Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Extraction and Collection
2.3. Planning and Conducting the Review
3. Results
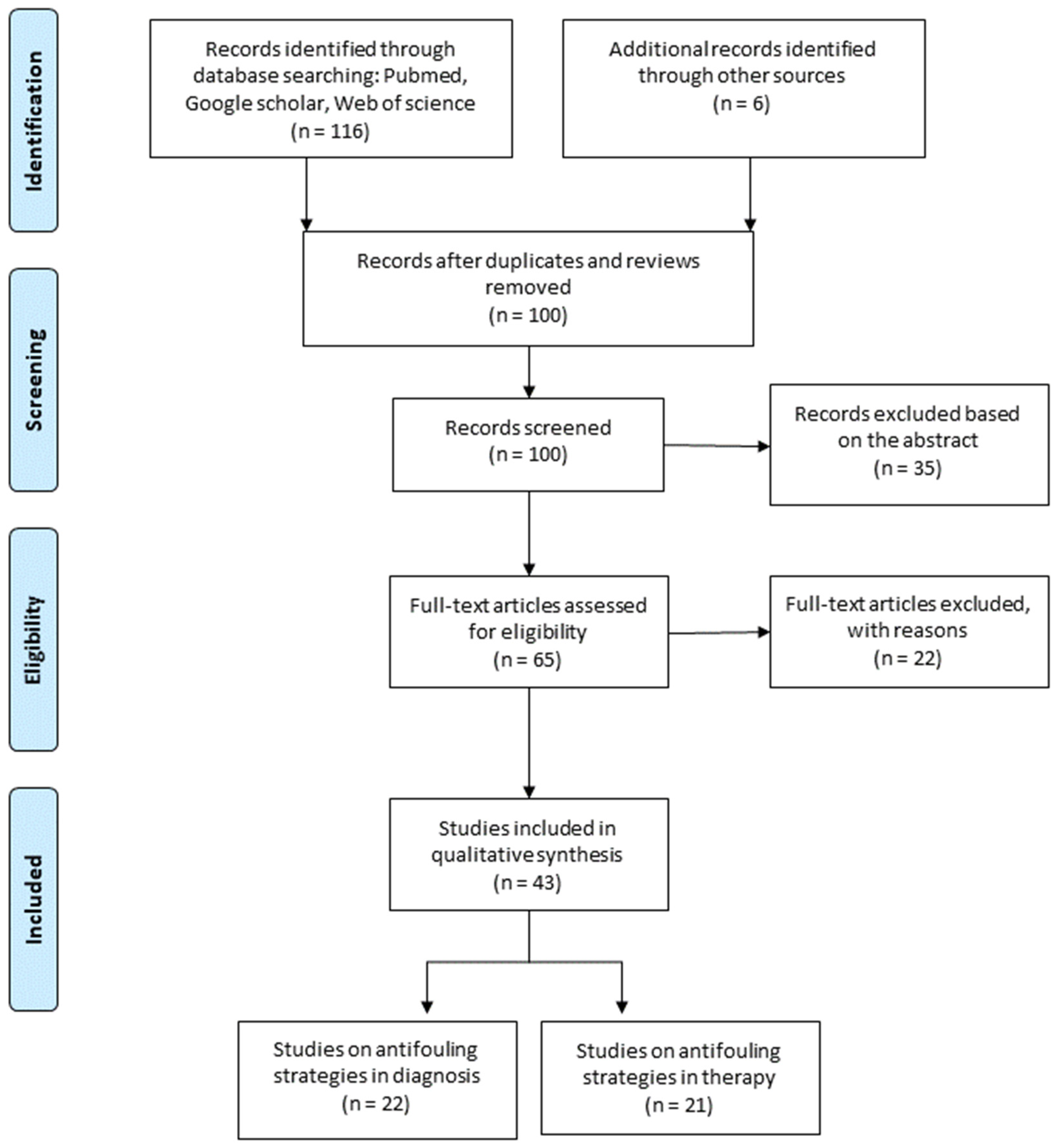
3.1. Study Selection
3.2. Antifouling Strategies for Diagnostic Purpose
3.2.1. PEGylation Strategy
3.2.2. Zwitterionic Strategy
3.2.3. Other Strategies
3.3. Antifouling Strategies for Therapeutic Purpose
3.3.1. PEGylation Strategy
3.3.2. Zwitterionic Strategy
3.3.3. Other Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of Nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Dausend, J.; Musyanovych, A.; Dass, M.; Walther, P.; Schrezenmeier, H.; Landfester, K.; Mailaender, V. Uptake Mechanism of Oppositely Charged Fluorescent Nanoparticles in HeLa Cells. Macromol. Biosci. 2008, 8, 1135–1143. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Hua, C.; Liu, Z.; Bohinc, K.; Guo, X. Spherical Polyelectrolyte Brushes as Templates to Prepare Hollow Silica Spheres Encapsulating Metal Nanoparticles. Nanomaterials 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, S.; Landfester, K.; Mailaender, V. Controlling the Stealth Effect of Nanocarriers through Understanding the Protein Corona. Angew. Chem. Int. Ed. 2016, 55, 8806–8815. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A Decade of the Protein Corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef]
- Safi, M.; Courtois, J.; Seigneuret, M.; Conjeaud, H.; Berret, J.F. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials 2011, 32, 9353–9363. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Chung, H.-J.; Kim, H.-J.; Hong, S.-T. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomed.-Nanotechnol. Biol. Med. 2020, 23. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772-U1000. [Google Scholar] [CrossRef]
- Filipovic, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283. [Google Scholar] [CrossRef] [PubMed]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling evolution of protein corona: A prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Docter, D.; Heine, M.; del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F.; et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016, 45, 2440–2457. [Google Scholar] [CrossRef]
- Sanchez-Cano, C.; Carril, M. Recent Developments in the Design of Non-Biofouling Coatings for Nanoparticles and Surfaces. Int. J. Mol. Sci. 2020, 21, 1007. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Cho, M.; Kim, S.R.; Choi, M.; Lee, J.Y.; Han, B.S.; Park, S.N.; Yu, M.K.; Jon, S.; Jeong, J. Pulmonary toxicity and kinetic study of Cy5.5-conjugated superparamagnetic iron oxide nanoparticles by optical imaging. Toxicol. Appl. Pharmacol. 2009, 239, 106–115. [Google Scholar] [CrossRef]
- Oh, M.H.; Lee, N.; Kim, H.; Park, S.P.; Piao, Y.; Lee, J.; Jun, S.W.; Moon, W.K.; Choi, S.H.; Hyeon, T. Large-Scale Synthesis of Bioinert Tantalum Oxide Nanoparticles for X-ray Computed Tomography Imaging and Bimodal Image-Guided Sentinel Lymph Node Mapping. J. Am. Chem. Soc. 2011, 133, 5508–5515. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, K.; Liu, J.; Han, X.; Ren, J.; Qu, X. Anti-Biofouling Polymer-Decorated Lutetium-Based Nanoparticulate Contrast Agents for In Vivo High-Resolution Trimodal Imaging. Small 2014, 10, 2429–2438. [Google Scholar] [CrossRef]
- Jeong, K.; Lee, Y.-D.; Park, S.; Lee, E.; Lim, C.-K.; Lee, K.E.; Jeon, H.; Kim, J.; Kwon, I.C.; Park, C.R.; et al. Poly(oxyethylene sugaramide)s: Unprecedented multihydroxyl building blocks for tumor-homing nanoassembly. J. Mater. Chem. B 2013, 1, 3437–3442. [Google Scholar] [CrossRef]
- Li, Y.; Lin, R.; Wang, L.; Huang, J.; Wu, H.; Cheng, G.; Zhou, Z.; MacDonald, T.; Yang, L.; Mao, H. PEG-b-AGE polymer coated magnetic nanoparticle probes with facile functionalization and anti-fouling properties for reducing non-specific uptake and improving biomarker targeting. J. Mater. Chem. B 2015, 3, 3591–3603. [Google Scholar] [CrossRef]
- Tu, C.-C.; Chen, K.-P.; Yang, T.-A.; Chou, M.-Y.; Lin, L.Y.; Li, Y.-K. Silicon Quantum Dot Nanoparticles with Antifouling Coatings for Immunostaining on Live Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 13714–13723. [Google Scholar] [CrossRef]
- Suarez-Garcia, S.; Esposito, T.V.F.; Neufeld-Peters, J.; Bergamo, M.; Yang, H.; Saatchi, K.; Schaffer, P.; Hafeli, U.O.; Ruiz-Molina, D.; Rodriguez-Rodriguez, C.; et al. Hybrid Metal-Phenol Nanoparticles with Polydopamine-like Coating for PET/SPECT/CT Imaging. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Elsabahy, M.; Song, Y.; Wang, H.; Su, L.; Letteri, R.A.; Khan, S.; Heo, G.S.; Sun, G.; Liu, Y.; et al. Functional, Degradable Zwitterionic Polyphosphoesters as Biocompatible Coating Materials for Metal Nanostructures. Langmuir 2019, 35, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Li, Y.; Li, Y.; Jin, H.; Zheng, Y.; Huang, W.; Chen, S. Tumor-specific design of PEGylated gadolinium-based nanoscale particles: Facile synthesis, characterization, and improved magnetic resonance imaging of metastasis lung cancer. J. Photochem. Photobiol. B-Biol. 2020, 202. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, J.; Zhou, B.; Hu, Y.; Xing, L.; Xu, F.; Shen, M.; Zhang, G.; Shi, X. Antifouling Manganese Oxide Nanoparticles: Synthesis, Characterization, and Applications for Enhanced MR Imaging of Tumors. ACS Appl. Mater. Interfaces 2017, 9, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, J.; Luo, Y.; Wang, H.; Shi, X. Zwitterion-coated ultrasmall iron oxide nanoparticles for enhanced T-1-weighted magnetic resonance imaging applications. J. Mater. Chem. B 2017, 5, 7267–7273. [Google Scholar] [CrossRef]
- Wang, P.; Xu, X.; Wang, Y.; Zhou, B.; Qu, J.; Li, J.; Shen, M.; Xia, J.; Shi, X. Zwitterionic Polydopamine-Coated Manganese Oxide Nanoparticles with Ultrahigh Longitudinal Relaxivity for Tumor-Targeted MR Imaging. Langmuir 2019, 35, 4336–4341. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Y.; Zhu, J.; Li, X.; He, Y.; Qu, J.; Shen, M.; Xia, J.; Shi, X. Dendrimers meet zwitterions: Development of a unique antifouling nanoplatform for enhanced blood pool, lymph node and tumor CT imaging. Nanoscale 2017, 9, 12295–12301. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.; Zhang, J.; Peng, C.; Klajnert-Maculewicz, B.; Shen, M.; Shi, X. Zwitterionic Gadolinium(III)-Complexed Dendrimer-Entrapped Gold Nanoparticles for Enhanced Computed Tomography/Magnetic Resonance Imaging of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 15212–15221. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, L.; Fan, Y.; Zhao, J.; Shi, X.; Shen, M. I-131-Labeled Multifunctional Polyphosphazene Nanospheres for SPECT Imaging-Guided Radiotherapy of Tumors. Adv. Healthc. Mater. 2019, 8, 1901299. [Google Scholar] [CrossRef]
- Ferretti, A.M.; Usseglio, S.; Mondini, S.; Drago, C.; La Mattina, R.; Chini, B.; Verderio, C.; Leonzino, M.; Cagnoli, C.; Joshi, P.; et al. Towards bio-compatible magnetic nanoparticles: Immune-related effects, in-vitro internalization, and in-vivo bio-distribution of zwitterionic ferrite nanoparticles with unexpected renal clearance. J. Colloid Interface Sci. 2021, 582, 678–700. [Google Scholar] [CrossRef]
- Tasso, M.; Giovanelli, E.; Zala, D.; Bouccara, S.; Fragola, A.; Hanafi, M.; Lenkei, Z.; Pons, T.; Lequeux, N. Sulfobetaine-Vinylimidazole Block Copolymers: A Robust Quantum Dot Surface Chemistry Expanding Bioimaging’s Horizons. ACS Nano 2015, 9, 11479–11489. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, X.; Zhang, B.; Liu, F.; Cheng, Y.; Shi, D. Surface engineered antifouling optomagnetic SPIONs for bimodal targeted imaging of pancreatic cancer cells. Int. J. Nanomed. 2014, 9, 1601–1615. [Google Scholar] [CrossRef]
- Balagurunathan, Y.; Kumar, V.; Gu, Y.H.; Kim, J.; Wang, H.; Liu, Y.; Goldgof, D.B.; Hall, L.O.; Korn, R.; Zhao, B.S.; et al. Test-Retest Reproducibility Analysis of Lung CT Image Features. J. Digit. Imaging 2014, 27, 805–823. [Google Scholar] [CrossRef]
- Cotin, G.; Blanco-Andujar, C.; Nguyen, D.V.; Affolter, C.; Boutry, S.; Boos, A.; Ronot, P.; Uring-Lambert, B.; Choquet, P.; Zorn, P.E.; et al. Dendron based antifouling, MRI and magnetic hyperthermia properties of different shaped iron oxide nanoparticles. Nanotechnology 2019, 30, 374002. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Chao, Y.; Park, J.-H.; Sailor, M.J.; Ruoslahti, E.; Esener, S.C.; Simberg, D. Different Effect of Hydrogelation on Antifouling and Circulation Properties of Dextran-Iron Oxide Nanoparticles. Mol. Pharm. 2012, 9, 539–545. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Yu, Q.; Qian, W.; Tiwari, D.; Yi, H.; Wang, A.Y.; Huang, J.; Yang, L.; Mao, H. Anti-HER2 antibody and ScFvEGFR-conjugated antifouling magnetic iron oxide nanoparticles for targeting and magnetic resonance imaging of breast cancer. Int. J. Nanomed. 2013, 8, 3781–3794. [Google Scholar] [CrossRef]
- Lv, L.; Zhuang, Y.-X.; Zhang, H.-W.; Tian, N.-N.; Dang, W.-Z.; Wu, S.-Y. Capsaicin-loaded folic acid-conjugated lipid nanoparticles for enhanced therapeutic efficacy in ovarian cancers. Biomed. Pharmacother. 2017, 91, 999–1005. [Google Scholar] [CrossRef]
- Park, S.; Jeong, K.; Lee, E.; Lee, J.H.; Yhee, J.Y.; Singh, A.; Koh, J.; Lee, S.; Kim, K.; Kwon, I.C.; et al. Amphiphilized poly(ethyleneimine) nanoparticles: A versatile multi-cargo carrier with enhanced tumor-homing efficiency and biocompatibility. J. Mater. Chem. B 2015, 3, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wu, M.; Zhang, C.; Lin, X.; Wei, Z.; Zheng, Y.; Zhang, D.; Zhang, Z.; Liu, X. Photoresponsive lipid-polymer hybrid nanoparticles for controlled doxorubicin release. Nanotechnology 2017, 28, 255101. [Google Scholar] [CrossRef]
- Elsabahy, M.; Zhang, S.; Zhang, F.; Deng, Z.J.; Lim, Y.H.; Wang, H.; Parsamian, P.; Hammond, P.T.; Wooley, K.L. Surface Charges and Shell Crosslinks Each Play Significant Roles in Mediating Degradation, Biofouling, Cytotoxicity and Immunotoxicity for Polyphosphoester-based Nanoparticles. Sci. Rep. 2013, 3, 3313. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chu, S.-H.; Li, C.-H.; Lee, T.R. Surface modification with zwitterionic cysteine betaine for nanoshell-assisted near-infrared plasmonic hyperthermia. Colloids Surf. B-Biointerfaces 2016, 145, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Wu, D.; Fan, M.; Wang, H.; Liao, Y.; Wang, Q.; Yang, Y. Injectable zwitterionic thermosensitive hydrogels with low-protein adsorption and combined effect of photothermal-chemotherapy. J. Mater. Chem. B 2020, 8, 10637–10649. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kang, K.; Zhang, Y.; Yi, Q.; Gu, Z. Detachable Polyzwitterion-Coated Ternary Nanoparticles Based on Peptide Dendritic Carbon Dots for Efficient Drug Delivery in Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 43923–43935. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Alves, C.S.; Wang, J.; Li, A.; Liu, J.; Shen, M.; Rodrigues, J.; Tomas, H.; Shi, X. Zwitterion-functionalized dendrimer-entrapped gold nanoparticles for serum-enhanced gene delivery to inhibit cancer cell metastasis. Acta Biomater. 2019, 99, 320–329. [Google Scholar] [CrossRef]
- Ding, F.; Yang, S.; Gao, Z.; Guo, J.; Zhang, P.; Qiu, X.; Li, Q.; Dong, M.; Hao, J.; Yu, Q.; et al. Antifouling and pH-Responsive Poly(Carboxybetaine)-Based Nanoparticles for Tumor Cell Targeting. Front. Chem. 2019, 7, 770. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.; Zhang, K.; Lin, Q.; Ye, E.; Poma, A.; Battaglia, G.; Loh, X.J.; Lee, T.-C. Biocompatible pH-responsive nanoparticles with a core-anchored multilayer shell of triblock copolymers for enhanced cancer therapy. J. Mater. Chem. B 2017, 5, 4421–4425. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Z.; Tong, Z.; Zhang, S.; Min, J.; Xu, Q.; Zhou, L.; Mao, Z.; Xia, H.; Wang, W. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics 2020, 10, 5195–5208. [Google Scholar] [CrossRef]
- Wu, J.; Han, H.; Jin, Q.; Li, Z.; Li, H.; Ji, J. Design and Proof of Programmed 5-Aminolevulinic Acid Prodrug Nanocarriers for Targeted Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 14596–14605. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Y.; Li, H.; Jin, Q.; Ji, J. Zwitterionic stealth peptide-capped 5-aminolevulinic acid prodrug nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 2017, 485, 251–259. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Xu, X.; Fan, Y.; Xue, X.; Shen, M.; Shi, X. Targeted Combination of Antioxidative and Anti-Inflammatory Therapy of Rheumatoid Arthritis using Multifunctional Dendrimer-Entrapped Gold Nanoparticles as a Platform. Small 2020, 16. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Zhang, X.; Gooding, J.J.; Zhou, Y. Targeted Drug Delivery: Carbon-Quantum-Dots-Loaded Mesoporous Silica Nanocarriers with pH-Switchable Zwitterionic Surface and Enzyme-Responsive Pore-Cap for Targeted Imaging and Drug Delivery to Tumor (Adv. Healthcare Mater. 12/2016). Adv. Healthc. Mater. 2016, 5, 1380. [Google Scholar] [CrossRef]
- Chen, D.; Huang, Y.; Xu, S.; Jiang, H.; Wu, J.; Jin, X.; Zhu, X. Self-Assembled Polyprodrug Amphiphile for Subcutaneous Xenograft Tumor Inhibition with Prolonged Acting Time In Vivo. Macromol. Biosci. 2017, 17, 1700174. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, H.; Guo, D.; Yasen, W.; Ao, J.; Su, Y.; Pan, D.; Jin, X.; Zhu, X. Anti-biofouling therapeutic nanoparticles with removable shell and highly efficient internalization by cancer cells. Biomater. Sci. 2019, 7, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, C.; Wang, X.; Guo, D.; Duncan, T.M.; Luo, J. Zwitterionic Janus Dendrimer with distinct functional disparity for enhanced protein delivery. Biomaterials 2019, 215, 119233. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, J.; Zhang, F.; Zhang, J.; Paholak, H.; Sun, D. Highly crystallized iron oxide nanoparticles as effective and biodegradable mediators for photothermal cancer therapy. J. Mater. Chem. B 2014, 2, 757–765. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef]
- Huang, B.; Yang, Z.; Fang, S.; Li, Y.; Zhong, Z.; Zheng, R.; Zhang, J.; Wang, H.; Wang, S.; Zou, Q.; et al. Amphoteric natural starch-coated polymer nanoparticles with excellent protein corona-free and targeting properties. Nanoscale 2020, 12, 5834–5847. [Google Scholar] [CrossRef] [PubMed]
- Chrastina, A.; Massey, K.A.; Schnitzer, J.E. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Scherphof, G.L.; Kamps, J. Receptor versus non-receptor mediated clearance of liposomes. Adv. Drug Deliv. Rev. 1998, 32, 81–97. [Google Scholar] [CrossRef]
- Jain, P.; Pawar, R.S.; Pandey, R.S.; Madan, J.; Pawar, S.; Lakshmi, P.K.; Sudheesh, M.S. In-vitro in-vivo correlation (IVIVC) in nanomedicine: Is protein corona the missing link? Biotechnol. Adv. 2017, 35, 889–904. [Google Scholar] [CrossRef]
- Peng, Q.; Mu, H.L. The potential of protein-nanomaterial interaction for advanced drug delivery. J. Control. Release 2016, 225, 121–132. [Google Scholar] [CrossRef]
- Garcia, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Brooks, D.E. In vivo biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials 2007, 28, 4779–4787. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Estephan, Z.G.; Schlenoff, P.S.; Schlenoff, J.B. Zwitteration As an Alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.S.; Deschatelets, P.; Whitesides, G.M. Kosmotropes form the basis of protein-resistant surfaces. Langmuir 2003, 19, 2388–2391. [Google Scholar] [CrossRef]

| Author | Year | Nanostructure | Antifouling Moiety | In Vitro Model | In Vivo Model | Application |
|---|---|---|---|---|---|---|
| Cho et al. | 2009 [15] | Iron Oxide NPs (TCL-SPION) | poly(3-(trimethoxysilyl)propyl methacrylate-r-PEG methylethermethacrylate-r-N-acryloxysuccinimide) | - | BALB/c mice | Optical imaging (OI) |
| Oh et al. | 2011 [16] | Tantalum Oxide (TaOx NPs) | Polyethylen Glycol (PEG) | RAW264.7 | Rats | Bimodal imaging (Computed Tomography (CT) and OI) |
| Liu et al. | 2014 [17] | Hybrid Lutetium Oxide NPs (UCNPs) | PEG | MCF-7 | Kunming mice, C57BL/6 mice, and wister rats | Multimodal imaging (up-conversion luminescent, X-ray and Magnetic Resonance Imaging (MRI)) |
| Joeng et al. | 2013 [18] | Poly (oxyethylene galactaramide)s (PEGA) NPs | PEGA | Hela | SCC7 tumour-bearing mice | OI |
| Li et al. | 2015 [19] | Magnetic Iron Oxide NPs (IONPs) | PEG and allyl glycidyl ether (PEG-b-AGE) | RAW264.7, D556, Daoy, U87MG, MDA-MB-231, MCF7 and A549 | - | Theranostic |
| Tu et al. | 2016 [20] | Silicon Quantum DotNanoparticles (SiQD-NPs) | PEG and Bovine Serum Albumin (BSA) | CHO, SKOV3 | - | OI |
| Suàrez-Garzìa | 2021 [21] | Metal-phenolic NPs (MPS) | PEG | CT26, HeLa, 3T3 | bearing CT26 tumour-bearing mice | SPECT/PET |
| Li et al. | 2019 [22] | Gold NPs (AuNPs) | L-cysteine-functionalized with poly(but-3-yn-1-yloxy)-2-oxo- 1,3,2-dioxaphospholane (zPBYP) | RAW264.7 | - | Theranostic |
| Sui et al. | 2020 [23] | Ultrasmall Gadolinium oxide NPs Gd2O3 NPs) | PEG-L-cysteine | RAW264.7 | B16 lung cancer metastasis mouse model | MRI |
| Wang et al. | 2017 [24] | Manganese Oxide NPs (Mn3O4NPs) | PEG-L-cysteine | C6 and Raw 264.7 | Mouse | MRI |
| Ma et al. | 2017 [25] | Iron Oxide NPs (Fe3O4NPs) | PEG-L-cysteine | L929 | Rats | MRI |
| Wang et al. | 2019 [26] | Mn3O4 NPs | L-lysine | KB | Mouse | MRI |
| Xiong et al. | 2017 [27] | Dendrimer-entrapped gold NPs (Au DENPs) | Carboxybetaine Acrylamide (CBAA) | U87MG | U87MG tumour-bearing mice | X-ray CT |
| Liu et al. | 2019 [28] | Gadolinium(-Complexed Dendrimer-Entrapped Gold NPs (Gd-Au DEN-PS.) | CBAA, 2-methacryloyloxyethyl phosphorylcholine (MPC) or 1,3-propane sultone (1,3-PS) | Macrophage | B16 lung cancer metastasis mouse model | Bimodal imaging (X-ray CT and MRI) |
| Zhu et al. | 2019 [29] | poly(cyclotriphosphazene-co-polyethylenimine) nanospheres (PNSs) | 1,3-PS | 4T1 cells | 4T1 tumor-bearing mouse | Theranostic |
| Ferretti et al. | 2020 [30] | IONPs | Zwitterionic Dopamine Sulfonate (ZDS) | BV2 and glial cells | CD-1 mice | MRI |
| Tasso et al. | 2015 [31] | Quantum dot (QD) | Poly(methacrylamidosulfobetaine-block-4-vinylimidazole) | HEK293 | - | OI |
| Wang et al. | 2014 [32] | Superparamagnetic Iron Oxide NPs (SPIO) | BSA | PDAC, Panc-1 and L02 | - | MRI |
| Lamanna et al. | 2011 [33] | IONPs | Phosphonate | U87MG | Rats | Multimodal imaging (OI and MRI) |
| Cotin et al. | 2019 [34] | IONPs | Dendron coating | Huh7 | CD-1 mice | Theranostic |
| Karmali et al. | 2012 [35] | SPIO | Crosslinked dextran | - | C57BL/6J mice | MRI |
| Chen et al. | 2013 [36] | IONPs | Poly(ethylene oxide)-block-poly(γ-methacryloxypropyltrimethoxysilane) (PEO-b-PγMPS) | SK-BR-3, MDA-MB-231, MCF-7, MDA-MB-453, 4T1 and RAW264.7 | 4T1 mice | MRI |
| Author | Year | Nanostructure | Antifouling Moiety | In Vitro Model | In Vivo Model | Application |
|---|---|---|---|---|---|---|
| Lv et al. | 2017 [37] | Lipidic NPs | polyethylene glycol (PEG) | SKOV-3 | - | Targeted drug delivery |
| Park et al. | 2015 [38] | poly(ethyleneimine) (aPEI) NPs | PEG | Hela | SCC7 tumor bearing mice | Drug delivery |
| Yao et al. | 2017 [39] | Poly(D,L-lactide-co-glycolide) (PLGA) NPs | PEG-hydrophilic block, hexadecyl hydrophobic block, and a 2-nitrobenzyl linker | HepG2 and HeLa | HepG2 tumour-bearing nude Balb/c mice | Drug delivery |
| Elsabahy et al. | 2013 [40] | Polyphosphoester (PPE) micelle | Zwitterionic diblock copolymers (acrylic acid/amino group (1:1)) | RAW 264.7 | - | Drug delivery |
| Huang et al. | 2016 [41] | Hollow gold-silver nanoshells | Cysteine betaine (Cys-b) | MDA-MB-453 | - | Hyperthermia |
| Zheng et al. | 2020 [42] | Poly(N-isopropylacrylamide) (PNIPAM) Nanogels | Sulfobetaine methacrylate (SBMA) | L929 and HepG2 | H22-bearing mice | Photothermal drug delivery |
| Ma et al. | 2018 [43] | Dendritic carbon dots (CDs) | Poly(carboxybetaine methacrylate) (pCBMA) | 4T1 and HepG2 | BALB/c mice | Drug delivery |
| Xiong et al. | 2019 [44] | Dendrimer-entrapped gold NPs (Au DENPs) | Carboxybetaine acrylamide (CBAA) | Hela | - | Gene delivery |
| Ding et al. | 2019 [45] | poly(2-(diisopropylamino)ethyl methacrylate) (PDPA)NPs | pCBMA | RAW 264.7, HeLa, and U87 | - | Targeted drug delivery |
| Ellis et al. | 2017 [46] | Gold NPs (Au NPs) | Poly(2-(methacryloyloxy)ethyl phosphorylcholine) pMPC | MCF-7 | - | Drug delivery |
| Ding et al. | 2020 [47] | Au NPs | Peptide sequence of glutamic acid and lysine | LM3 | LM3 Tumour-bearing mice | Radiotherapy |
| Wu et al. | 2017 [48] | Au NPs | Peptide sequence of glutamic acid and lysine | SCC-7 | SCC-7 tumour-bearing mice | Photodynamic therapy |
| Wu et al. | 2017 [49] | Au NPs | Peptide sequence of glutamic acid and lysine and RGD moieties | A549 | - | Photodynamic therapy |
| Li et al. | 2020 [50] | Au DENPs | 1,3-propane sultone (1,3-PS) | RAW264.7 | Collagen-induced arthritis (CIA) mouse | Targeted drug delivery |
| Liu et al. | 2016 [51] | Mesoporous Silica NPs | -COO− and -HN+(Me)2 | SCC, HaCaT | - | Theranostic |
| Chen et al. | 2017 [52] | poly(10-hydroxy-camptothecin methacrylate (pMPC-b-pHCPT) Micelles | pMPC | HeLa and L929 | Kunming mice and nude mice | Drug delivery |
| Chen et al. | 2019 [53] | Cationic gelatin NPs ((+)GNPs) | pMPC | HeLa | SD rats and nude mice | Targeted drug delivery |
| Wang et al. | 2019 [54] | Janus dendrimer (JD GPC) | Glycerylphosphorylcholine (GPC) | HT-29, SKOV-3, U87 | BALB/c mice, | Drug delivery |
| Chen et al. | 2014 [55] | Crystallized iron NPs (HCIONPs) | Poly(ethylene oxide)-block-poly(γ-methacryloxypropyltrimethoxysilane) (PEO-b-PγMPS) | SUM-159 | SUM-159 tumor-bearing BALB/c mice | Targeted drug delivery |
| Shi et al. | 2018 [56] | Silver NPs (Ag NPs) | Chitosan and dextran | NIH 3T3 | SD rats | Targeted drug therapy |
| Huang et al. | 2020 [57] | (Etherified starch-coated poly(methyl methacrylate- co-acrylic acid) Micelles | Amphoteric starch | SW480 and A549 | - | Targeted drug therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevilacqua, P.; Nuzzo, S.; Torino, E.; Condorelli, G.; Salvatore, M.; Grimaldi, A.M. Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials 2021, 11, 780. https://doi.org/10.3390/nano11030780
Bevilacqua P, Nuzzo S, Torino E, Condorelli G, Salvatore M, Grimaldi AM. Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials. 2021; 11(3):780. https://doi.org/10.3390/nano11030780
Chicago/Turabian StyleBevilacqua, Paolo, Silvia Nuzzo, Enza Torino, Gerolama Condorelli, Marco Salvatore, and Anna Maria Grimaldi. 2021. "Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature" Nanomaterials 11, no. 3: 780. https://doi.org/10.3390/nano11030780
APA StyleBevilacqua, P., Nuzzo, S., Torino, E., Condorelli, G., Salvatore, M., & Grimaldi, A. M. (2021). Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials, 11(3), 780. https://doi.org/10.3390/nano11030780







