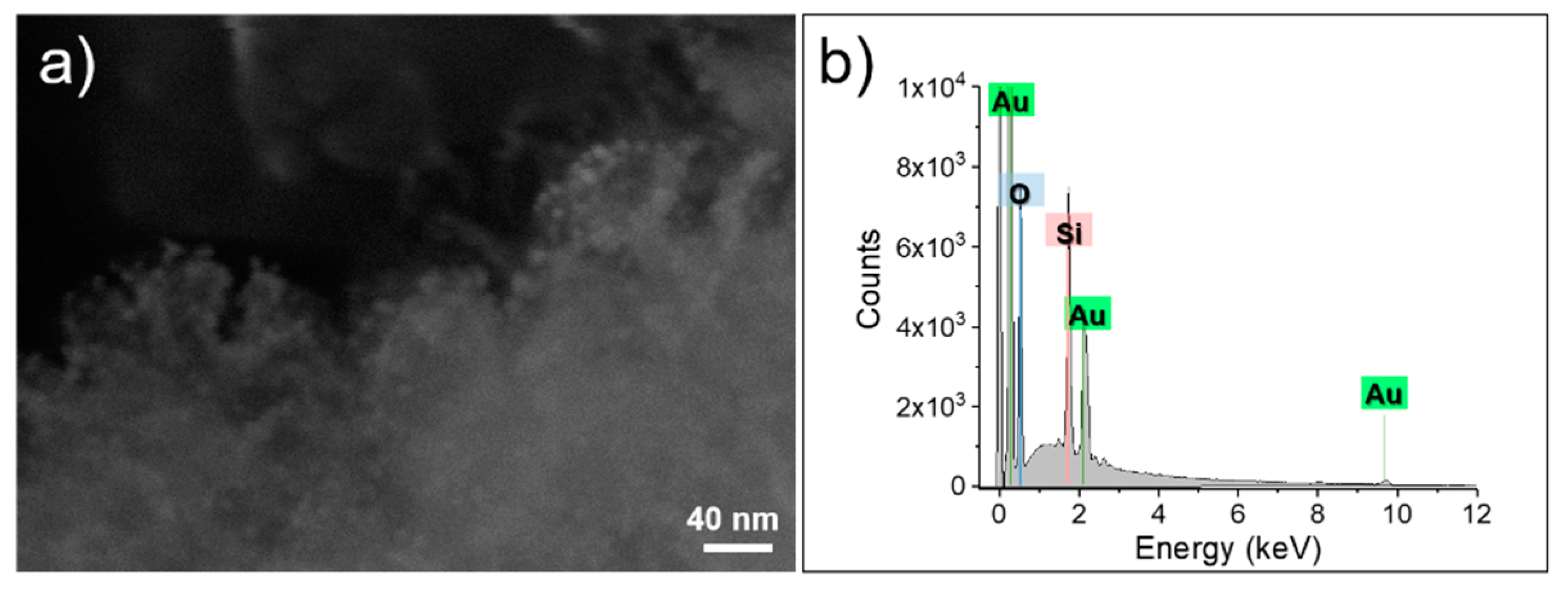
3.1. Silica Star-Shaped Nanoparticles: Synthesis, Functionalization, and Au Nanoseeds Growth
3.2. s-MSN, s-MSN-NH2, and s-MSN-Au and Mesenchymal Stem Cell Behavior and Interaction
3.2.1. Stem Cell Proliferation and Viability
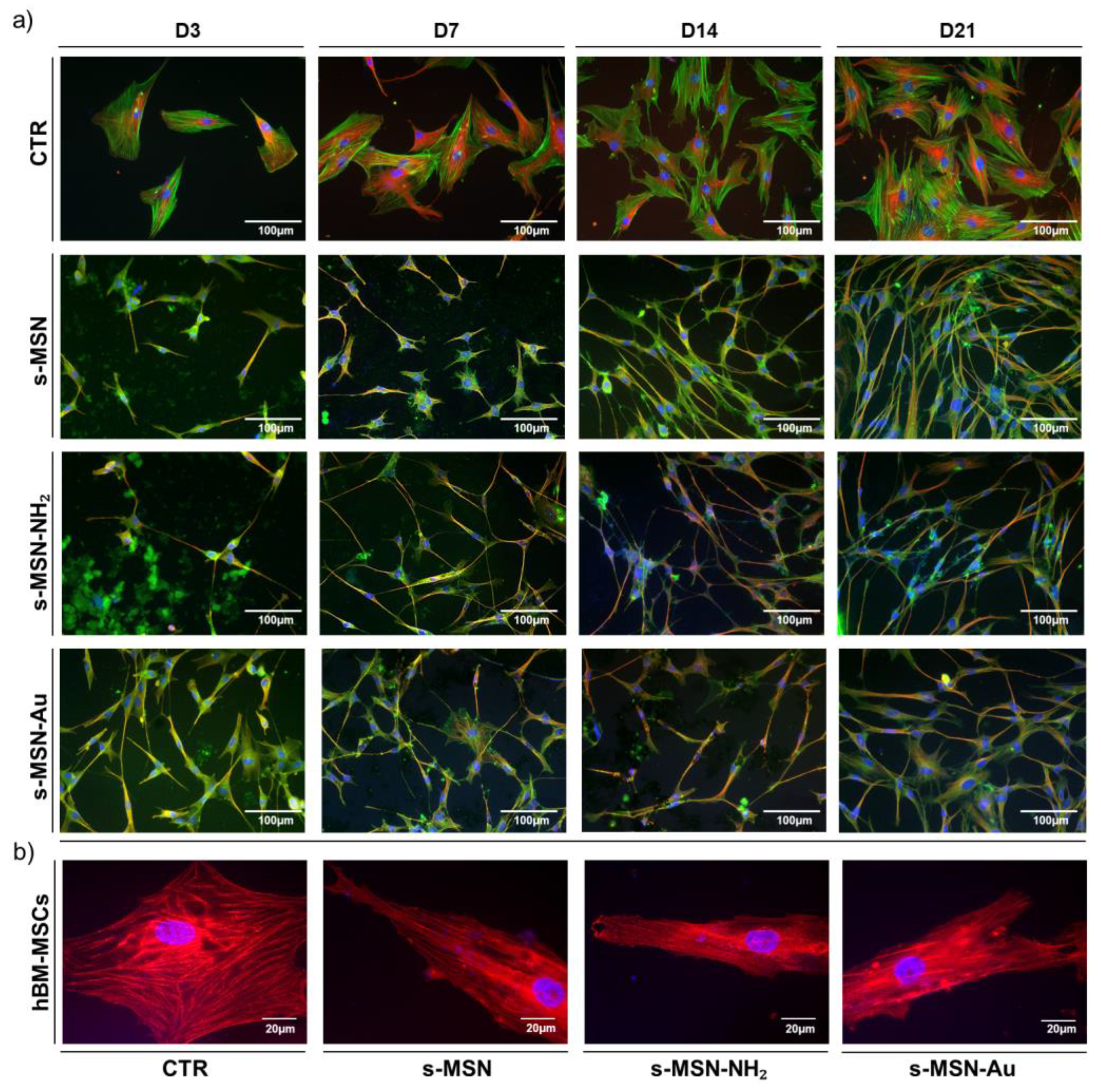
3.2.2. Stem Cell Shape on s-MSN, s-MSN-NH2 and s-MSN-Au
3.2.3. Stem Cell Adhesion on s-MSN, s-MSN-NH2, and s-MSN-Au
3.2.4. Stem Cell Differentiation on s-MSN, s-MSN-NH2 and s-MSN-Au
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolonduro, O.A.; Duffy, B.M.; Rao, A.A.; Black, L.D.; Timko, B.P. From biomimicry to bioelectronics: Smart materials for cardiac tissue engineering. Nano Res. 2020, 13, 1253–1267. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Zhao, M.; Liu, G.; Wu, J. 2D nanomaterials for tissue engineering application. Nano Res. 2020, 13, 2019–2034. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Argentati, C.; Morena, F.; Tortorella, I.; Bazzucchi, M.; Porcellati, S.; Emiliani, C.; Martino, S. Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int. J. Mol. Sci. 2019, 20, 5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morena, F.; Armentano, I.; Montanucci, P.; Argentati, C.; Fortunati, E.; Montesano, S.; Bicchi, I.; Pescara, T.; Pennoni, I.; Mattioli, S.; et al. Design of a nanocomposite substrate inducing adult stem cell assembly and progression toward an Epiblast-like or Primitive Endoderm-like phenotype via mechanotransduction. Biomaterials 2017, 144, 211–229. [Google Scholar] [CrossRef]
- Góra, A.; Tian, L.; Ramakrishna, S.; Mukherjee, S. Design of novel perovskite-based polymeric poly(L-lactide-co-glycolide) nanofibers with anti-microbial properties for tissue engineering. Nanomaterials 2020, 10, 1127. [Google Scholar] [CrossRef]
- Cristallini, C.; Vitale, E.; Giachino, C.; Rastaldo, R. Nanoengineering in cardiac regeneration: Looking back and going forward. Nanomaterials 2020, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Costa Fernandes, C.; Pinto, T.S.; Kang, H.R.; Magalhães Padilha, P.; Koh, I.H.J.; Constantino, V.R.L.; Zambuzzi, W.F. Layered Double Hydroxides Are Promising Nanomaterials for Tissue Bioengineering Application. Adv. Biosyst. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Liu, X.L.; Chen, S.; Zhang, H.; Zhou, J.; Fan, H.M.; Liang, X.J. Magnetic Nanomaterials for Advanced Regenerative Medicine: The Promise and Challenges. Adv. Mater. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Chan, M.H.; Lai, C.Y.; Chan, Y.C.; Hsiao, M.; Chung, R.J.; Chen, X.; Liu, R.S. Development of upconversion nanoparticle-conjugated indium phosphide quantum dot for matrix metalloproteinase-2 cancer transformation sensing. Nanomedicine 2019, 14, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Sengupta, S. Transcellular transfer of nanomedicine. Nat. Nanotechnol. 2019, 14, 731–732. [Google Scholar] [CrossRef]
- Fiorini, F.; Prasetyanto, E.A.; Taraballi, F.; Pandolfi, L.; Monroy, F.; López-Montero, I.; Tasciotti, E.; De Cola, L. Nanocomposite Hydrogels as Platform for Cells Growth, Proliferation, and Chemotaxis. Small 2016, 12, 4881–4893. [Google Scholar] [CrossRef] [PubMed]
- Andrée, L.; Barata, D.; Sutthavas, P.; Habibovic, P.; van Rijt, S. Guiding mesenchymal stem cell differentiation using mesoporous silica nanoparticle-based films. Acta Biomater. 2019, 96, 557–567. [Google Scholar] [CrossRef]
- Mashayekhi, S.; Rasoulpoor, S.; Shabani, S.; Esmaeilizadeh, N.; Serati-Nouri, H.; Sheervalilou, R.; Pilehvar-Soltanahmadi, Y. Curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for supporting long-term proliferation and stemness preservation of adipose-derived stem cells. Int. J. Pharm. 2020, 587, 119656. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110267. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, X.; Gao, Y.E.; Hou, M.; Xue, P.; Li, C.M.; Kang, Y. Multifunctional silica nanoparticles as a promising theranostic platform for biomedical applications. Mater. Chem. Front. 2017, 1, 1257–1272. [Google Scholar] [CrossRef]
- Zampini, G.; Matino, D.; Quaglia, G.; Tarpani, L.; Gargaro, M.; Cecchetti, F.; Iorio, A.; Fallarino, F.; Latterini, L. Experimental evidences on the role of silica nanoparticles surface morphology on the loading, release and activity of three proteins. Microporous Mesoporous Mater. 2019, 287, 220–227. [Google Scholar] [CrossRef]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- Tarpani, L.; Morena, F.; Gambucci, M.; Zampini, G.; Massaro, G.; Argentati, C.; Emiliani, C.; Martino, S.; Latterini, L. The influence of modified silica nanomaterials on adult stem cell culture. Nanomaterials 2016, 6, 104. [Google Scholar] [CrossRef] [Green Version]
- Gambucci, M.; Tarpani, L.; Zampini, G.; Massaro, G.; Nocchetti, M.; Sassi, P.; Latterini, L. Fluorimetric Studies of a Transmembrane Protein and Its Interactions with Differently Functionalized Silver Nanoparticles. J. Phys. Chem. B 2018, 122, 6872–6879. [Google Scholar] [CrossRef]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Antosova, A.; Gambucci, M.; Bednarikova, Z.; Albonetti, C.; Valle, F.; Sassi, P.; Latterini, L.; Gazova, Z.; Bystrenova, E. Effect of metallic nanoparticles on amyloid fibrils and their influence to neural cell toxicity. Nano Res. 2020, 13, 1081–1089. [Google Scholar] [CrossRef]
- Zampini, G.; Tarpani, L.; Massaro, G.; Gambucci, M.; Peli, E.; Latterini, L. Controlled assembly of metal colloids on dye-doped silica particles to tune the photophysical properties of organic molecules. Photochem. Photobiol. Sci. 2018, 17, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Latterini, L.; Tarpani, L. Hierarchical Assembly of Nanostructures to Decouple Fluorescence and Photothermal Effect. J. Phys. Chem. C 2011, 115, 21098–21104. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef]
- Donnelly, E.M.; Kubelick, K.P.; Dumani, D.S.; Emelianov, S.Y. Photoacoustic Image-Guided Delivery of Plasmonic-Nanoparticle-Labeled Mesenchymal Stem Cells to the Spinal Cord. Nano Lett. 2018, 18, 6625–6632. [Google Scholar] [CrossRef]
- Tham, A.Y.; Gandhimathi, C.; Praveena, J.; Venugopal, J.R.; Ramakrishna, S.; Dinesh Kumar, S. Minocycline loaded hybrid composites nanoparticles for mesenchymal stem cells differentiation into osteogenesis. Int. J. Mol. Sci. 2016, 17, 1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollands, P. Application of nanomaterials in stem cells, tissue engineering and regenerative medicine. In Applications of Nanomaterials in Human Health; Khan, F.A., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 65–81. ISBN 9789811548024. [Google Scholar]
- Morena, F.; Argentati, C.; Soccio, M.; Bicchi, I.; Luzi, F.; Torre, L.; Munari, A.; Emiliani, C.; Gigli, M.; Lotti, N.; et al. Unpatterned Bioactive Poly(Butylene 1,4-Cyclohexanedicarboxylate)-Based Film Fast Induced Neuronal-Like Differentiation of Human Bone Marrow-Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 9274. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Armentano, I.; Mattioli, S.; Crispoltoni, L.; Tiribuzi, R.; Cerulli, G.G.; Palmerini, C.A.; Kenny, J.M.; Martino, S.; Orlacchio, A. Micropatterned hydrogenated amorphous carbon guides mesenchymal stem cells towards neuronal differentiation. Eur. Cells Mater 2010, 20, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.S.; Choi, S.M.; Kim, H.O.; Kim, E.H.; You, J.; Park, T.; Kim, E.; Kim, H.S. Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience 2013, 238, 305–318. [Google Scholar] [CrossRef]
- Poudineh, M.; Wang, Z.; Labib, M.; Ahmadi, M.; Zhang, L.; Das, J.; Ahmed, S.; Angers, S.; Kelley, S.O. Three-Dimensional Nanostructured Architectures Enable Efficient Neural Differentiation of Mesenchymal Stem Cells via Mechanotransduction. Nano Lett. 2018, 18, 7188–7193. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone Marrow Mesenchymal Stem Cells: Aging and Tissue Engineering Applications to Enhance Bone Healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2602. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, M.; Di Taranto, G.; Lattanzi, W. Adipose-derived stem cell therapies for bone regeneration. Expert Opin. Biol. Ther. 2017, 17, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; D’Angelo, F.; Armentano, I.; Kenny, J.M.; Orlacchio, A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012, 30, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A New Hydrosol of Gold Clusters. 1. Formation and Particle Size Variation. Langmuir 1993, 9, 2301–2309. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Montanucci, P.; Rallini, M.; Basta, G.; Calabrese, N.; Calafiore, R.; Cordellini, M.; Emiliani, C.; Armentano, I.; et al. Surface hydrophilicity of poly(L-lactide) acid polymer film changes the human adult adipose stem cell architecture. Polymers 2018, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Martino, S.; D’Angelo, F.; Armentano, I.; Tiribuzi, R.; Pennacchi, M.; Dottori, M.; Mattioli, S.; Caraffa, A.; Cerulli, G.G.; Kenny, J.M.; et al. Hydrogenated amorphous carbon nanopatterned film designs drive human bone marrow mesenchymal stem cell cytoskeleton architecture. Tissue Eng. Part A 2009, 15, 3139–3149. [Google Scholar] [CrossRef]
- D’Angelo, F.; Armentano, I.; Cacciotti, I.; Tiribuzi, R.; Quattrocelli, M.; Del Gaudio, C.; Fortunati, E.; Saino, E.; Caraffa, A.; Cerulli, G.G.; et al. Tuning Multi/Pluri-Potent Stem Cell Fate by Electrospun Poly( l -lactic acid)-Calcium-Deficient Hydroxyapatite Nanocomposite Mats. Biomacromolecules 2012, 13, 1350–1360. [Google Scholar] [CrossRef]
- Luzi, F.; Tortorella, I.; Di Michele, A.; Dominici, F.; Argentati, C.; Morena, F.; Torre, L.; Puglia, D.; Martino, S. Novel nanocomposite PLA films with lignin/zinc oxide hybrids: Design, characterization, interaction with mesenchymal stem cells. Nanomaterials 2020, 10, 2176. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Bazzucchi, M.; Armentano, I.; Emiliani, C.; Martino, S. Adipose stem cell translational applications: From bench-to-bedside. Int. J. Mol. Sci. 2018, 19, 3475. [Google Scholar] [CrossRef] [Green Version]
- Morena, F.; Argentati, C.; Calzoni, E.; Cordellini, M.; Emiliani, C.; D’Angelo, F.; Martino, S. Ex-Vivo Tissues Engineering Modeling for Reconstructive Surgery Using Human Adult Adipose Stem Cells and Polymeric Nanostructured Matrix. Nanomaterials 2016, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Rescignano, N.; Tarpani, L.; Tiribuzi, R.; Montesano, S.; Martino, S.; Latterini, L.; Kenny, J.M.; Armentano, I. Protein encapsulation in biodegradable polymeric nanoparticles: Morphology, fluorescence behaviour and stem cell uptake. Macromol. Biosci. 2013, 13, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, V.; Lattanzi, A.; Tiradani, L.; Bravo, G.; Morena, F.; Sanvito, F.; Calabria, A.; Bringas, J.; Fisher-Perkins, J.M.; Dufour, J.P.; et al. Pervasive supply of therapeutic lysosomal enzymes in the CNS of normal and Krabbe-affected non-human primates by intracerebral lentiviral gene therapy. EMBO Mol. Med. 2016, 8, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Argentati, C.; Trotta, R.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; di Baldassarre, A.; Calafiore, R.; Montanucci, P.; Basta, G.; et al. A Comparison of Lysosomal Enzymes Expression Levels in Peripheral Blood of Mild- and Severe-Alzheimer’s Disease and MCI Patients: Implications for Regenerative Medicine Approaches. Int. J. Mol. Sci. 2017, 18, 1806. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; Cavalieri, C.; Emiliani, C.; Dolcetta, D.; De Cusella Angelis, M.G.; Chigorno, V.; Severini, G.M.; Sandhoff, K.; Bordignon, C.; Sonnino, S.; et al. Restoration of the GM2 ganglioside metabolism in bone marrow-derived stromal cells from Tay-Sachs disease animal model. Neurochem. Res. 2002, 27, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Tiribuzi, R.; Orlacchio, A.; Crispoltoni, L.; Maiotti, M.; Zampolini, M.; De Angelis, M.; Mecocci, P.; Cecchetti, R.; Bernardi, G.; Datti, A.; et al. Lysosomal β-galactosidase and β-hexosaminidase activities correlate with clinical stages of dementia associated with Alzheimer’s disease and type 2 diabetes mellitus. J. Alzheimer’s Dis. 2011, 24, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Argentati, C.; Acquati, S.; DeWall, S.; Kelly, F.; Calbi, V.; Fumagalli, F.; Zancan, S.; Biffi, A.; Aiuti, A.; et al. Toward Reference Intervals of ARSA Activity in the Cerebrospinal Fluid: Implication for the Clinical Practice of Metachromatic Leukodystrophy. J. Appl. Lab. Med. 2020, 6, 354–366. [Google Scholar] [CrossRef]
- Morena, F.; di Girolamo, I.; Emiliani, C.; Gritti, A.; Biffi, A.; Martino, S. A new analytical bench assay for the determination of arylsulfatase a activity toward galactosyl-3-sulfate ceramide: Implication for metachromatic leukodystrophy diagnosis. Anal. Chem. 2014, 86, 473–481. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji—An Open platform for biological image analysis. Nat. Methods 2009, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Schwendy, M.; Unger, R.E.; Bonn, M.; Parekh, S.H. Automated cell segmentation in FIJI® using the DRAQ5 nuclear dye. BMC Bioinform. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, L.L.; Jiang, J.G.; Calin, N.; Lam, K.F.; Zhang, S.J.; Wu, H.H.; Wu, G.D.; Albela, B.; Bonneviot, L.; et al. Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Luechinger, M.; Pirngruber, G.D.; Lindlar, B.; Laggner, P.; Prins, R. The effect of the hydrophobicity of aromatic swelling agents on pore size and shape of mesoporous silicas. Microporous Mesoporous Mater. 2005, 79, 41–52. [Google Scholar] [CrossRef]
- Zampini, G.; Planas, O.; Marmottini, F.; Gulías, O.; Agut, M.; Nonell, S.; Latterini, L. Morphology effects on singlet oxygen production and bacterial photoinactivation efficiency by different silica-protoporphyrin IX nanocomposites. RSC Adv. 2017, 7, 14422–14429. [Google Scholar] [CrossRef] [Green Version]
- Tainer, J.A.; Getzoff, E.D.; Beem, K.M.; Richardson, J.S.; Richardson, D.C. Determination and analysis of the 2 Å structure of copper, zinc superoxide dismutase. J. Mol. Biol. 1982, 160, 181–217. [Google Scholar] [CrossRef]
- Altobelli, G.G.; Van Noorden, S.; Balato, A.; Cimini, V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front. Med. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.F.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Yang, S.C.; Tang, Y.M.; Hwang, T.I.S. Acridine orange exhibits photodamage in human bladder cancer cells under blue light exposure. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thomé, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent Advances of Gold Nanoparticles in Biomedical Applications: State of the Art. Cell Biochem. Biophys. 2019, 77, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, S.; Xia, P.; Chang, Y.; Fu, C.; Kong, W.; Yu, Z.; Wang, K.; Yang, X.; Qi, Z. Advances in the application of gold nanoparticles in bone tissue engineering. J. Biol. Eng. 2020, 14, 1–15. [Google Scholar] [CrossRef]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. 2014, 6, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Lopes, T.S.; Alves, G.G.; Pereira, M.R.; Granjeiro, J.M.; Leite, P.E.C. Advances and potential application of gold nanoparticles in nanomedicine. J. Cell. Biochem. 2019, 120, 16370–16378. [Google Scholar] [CrossRef] [PubMed]
- Krohn-Grimberghe, M.; Mitchell, M.J.; Schloss, M.J.; Khan, O.F.; Courties, G.; Guimaraes, P.P.G.; Rohde, D.; Cremer, S.; Kowalski, P.S.; Sun, Y.; et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat. Biomed. Eng. 2020, 4, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Chen, W.; Hung, Y.; Mou, C.Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef] [PubMed]
- Yah, C.S. The toxicity of gold nanoparticles in relation to their physiochemical properties. Biomed. Res. 2013, 24, 400–413. [Google Scholar]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauß, S.; Neumeister, A.; Barcikowski, S.; Kracht, D.; Kuhbier, J.W.; Radtke, C.; Reimers, K.; Vogt, P.M. Adhesion, Vitality and Osteogenic Differentiation Capacity of Adipose Derived Stem Cells Seeded on Nitinol Nanoparticle Coatings. PLoS ONE 2013, 8, e53309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicchi, I.; Emiliani, C.; Vescovi, A.; Martino, S. The Big Bluff of Amyotrophic Lateral Sclerosis Diagnosis: The Role of Neurodegenerative Disease Mimics. Neurodegener. Dis. 2015, 15, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Barnard, S.; Moquet, J.; Ellender, M.; Rana, Z.; Burdak-Rothkamm, S. DNA Damage Foci: Meaning and Significance. Environ. Mol. Mutagen. 2015, 56, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of nanomaterials: Advanced in vitro models and high throughput methods for human hazard assessment—A review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Effect of surface coating of gold nanoparticles on cytotoxicity and cell cycle progression. Nanomaterials 2018, 8, 1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Li, Z.; Wang, Y.; Liu, H. Gold Nanoparticles Promote Proliferation of Human Periodontal Ligament Stem Cells and Have Limited Effects on Cells Differentiation. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Liu, K.; Niu, W.; Chen, M.; Wang, M.; Xue, Y.; Gao, C.; Ma, P.X.; Lei, B. Gold and gold-silver alloy nanoparticles enhance the myogenic differentiation of myoblasts through p38 MAPK signaling pathway and promote in vivo skeletal muscle regeneration. Biomaterials 2018, 175, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold nanoparticle-induced cell death and potential applications in nanomedicine. Int. J. Mol. Sci. 2018, 19, 754. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.; Lauer, J.; Selig, M.; Hanak, M.; Walters, B.; Rolauffs, B. Shaping the Cell and the Future: Recent Advancements in Biophysical Aspects Relevant to Regenerative Medicine. J. Funct. Morphol. Kinesiol. 2017, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Joglekar, M.; Roggers, R.A.; Zhao, Y.; Trewyn, B.G. Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells. RSC Adv. 2013, 3, 2454–2461. [Google Scholar] [CrossRef]
- Böcking, D.; Wiltschka, O.; Niinimäki, J.; Shokry, H.; Brenner, R.; Lindén, M.; Sahlgren, C. Mesoporous silica nanoparticle-based substrates for cell directed delivery of Notch signalling modulators to control myoblast differentiation. Nanoscale 2014, 6, 1490–1498. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argentati, C.; Morena, F.; Fontana, C.; Tortorella, I.; Emiliani, C.; Latterini, L.; Zampini, G.; Martino, S. Functionalized Silica Star-Shaped Nanoparticles and Human Mesenchymal Stem Cells: An In Vitro Model. Nanomaterials 2021, 11, 779. https://doi.org/10.3390/nano11030779
Argentati C, Morena F, Fontana C, Tortorella I, Emiliani C, Latterini L, Zampini G, Martino S. Functionalized Silica Star-Shaped Nanoparticles and Human Mesenchymal Stem Cells: An In Vitro Model. Nanomaterials. 2021; 11(3):779. https://doi.org/10.3390/nano11030779
Chicago/Turabian StyleArgentati, Chiara, Francesco Morena, Chiara Fontana, Ilaria Tortorella, Carla Emiliani, Loredana Latterini, Giulia Zampini, and Sabata Martino. 2021. "Functionalized Silica Star-Shaped Nanoparticles and Human Mesenchymal Stem Cells: An In Vitro Model" Nanomaterials 11, no. 3: 779. https://doi.org/10.3390/nano11030779
APA StyleArgentati, C., Morena, F., Fontana, C., Tortorella, I., Emiliani, C., Latterini, L., Zampini, G., & Martino, S. (2021). Functionalized Silica Star-Shaped Nanoparticles and Human Mesenchymal Stem Cells: An In Vitro Model. Nanomaterials, 11(3), 779. https://doi.org/10.3390/nano11030779