Multifunctional Magnetic Nanocolloids for Hybrid Solar-Thermoelectric Energy Harvesting
Abstract
:1. Introduction
2. Concepts
2.1. Basic Concepts on the Thermoelectric Energy Conversion in Liquid Electrolytes and Colloidal Suspensions: ‘Thermocell’
2.1.1. Thermogalvanic Effect
2.1.2. Thermoelectro-Diffusion Effect
3. Experimental
3.1. Sample Preparation and Characterization
3.2. Thermoelectric and Electrical Characterizations
3.3. Optical Characterization
4. Results and Discussion
4.1. Ionic Conductivity and Determination of Effective Dynamic Charge
4.2. Thermoelectric Properties
4.2.1. Seebeck Coefficient
4.2.2. Power Output
Low Redox Concentration, No-Convection Regime
High Redox Couple Concentration, No-Convection Regime
High Redox Couple Concentration, Convective Regime
4.3. Optical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinning, C. An inconvenient truth about thermoelectrics. Nat. Mater. 2009, 8, 83–85. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.; Shafii, M.; Mosleh, H.J. A novel concentrating photovoltaic/thermal solar system combined with thermoelectric module in an integrated design. Renew. Energy 2017, 113, 822–834. [Google Scholar] [CrossRef]
- Lertsatitthanakorn, C.; Khasee, N.; Atthajariyakul, S.; Soponronnarit, S.; Therdyothin, A.; Suzuki, R.O. Performance analysis of a double-pass thermoelectric solar air collector. Sol. Energy Mater. Sol. Cells 2008, 92, 1105–1109. [Google Scholar] [CrossRef] [Green Version]
- Narducci, D.; Lorenzi, B. Challenges and Perspectives in Tandem Thermoelectric–Photovoltaic Solar Energy Conversion. IEEE Trans. Nanotechnol. 2016, 15, 348–355. [Google Scholar] [CrossRef]
- Sundarraj, P.; Maity, D.; Roy, S.S.; Taylor, R.A. Recent advances in thermoelectric materials and solar thermoelectric generators—A critical review. RSC Adv. 2014, 4, 46860–46874. [Google Scholar] [CrossRef]
- Allouhi, A. Advances on solar thermal cogeneration processes based on thermoelectric devices: A review. Sol. Energy Mater. Sol. Cells 2019, 200, 109954. [Google Scholar] [CrossRef]
- Zhang, M.; Miao, L.; Kang, Y.P.; Tanemura, S.; Fisher, C.A.; Xu, G.; Li, C.X.; Fan, G.Z. Efficient, low-cost solar thermoelectric cogenerators comprising evacuated tubular solar collectors and thermoelectric modules. Appl. Energy 2013, 109, 51–59. [Google Scholar] [CrossRef]
- Minardi, J.E.; Chuang, H.N. Performance of a ‘black’ liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef] [Green Version]
- Moradi, A.; Sani, E.; Simonetti, M.; Francini, F.; Chiavazzo, E.; Asinari, P. Carbon-Nanohorn Based Nanofluids for a Direct Absorption Solar Collector for Civil Application. J. Nanosci. Nanotechnol. 2015, 15, 3488–3495. [Google Scholar] [CrossRef] [Green Version]
- Lenert, A.; Wang, E.N. Optimization of nanofluid volumetric receivers for solar thermal energy conversion. Sol. Energy 2012, 86, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Wang, C.; Wei, W.; Xiao, G.; Ni, M. Performance improvement of a nanofluid solar collector based on direct absorption collection (DAC) concepts. Int. J. Heat Mass Transf. 2014, 75, 262–271. [Google Scholar] [CrossRef]
- Sani, E.; Barison, S.; Pagura, C.; Mercatelli, L.; Sansoni, P.; Fontani, D.; Jafrancesco, D.; Francini, F. Carbon nanohorns-based nanofluids as direct sunlight absorbers. Opt. Express 2010, 18, 5179–5187. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.; Mercatelli, L.; Barison, S.; Pagura, C.; Agresti, F.; Colla, L.; Sansoni, P. Potential of carbon nanohorn-based suspensions for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2011, 95, 2994–3000. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Adrian, R.; Prasher, R. Nanofluid optical property characterization: Towards efficient direct absorption solar collectors. Nanoscale Res. Lett. 2011, 6, 225. [Google Scholar] [CrossRef] [Green Version]
- Nazari, M.; Rasoulifard, M.; Hosseini, H. Dielectric breakdown strength of magnetic nanofluid based on insulation oil after impulse test. J. Magn. Magn. Mater. 2016, 399, 1–4. [Google Scholar] [CrossRef]
- Shima, P.D.; Philip, J. Tuning of Thermal Conductivity and Rheology of Nanofluids Using an External Stimulus. J. Phys. Chem. C 2011, 115, 20097–20104. [Google Scholar] [CrossRef]
- Bahiraei, M.; Hangi, M. Flow and heat transfer characteristics of magnetic nanofluids: A review. J. Magn. Magn. Mater. 2015, 374, 125–138. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Rodríguez-Schwendtner, E.; Díaz-Herrera, N.; Navarrete, M.C.; González-Cano, A.; Esteban, Ó. Plasmonic sensor based on tapered optical fibers and magnetic fluids for measuring magnetic fields. Sensors Actuators A Phys. 2017, 264, 58–62. [Google Scholar] [CrossRef]
- Horng, H.E.; Chen, C.S.; Fang, K.L.; Yang, S.Y.; Chieh, J.J.; Hong, C.-Y.; Yang, H.C. Tunable optical switch using magnetic fluids. Appl. Phys. Lett. 2004, 85, 5592–5594. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yan, Y.; Fu, R.; Alsaady, M. Enhancement of solar energy collection with magnetic nanofluids. Therm. Sci. Eng. Prog. 2018, 8, 130–135. [Google Scholar] [CrossRef]
- Anu, K.; Hemalatha, J. Magnetically tuned thermoelectric behavior of Zn-doped magnetite nanofluids. Nanotechnology 2020, 32, 025707. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Sarkar, M.; Salez, T.J.; Nakamae, S.; Demouchy, G.; Cousin, F.; Dubois, E.; Michot, L.; Perzynski, R.; Peyre, V. Structural, Thermodiffusive and Thermoelectric Properties of Maghemite Nanoparticles Dispersed in Ethylammonium Nitrate. ChemEngineering 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Salez, T.J.; Kouyaté, M.; Filomeno, C.L.; Bonetti, M.; Roger, M.; Demouchy, G.; Dubois, E.; Perzynski, R.; Cebers, A.; Nakamae, S. Magnetically enhancing the Seebeck coefficient in ferrofluids. Nanoscale Adv. 2019, 1, 2979–2989. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.T.; Roger, M.; Bonetti, M.; Salez, T.J.; Wiertel-Gasquet, C.; Dubois, E.; Gomes, R.C.; Demouchy, G.; Mériguet, G.; Peyre, V.; et al. Thermoelectricity and thermodiffusion in charged colloids. J. Chem. Phys. 2015, 143, 054902. [Google Scholar] [CrossRef] [PubMed]
- Salez, T.J.; Huang, B.T.; Rietjens, M.; Bonetti, M.; Wiertel-Gasquet, C.; Roger, M.; Filomeno, C.L.; Dubois, E.; Perzynski, R.; Nakamae, S. Can charged colloidal particles increase the thermoelectric energy conversion efficiency? Phys. Chem. Chem. Phys. 2017, 19, 9409–9416. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kim, T.; Li, N.; Kang, T.J.; Chen, J.; Pringle, J.M.; Zhang, M.; Kazim, A.H.; Fang, S.; Haines, C.; et al. High Power Density Electrochemical Thermocells for Inexpensively Harvesting Low-Grade Thermal Energy. Adv. Mater. 2017, 29, 1605652. [Google Scholar] [CrossRef]
- Dupont, M.F.; Macfarlane, D.R.; Pringle, J.M. Thermo-electrochemical cells for waste heat harvesting—Progress and perspectives. Chem. Commun. 2017, 53, 6288–6302. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Zou, X.; Liang, Y.; Kimizuka, N. A supramolecular thermocell consisting of ferrocenecarboxylate and β-cyclodextrin that has a negative Seebeck coefficient. Polym. J. 2018, 50, 771–774. [Google Scholar] [CrossRef]
- Hu, R.; Cola, B.A.; Haram, N.; Barisci, J.N.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting Waste Thermal Energy Using a Carbon-Nanotube-Based Thermo-Electrochemical Cell. Nano Lett. 2010, 10, 838–846. [Google Scholar] [CrossRef]
- Keppner, H.; Uhl, S.; Laux, E.; Jeandupeux, L.; Tschanz, J.; Journot, T. Ionic Liquid-based Thermoelectric Generator: Links between Liquid Data and Generator Characteristics. Mater. Today Proc. 2015, 2, 680–689. [Google Scholar] [CrossRef]
- Nernst, W. Die elektromotorische Wirksamkeit der Jonen. Z. Für Phys. Chem. 1889, 4, 129–181. [Google Scholar]
- Salez, T.J.; Nakamae, S.; Perzynski, R.; Mériguet, G.; Cebers, A.; Roger, M. Thermoelectricity and Thermodiffusion in Magnetic Nanofluids: Entropic Analysis. Entropy 2018, 20, 405. [Google Scholar] [CrossRef] [Green Version]
- De Groot, S. Sur la thermodynamique de quelques processus irréversibles. II. Diffusion thermique et phénomènes connexes. J. Phys. Radium 1947, 8, 193–200. [Google Scholar] [CrossRef]
- Agar, J. Thermogalvanic Cells. In Advances in Electrochemistry and Electrochemical Engineering; Delahay, P., Ed.; Interscience: New York, NY, USA, 1963; pp. 31–121. [Google Scholar]
- Revil, A. Ionic Diffusivity, Electrical Conductivity, Membrane and Thermoelectric Potentials in Colloids and Granular Porous Media: A Unified Model. J. Colloid Interface Sci. 1999, 212, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Agar, J.N.; Turner, J.C.R. Thermal diffusion in solutions of electrolytes. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1960, 255, 307–330. [Google Scholar]
- Guibert, C. Etude des Propriétés D’hyperthermie de Nanoparticules Dispersées Dans des Systèmes Complexes. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2015. [Google Scholar]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Guibert, C.; Dupuis, V.; Peyre, V.; Fresnais, J. Hyperthermia of Magnetic Nanoparticles: Experimental Study of the Role of Aggregation. J. Phys. Chem. C 2015, 119, 28148–28154. [Google Scholar] [CrossRef] [Green Version]
- Bacri, J.-C.; Perzynski, R.; Salin, D.; Cabuil, V.; Massart, R. Magnetic colloidal properties of ionic ferrofluids. J. Magn. Magn. Mater. 1986, 62, 36–46. [Google Scholar] [CrossRef]
- Sehgal, A.; Lalatonne, Y.; Berret, J.-F.; Morvan, M. Precipitation−Redispersion of Cerium Oxide Nanoparticles with Poly(acrylic acid): Toward Stable Dispersions. Langmuir 2005, 21, 9359–9364. [Google Scholar] [CrossRef] [Green Version]
- Fresnais, J.; Yan, M.; Courtois, J.; Bostelmann, T.; Bée, A.; Berret, J.-F. Poly(acrylic acid)-coated iron oxide nanoparticles: Quantitative evaluation of the coating properties and applications for the removal of a pollutant dye. J. Colloid Interface Sci. 2013, 395, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Sani, E.; Dell’Oro, A. Optical constants of ethylene glycol over an extremely wide spectral range. Opt. Mater. 2014, 37, 36–41. [Google Scholar] [CrossRef]
- Sani, E.; Dell’Oro, A. Spectral optical constants of ethanol and isopropanol from ultraviolet to far infrared. Opt. Mater. 2016, 60, 137–141. [Google Scholar] [CrossRef]
- ASTM International. Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface; Active Standard ASTM G173. ASTM G173-03; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Vanysek, P. Activity Coefficients of Acids, Bases, and Salts. In Section 5: Thermochemistry, Electrochemistry, and Solution Chemistry; CRC Press: Boca Raton, FL, USA, 2014; pp. 104–105. [Google Scholar]
- Kang, T.J.; Fang, S.; Kozlov, M.E.; Haines, C.S.; Li, N.; Kim, Y.H.; Chen, Y.; Baughman, R.H. Electrical Power From Nanotube and Graphene Electrochemical Thermal Energy Harvesters. Adv. Funct. Mater. 2011, 22, 477–489. [Google Scholar] [CrossRef]
- Hupp, J.T.; Weaver, M.J. Solvent, ligand, and ionic charge effects on reaction entropies for simple transition-metal redox couples. Inorg. Chem. 1984, 23, 3639–3644. [Google Scholar] [CrossRef]
- Burrows, B. Discharge Behavior of Redox Thermogalvanic Cells. J. Electrochem. Soc. 1976, 123, 154. [Google Scholar] [CrossRef]
- Quickenden, T.; Vernon, C. Thermogalvanic conversion of heat to electricity. Sol. Energy 1986, 36, 63–72. [Google Scholar] [CrossRef]
- Quickenden, T.I.; Mua, Y. A Review of Power Generation in Aqueous Thermogalvanic Cells. J. Electrochem. Soc. 1995, 142, 3985–3994. [Google Scholar] [CrossRef]
- Ikeshoji, T. Thermoelectric Conversion by Thin-Layer Thermogalvanic Cells with Soluble Redox Couples. Bull. Chem. Soc. Jpn. 1987, 60, 1505–1514. [Google Scholar] [CrossRef]
- Salazar, P.F.; Kumar, S.; Cola, B.A. Design and optimization of thermo-electrochemical cells. J. Appl. Electrochem. 2013, 44, 325–336. [Google Scholar] [CrossRef]
- Mua, Y.; Quickenden, T.I. Power Conversion Efficiency, Electrode Separation, and Overpotential in the Ferricyanide/Ferrocyanide Thermogalvanic Cell. J. Electrochem. Soc. 1996, 143, 2558–2564. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lin, K.-F.; Huang, S.-R.; Liu, K.-Y. Enhancing photovoltaic performance of all-solid-state dye-sensitized solar cells by incorporating ionic liquid-physisorbed MWCNT. J. Mater. Chem. 2012, 22, 15592. [Google Scholar] [CrossRef]
- Vahlman, H.; Halme, J.; Korhonen, J.; Aitola, K.; Patakangas, J. On the Mass Transport in Apparently Iodine-Free Ionic Liquid Polyaniline-Coated Carbon Black Composite Electrolytes in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 11920–11929. [Google Scholar] [CrossRef]
- Salazar, P.F.; Stephens, S.T.; Kazim, A.H.; Pringle, J.M.; Cola, B.A. Enhanced thermo-electrochemical power using carbon nanotube additives in ionic liquid redox electrolytes. J. Mater. Chem. A 2014, 2, 20676–20682. [Google Scholar] [CrossRef] [Green Version]
- Salazar, P.F.; Chan, K.J.; Stephens, S.T.; Cola, B.A. Enhanced Electrical Conductivity of Imidazolium-Based Ionic Liquids Mixed with Carbon Nanotubes: A Spectroscopic Study. J. Electrochem. Soc. 2014, 161, H481–H486. [Google Scholar] [CrossRef] [Green Version]
- Kouyaté, M.; Filomeno, C.L.; Demouchy, G.; Mériguet, G.; Nakamae, S.; Peyre, V.; Roger, M.; Cēbers, A.; Depeyrot, J.; Dubois, E.; et al. Thermodiffusion of citrate-coated gamma-Fe2O3 nanoparticles in aqueous dispersions with tuned counter-ions–Anisotropy of the Soret coefficient under magnetic field. Phys. Chem. Chem. Phys. 2019, 21, 1895–1903. [Google Scholar]
- Lucas, I.T.; Dubois, E.; Chevalet, J.; Durand-Vidal, S.; Joiret, S. Reactivity of nanocolloidal particles γ-Fe2O3 at charged interfaces: Part 2. Electrochemical conversion. Role of the electrode material. Phys. Chem. Chem. Phys. 2008, 10, 3274–3286. [Google Scholar] [CrossRef] [Green Version]
- Salez, T.J. Effets Thermoélectriques Dans des fluides Complexes: Liquides Ioniques et Ferrofluides. Ph.D. Thesis, L’Ecole Normale Supérieure, Paris, France, 2017. [Google Scholar]
- Joubert, J.; Sharifpur, M.; Solomon, A.B.; Meyer, J. Enhancement in heat transfer of a ferrofluid in a differentially heated square cavity through the use of permanent magnets. J. Magn. Magn. Mater. 2017, 443, 149–158. [Google Scholar] [CrossRef]
- Kazim, A.H.; Booeshaghi, A.S.; Stephens, S.T.; Cola, B.A. Thermo-electrochemical generator: Energy harvesting & thermoregulation for liquid cooling applications. Sustain. Energy Fuels 2017, 1, 1381–1389. [Google Scholar] [CrossRef]
- Bellos, E.; Mathioulakis, E.; Tzivanidis, C.; Belessiotis, V.; Antonopoulos, K.A. Experimental and numerical investigation of a linear Fresnel solar collector with flat plate receiver. Energy Convers. Manag. 2016, 130, 44–59. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Regier, T.Z.; Peak, D.; Nagy, S.; Shao-Horn, Y. Reversibility of Ferri-Ferrocyanide Redox during in-Situ Soft X-Ray Spectroscopy. ECS Meet. Abstr. 2015, 119, 18903–18910. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Querry, M.R. Optical Constants, Contractor Report, CRDC-CR-85034; Missouri University: Columbia, MO, USA, 1985. [Google Scholar]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-µm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.P.; Mercatelli, L.; Martina, M.R.; Di Rosa, D.; Dell’Oro, A.; Lugo, L.; Sani, E. Comparative study of different functionalized graphene-nanoplatelet aqueous nanofluids for solar energy applications. Renew. Energy 2019, 141, 791–801. [Google Scholar] [CrossRef]










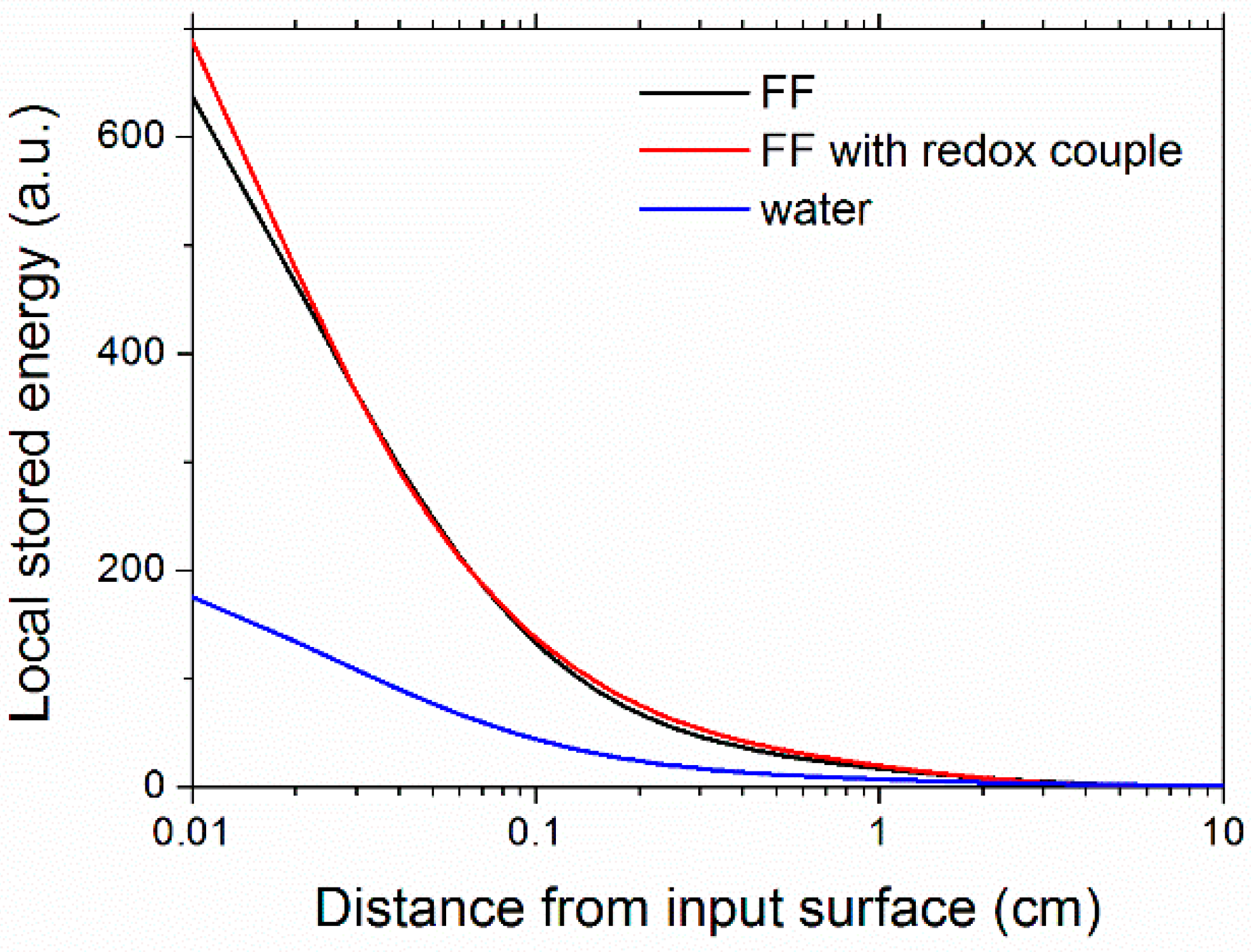
| Φ | Ri (Ω) (exp) | RO(Ω) (exp) | RMT(Ω) (calc) | RCT(Ω) [57] |
|---|---|---|---|---|
| 3 mM redox—Hot over cold configuration | ||||
| 0 | 34,000 ± 1000 | 700 | ~27,000 | <<1 |
| 0.5% vol. | 12,900± 1000 | 300 | ~27,000 | <<1 |
| 400 mM redox—Hot-over-cold configuration | ||||
| 0 | 438 ± 6 | 9.75 ± 0.5 | ~200 | <<1 |
| 0.5% vol. | 219 ± 5 | 9.75 ± 0.5 | ~200 | <<1 |
| 400 mM redox—Cold-over-hot configuration | ||||
| 0 | 23 ± 1 | 9.75 ± 0.5 | - | <<1 |
| 0.5% vol. | 23 ± 1 | 9.75 ± 0.5 | - | <<1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sani, E.; Martina, M.R.; Salez, T.J.; Nakamae, S.; Dubois, E.; Peyre, V. Multifunctional Magnetic Nanocolloids for Hybrid Solar-Thermoelectric Energy Harvesting. Nanomaterials 2021, 11, 1031. https://doi.org/10.3390/nano11041031
Sani E, Martina MR, Salez TJ, Nakamae S, Dubois E, Peyre V. Multifunctional Magnetic Nanocolloids for Hybrid Solar-Thermoelectric Energy Harvesting. Nanomaterials. 2021; 11(4):1031. https://doi.org/10.3390/nano11041031
Chicago/Turabian StyleSani, Elisa, Maria Raffaella Martina, Thomas J. Salez, Sawako Nakamae, Emmanuelle Dubois, and Véronique Peyre. 2021. "Multifunctional Magnetic Nanocolloids for Hybrid Solar-Thermoelectric Energy Harvesting" Nanomaterials 11, no. 4: 1031. https://doi.org/10.3390/nano11041031
APA StyleSani, E., Martina, M. R., Salez, T. J., Nakamae, S., Dubois, E., & Peyre, V. (2021). Multifunctional Magnetic Nanocolloids for Hybrid Solar-Thermoelectric Energy Harvesting. Nanomaterials, 11(4), 1031. https://doi.org/10.3390/nano11041031






