Self-Assembly Magnetic Micro- and Nanospheres and the Effect of Applied Magnetic Fields
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Magnetic Measurements
3.2. Drop-Casting
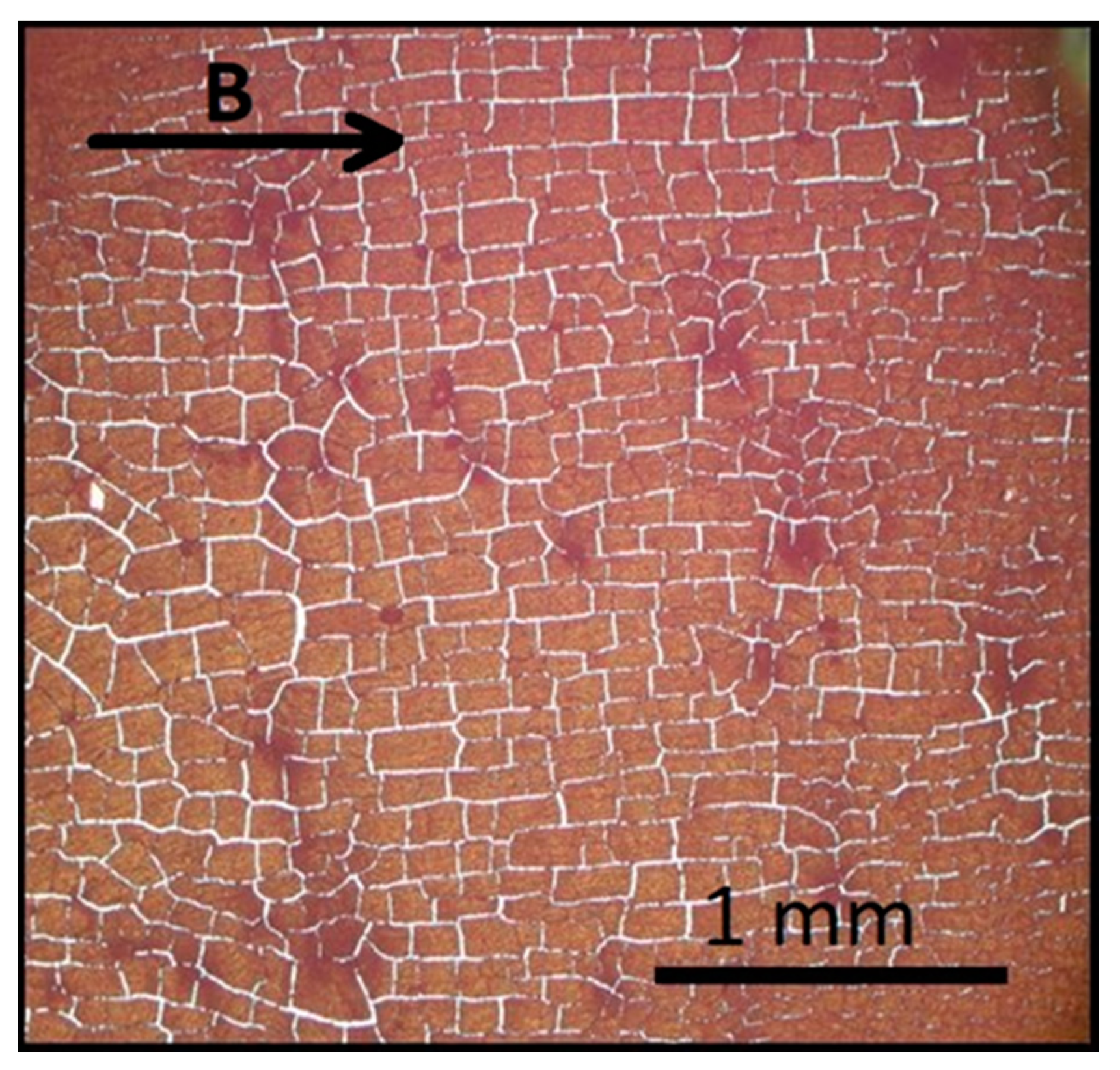
3.2.1. Self-Assembly under Zero Magnetic Field
3.2.2. Self-Assembly in the Presence of Magnetic Field
- In-Plane Field
- Perpendicular Field
3.3. Spin Coating
3.3.1. Spin Coating under Zero Field
3.3.2. Spin Coating in Magnetic Field
- In–Plane Field
- Perpendicular Field
3.4. Spin Coating Followed by SDS Post-Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markou, A.; Beltsios, K.G.; Gergidis, L.N.; Panagiotopoulos, I.; Bakas, T.; Ellinas, K.; Tserepi, A.; Stoleriu, L.; Tanasa, R.; Stancu, A. Magnetization reversal in triangular L10-FePt nanoislands. J. Magn. Magn. Mater. 2013, 344, 224–229. [Google Scholar] [CrossRef]
- Zhong, H.; Tarrach, G.; Wu, P.; Drechsler, A.; Wei, D.; Yuan, J. High resolution magnetic force microscopy of patterned L10-FePt dot arrays by nanosphere lithography. Nanotechnology 2008, 19, 095703. [Google Scholar] [CrossRef]
- Reyes, Y.; Campos-Terán, J.; Vázquez, F.; Duda, Y. Properties of films obtained from aqueous polymer dispersions: Study of drying rate and particle polydispersity effects. Model. Simul. Mater. Sci. Eng. 2007, 15, 355–368. [Google Scholar] [CrossRef]
- Albrecht, M.; Hu, G.; Guhr, I.L.; Ulbrich, T.C.; Boneberg, J.; Leiderer, P.; Schatz, G. Magnetic multilayers on nanospheres. Nat. Mater. 2005, 4, 203–206. [Google Scholar] [CrossRef]
- Colson, P.; Henrist, C.; Cloots, R. Nanosphere lithography: A powerful method for the controlled manufacturing of nanomaterials. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.L.; Nikolić, R.J.; Reinhardt, C.E.; Wang, T.F. Fabrication of nanopillars by nanosphere lithography. Nanotechnology 2006, 17, 1339–1343. [Google Scholar] [CrossRef]
- Zhang, C.; Cvetanovic, S.; Pearce, J.M. Fabricating ordered 2-D nano-structured arrays using nanosphere lithography. MethodsX 2017, 4, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, A.; Sibirev, N.V.; Dubrovskii, V.G.; Petty, M.C.; Gallant, A.J.; Zeze, D.A. Model for large-Area monolayer coverage of polystyrene nanospheres by spin coating. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Colson, P.; Cloots, R.; Henrist, C. Experimental design applied to spin coating of 2d colloidal crystal masks: A relevant method? Langmuir 2011, 27, 12800–12806. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, T.C.; Assmann, D.; Albrecht, M. Magnetic properties of Co/Pt multilayers on self-assembled particle arrays. J. Appl. Phys. 2008, 104, 084311. [Google Scholar] [CrossRef]
- Markou, A.; Panagiotopoulos, I.; Bakas, T.; Postolache, P.; Stoleriu, L.; Stancu, A. Magnetization reversal in graded anisotropy Co/Pt multilayers: A first order reversal curve study. J. Appl. Phys. 2012, 112. [Google Scholar] [CrossRef]
- Burmeister, F.; Schäfle, C.; Matthes, T.; Böhmisch, M.; Boneberg, J.; Leiderer, P. Colloid monolayers as versatile lithographic masks. Langmuir 1997, 13, 2983–2987. [Google Scholar] [CrossRef] [Green Version]
- Rakers, S.; Chi, L.F.; Fuchs, H. Influence of the evaporation rate on the packing order of polydisperse latex monofilms. Langmuir 1997, 13, 7121–7124. [Google Scholar] [CrossRef]
- Tripp, S.L.; Dunin-Borkowski, R.E.; Wei, A. Flux Closure in Self-Assembled Cobalt Nanoparticle Rings. Angew. Chem. 2003. [Google Scholar] [CrossRef]
- Thomas, J.R. Preparation and magnetic properties of colloidal cobalt particles. J. Appl. Phys. 1966, 37, 2914–2915. [Google Scholar] [CrossRef]
- Bishop, K.J.M.; Wilmer, C.E.; Soh, S.; Grzybowski, B.A. Nanoscale forces and their uses in self-assembly. Small 2009, 5, 1600–1630. [Google Scholar] [CrossRef]
- Singamaneni, S.; Bliznyuk, V.N.; Binek, C.; Tsymbal, E.Y. Magnetic nanoparticles: Recent advances in synthesis, self-assembly and applications. J. Mater. Chem. 2011, 21, 16819–16845. [Google Scholar] [CrossRef] [Green Version]
- Jadav, M.; Patel, R. Colloidal self assembly of non-magnetic particles in magnetic nanofluid. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2015; Volume 1665, p. 040015. [Google Scholar] [CrossRef]
- Abelmann, L.; Hageman, T.A.G.; Löthman, P.A.; Mastrangeli, M.; Elwenspoek, M.C. Three-dimensional self-assembly using dipolar interaction. Sci. Adv. 2020, 6, eaba2007. [Google Scholar] [CrossRef]
- Ahniyaz, A.; Sakamoto, Y.; Bergström, L. Magnetic field-induced assembly of oriented superlattices from maghemite nanocubes. Proc. Natl. Acad. Sci. USA 2007, 104, 17570–17574. [Google Scholar] [CrossRef] [Green Version]
- Slöetjes, S.D.; Urdahl, H.H.; Grepstad, J.K.; Folven, E. Tailoring the magnetic order in a supermagnetic metamaterial. AIP Adv. 2017, 7, 056325. [Google Scholar] [CrossRef] [Green Version]
- Pileni, M.P. Nanocrystal self-assemblies: Fabrication and collective properties. J. Phys. Chem. B 2001, 105, 3358–3371. [Google Scholar] [CrossRef]
- Favela-Camacho, S.E.; Samaniego-Benítez, E.J.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Varadan, V.K.; Chen, L.; Xie, J. Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors—Βιβλία Google. Available online: https://books.google.gr/books?hl=el&lr=&id=1sNpk-1ECIoC&oi=fnd&pg=PR5&ots=ydobBv4NOf&sig=wFfwRMvjiT8w0_EPdVWva0aPBtA&redir_esc=y#v=onepage&q&f=false (accessed on 14 January 2021).
- Rybczynski, J.; Ebels, U.; Giersig, M. Large-scale, 2D arrays of magnetic nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 219, 1–6. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [Green Version]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Járai-Szabó, F.; Aştilean, S.; Néda, Z. Understanding self-assembled nanosphere patterns. Chem. Phys. Lett. 2005, 408, 241–246. [Google Scholar] [CrossRef] [Green Version]













| Stage | RPM/s | Duration (s) | Phase | Effect |
|---|---|---|---|---|
| 1 | 150 | 120 | Solution spreads over substrate | |
| 2 | 250 | 120 | Spin-up | Coverage improvement |
| 3 | 800 | 60 | Spin-off | Disordered monolayer formation |
| 4 | 2500 | 20 | MSNs adhesion to substrate | |
| 5 | 5000 | 20 | Self-ordering | Hexagonal packing |
| 6 | 8000 | 360 | Drying | Enhanced hexagonal packing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourkas, A.; Zarlaha, A.; Kourkoumelis, N.; Panagiotopoulos, I. Self-Assembly Magnetic Micro- and Nanospheres and the Effect of Applied Magnetic Fields. Nanomaterials 2021, 11, 1030. https://doi.org/10.3390/nano11041030
Mourkas A, Zarlaha A, Kourkoumelis N, Panagiotopoulos I. Self-Assembly Magnetic Micro- and Nanospheres and the Effect of Applied Magnetic Fields. Nanomaterials. 2021; 11(4):1030. https://doi.org/10.3390/nano11041030
Chicago/Turabian StyleMourkas, Angelos, Angeliki Zarlaha, Nikolaos Kourkoumelis, and Ioannis Panagiotopoulos. 2021. "Self-Assembly Magnetic Micro- and Nanospheres and the Effect of Applied Magnetic Fields" Nanomaterials 11, no. 4: 1030. https://doi.org/10.3390/nano11041030
APA StyleMourkas, A., Zarlaha, A., Kourkoumelis, N., & Panagiotopoulos, I. (2021). Self-Assembly Magnetic Micro- and Nanospheres and the Effect of Applied Magnetic Fields. Nanomaterials, 11(4), 1030. https://doi.org/10.3390/nano11041030







