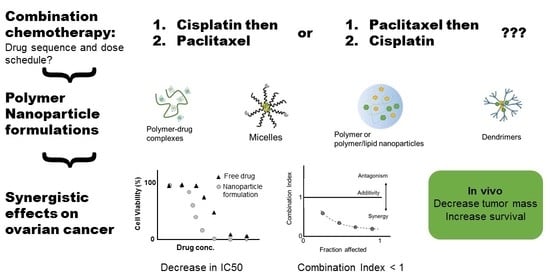
Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer
Abstract
1. Introduction
2. Background
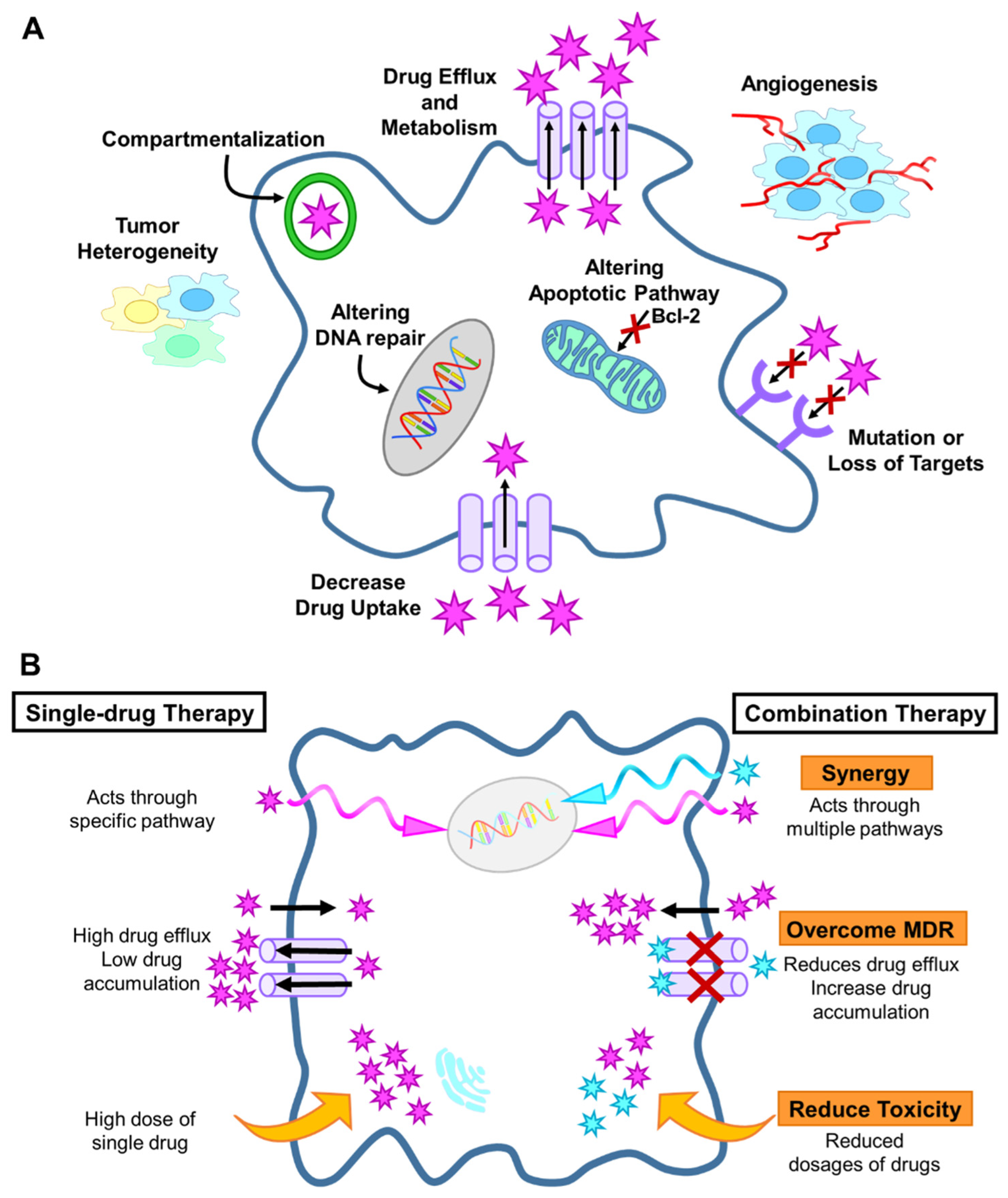
2.1. Drug Resistance Mechanisms
2.2. Quantification of Drug Interaction and Synergy
2.3. Sequence-Dependent Synergy of Free Chemotherapeutic Drugs
2.3.1. Platinum Based Combinations
2.3.2. Taxane Based Combinations
3. Nanoparticle Formulation of Drug Combinations
3.1. Polymer Nanoparticles and Micelles
3.2. Platinum Based Combinations
| Nanoparticle | Drugs | In Vitro | Key Results In Vitro | In Vivo | Key Results In Vivo | Source |
|---|---|---|---|---|---|---|
| Folic acid (FA)-PEGylated calix[4]arene nanoparticle | carboplatin/paclitaxel | SKOV-3, HO-8910 | Encapsulation increased the cell mortality rate of SKOV-3 by 2.5-fold; conjugation further increased the cell mortality rate by 3-fold in vitro | SKOV-3 xenograft (armpit) treated once every other day via intratumor injection | Reduced tumor volume compared to the free drug | [73] |
| Folic acid (FA)-PEGylated-polypeptide-nanogels | cisplatin/paclitaxel | A2780/Luc | 2-fold decrease in IC50 after 24 h using FA | A2780/Luc xenograft (IP) treated once every 4 days via tail vein injection | Increased cisplatin accumulation in tumor tissue; improved tumor inhibition and survival compared to single drug formulations | [75] |
| Poly(2-oxazoline) micelles | cisplatin/paclitaxel | A2780 and A2780cis (platinum resistant) | 40:3 ratios of PTX to C6CP resulted in combination indexes less than 0.2 in A2780CisR cells; CI highly dependent on the drug ratio | A2780/Luc xenograft (right flank) treated once every 4 days via tail vein injection | reduced tumor growth, increased survival compared to single drug loaded micelles | [76] |
| PEG-poly-(L-lysine) | Cisplatin/doxorubicin | A2780/A2780DDP (platinum resistant) | 2.5-to 3.3-fold decrease in IC50 of cisplatin, CI 0.21–55 | - | - | [77] |
| PLGA-PEG | cisplatin/paclitaxel | SKOV-3 | The co-loaded formulation was significantly more potent than prodrug stabilizer (3600-fold decrease in IC50) | - | - | [74] |
| PLGA-PEG NPs | cisplatin/wortmannin (DNA repair inhibitor) | A2780 and A2780cis (platinum resistant) | synergistically enhanced efficacy of A2780cis (CI ~ 0.04) with a 21-fold decrease in IC50, but were additive in A2780 cells (CI ~ 0.9–1.2) | A2780 and A2780cis xenograft (right flank) treated once by trail vein injection | reduced tumor growth rates compared to the free drugs; Increased cisplatin localization in the tumor | [78] |
| Hyaluronic acid micelles | cisplatin/EGCG | SKOV-3 | Slight decrease in cell viability compared to single drug loaded NPs. Intracellular uptake was possible facilitating Pt accumulation. | SKOV-3-Luc xenograft (IP) treated once a week for 3 weeks by IP injection | increased the Pt accumulation in the tumor and reduced tumor volume as well as increased survival rate compared to free cisplatin | [79] |
| PCL-based triblock co-polymer micelle carriers | oxoplatin/curcumin | A2780 | strong synergistic interaction (CI ~ 0.3) | - | - | [80] |
| poly(norbornene)-co-poly(ethylene glycol) | Cisplatin/doxorubicin camptothecin | OVCAR-3 | The triple drug co-loaded formulation was more potent than the single drug (cisplatin) or two drug loaded combination as indicated by the decrease in IC50 (up to 11-fold) | - | - | [81] |
3.3. Taxane Based Combinations
3.4. Other Drug Combinations
3.5. Lipid Nanoparticles

| Nanoparticle | Drugs | In Vitro | Key Results In Vitro | In Vivo | Key Results In Vivo | Source |
|---|---|---|---|---|---|---|
| Polyelectrolyte coated liposome | Cisplatin/olaparib or talazoparib | OVCAR-8 and COV362 | enhanced potency (reduced IC50) compared to free drugs | OVCAR-8 xenografts treated by tail vein injection once a week | Reduced tumor burden and metastasis as well as increasing survival | [101] |
| PEGylated lipid nanoemulsion | paclitaxel/curcumin | SKOV-3, SKOV-3TR (drug resistant) | enhanced cytotoxicity and increased apoptosis, slightly synergistic (CI = 0.93) in SKOV-3 and additive in SKOV-3TR compared to free drugs | - | - | [104] |
| PEG stabilized microemulsion | brucea oil/tripterine | SKOV-3 | CI = 0.90 at an 20:1 w:w ratio of brucea oil to tripterine | - | - | [105] |
| iRGD peptide Lipid-polymer hybrid nanoparticles | paclitaxel/tetrandrine | A2780/PTX cells (paclitaxel resistant) | CI 0.49–0.64 depending on drug ratios; increased intracellular paclitaxel accumulation apoptosis when co-loaded | - | - | [106] |
| Lipid-templated polymer-caged nanobins | cisplatin/doxorubicin | OVCAR-3 | enhanced cytotoxicity compared to both free drug and single-drug nanobins; CI between 0.27 and 0.67 depending on drug ratio compared | - | - | [107] |
| DSPE-PEG micelles | paclitaxel/tanespimycin (17-AAG) | - | - | SKOV-3 xenograft (flank) with sequential delivery of paclitaxel (free or NPs) once a week and followed by 17-AAG (free or NPs) 3 days later for 3 weeks, administed through tail vein injection | increased tumor accumulation by 2-fold,~2-fold reduction in tumor mass after 43 days significant tumor growth arrest compared to free drug combinations | [112] |
| Core-shell DOPA, DSPE-PEG, and cholesterol nanoparticles | carboplatin/gemcitabine | SKOV-3, A2780/CDPP (platinum resistant cells) | CI~0.5 comparable to free drugs | SKOV-3, A2780/CDDP (platinum-resistant) xenografts (right flank) injected by IP once every 3 days for a total of 3 injections | reduced tumor weight by 12-fold compared to free drug combination | [113] |
| mPEG-DPPE/calcium phosphate nanoparticle | triptolide/curcumin | SKOV-3 | Higher apoptosis rate compared to free drugs | SKOV-3 xenografts (upper left axillary fossa) treated twice a week for 24 days via tail vein injection | Reduced tumor volume compared to free drugs | [114] |
3.6. Dendrimers
| Nanoparticle | Drugs | In Vitro | Key Results In Vitro | In Vivo | Key Results In Vivo | Source |
|---|---|---|---|---|---|---|
| PEG 3-generation telodendrimer micelles | cisplatin/paclitaxel | SKOV-3, ES-2 | Antagonistic at 1:1 ratio; synergistic at 2:1 ratio of cisplatin to paclitaxel (CI = 0.21 for SKOV-3) | SKOV-3 xenograft (flank) treated 3 times at 4-day intervals via tail vein injection | highest accumulation in the tumor tissue, decreased tumor volume, increased survival time, and reduced renal toxicity compared to free cisplatin, cisplatin loaded telodendrimers, or paclitaxel loaded dendrimers | [119] |
| PLA/PLGA/PEG dendrimers | cisplatin prodrug/aspirin prodrug | A2780/CP70 (cisplatin resistant) | ~4-fold decreased IC50 in nanoparticle formulation | - | - | [121] |
| PEG dendrimers | Oxaliplatin/curcumin dendrimers | SKOV-3/OVCAR-3 | CI 0.855 in SKOV-3/CI 0.708 in OVCAR-3 after 48 h of treatment (IC50) | - | - | [122] |
| 3-generation PEG-PAMAM dendrimers | paclitaxel/borneol | A2780/PTX (paclitaxel resistant) | 3-fold lower IC50 value compared to the free drug combination | A2780/PTX xenograft (back) once every two days for 7 tail vein injections | decrease in tumor volume, compared with the free drug combination | [123] |
| Linear-dendritic telodendrimers | doxorubicin/bortezomib | SKOV-3 | Synergistic effects observed at bortezomib: doxorubicin ratios between 1:1 and 1:10; antagonistic at lower ratios | SKOV-3 xenograft (flank) treated every 4 days for a total of 3 tail vein injections | decreased toxicity delayed tumor growth compared to free drugs | [124] |
4. Outlook
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Deb, B.; Uddin, A.; Chakraborty, S. MiRNAs and Ovarian Cancer: An Overview. J. Cell. Physiol. 2018, 233, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Gouy, S.; Leary, A. Mucinous Ovarian Carcinoma. N. Engl. J. Med. 2019, 380, 1256–1266. [Google Scholar] [CrossRef]
- Miller, E.M.; Samec, T.M.; Alexander-Bryant, A.A. Nanoparticle Delivery Systems to Combat Drug Resistance in Ovarian Cancer. Nanomed. Nanotechnol. Biol. Med. 2021, 31, 102309. [Google Scholar] [CrossRef]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in Ovarian Cancer: Diagnosis and Treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Kim, S.; Han, Y.; Kim, S.I.; Kim, H.-S.; Kim, S.J.; Song, Y.S. Tumor Evolution and Chemoresistance in Ovarian Cancer. NPJ Precis. Oncol. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of Tumor Microenvironment in Ovarian Cancer Pathobiology. Oncotarget 2018, 9, 22832–22849. [Google Scholar] [CrossRef]
- Azaïs, H.; Estevez, J.P.; Foucher, P.; Kerbage, Y.; Mordon, S.; Collinet, P. Dealing with Microscopic Peritoneal Metastases of Epithelial Ovarian Cancer. A Surgical Challenge. Surg. Oncol. 2017, 26, 46–52. [Google Scholar] [CrossRef]
- Mancini, R.; Modlin, J. Chemotherapy Administration Sequence: A Review of the Literature and Creation of a Sequencing Chart. J. Hematol. Oncol. Pharm. 2011, 1, 17–25. [Google Scholar]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R. Nanomedicine-Based Multidrug Resistance Reversal Strategies in Cancer Therapy; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Brotto, L.; Brundage, M.; Hoskins, P.; Vergote, I.; Cervantes, A.; Casado, H.A.; Poveda, A.; Eisenhauer, E.; Tu, D. Randomized Study of Sequential Cisplatin-Topotecan/Carboplatin-Paclitaxel versus Carboplatin-Paclitaxel: Effects on Quality of Life. Support. Care Cancer 2016, 24, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Waltmire, C.N.; Alberts, D.S.; Dorr, R.T. Sequence-Dependent Cytotoxicity of Combination Chemotherapy Using Paclitaxel, Carboplatin and Bleomycin in Human Lung and Ovarian Cancer. Anticancer. Drugs 2001, 12, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Citardi, M.J.; Noe, D.A.; Donehower, R.C. Sequence-Dependent Cytotoxic Effects Due to Combinations of Cisplatin and the Antimicrotubule Agents Taxol and Vincristine. J. Cancer Res. Clin. Oncol. 1993, 119, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; Wang, S.; King-Jones, K. Spatial and Temporal Control of Gene Manipulation in Drosophila via Drug-Activated Cas9 Nucleases. Insect Biochem. Mol. Biol. 2020, 120, 103336. [Google Scholar] [CrossRef]
- Freimund, A.E.; Beach, J.A.; Christie, E.L.; Bowtell, D.D.L. Mechanisms of Drug Resistance in High-Grade Serous Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 983–996. [Google Scholar] [CrossRef]
- Tang, C.; Levit, S.; Zeevi, M.; Vasey, C.; Fromen, C. Polymer Colloids Enable Medical Applications. In Polymer Colloids: Formation, Characterization and Applications; Royal Society of Chemistry: London, UK, 2020; pp. 358–398. [Google Scholar]
- Correa, S.; Boehnke, N.; Barberio, A.E.; Deiss-Yehiely, E.; Shi, A.; Oberlton, B.; Smith, S.G.; Zervantonakis, I.; Dreaden, E.C.; Hammond, P.T. Tuning Nanoparticle Interactions with Ovarian Cancer through Layer-by-Layer Modification of Surface Chemistry. ACS Nano 2020, 14, 2224–2237. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer Nanoparticles for the Intravenous Delivery of Anticancer Drugs: The Checkpoints on the Road from the Synthesis to Clinical Translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable Polymers and Constructs: A Novel Approach in Drug Delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Norouzi-Barough, L.; Sarookhani, M.R.; Sharifi, M.; Moghbelinejad, S.; Jangjoo, S.; Salehi, R. Molecular Mechanisms of Drug Resistance in Ovarian Cancer. J. Cell. Physiol. 2018, 233, 4546–4562. [Google Scholar] [CrossRef]
- Tsibulak, I.; Zeimet, A.G.; Marth, C. Hopes and Failures in Front-Line Ovarian Cancer Therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 14–19. [Google Scholar] [CrossRef]
- Grisham, R.N.; Iyer, G. Low-Grade Serous Ovarian Cancer: Current Treatment Paradigms and Future Directions. Curr. Treat. Options Oncol. 2018, 19. [Google Scholar] [CrossRef]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing Treatment in Recurrent Epithelial Ovarian Cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Guppy, A.E.; Nelstrop, A.E.; Foster, T.; Agarwal, R.; Seckl, M.J.; Rustin, G.J.S. A Phase II Study of Sequential Carboplatin, Paclitaxel and Topotecan in Patients with Previously Untreated Advanced Ovarian Cancer. Br. J. Cancer 2004, 90, 810–814. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Lin, C.M.; Liu, Q.; Huang, L. Nanoformulations for Combination or Cascade Anticancer Therapy. Adv. Drug Deliv. Rev. 2017, 115, 3–22. [Google Scholar] [CrossRef]
- Rajora, A.K.; Ravishankar, D.; Zhang, H.; Rosenholm, J.M. Recent Advances and Impact of Chemotherapeutic and Antiangiogenic Nanoformulations for Combination Cancer Therapy. Pharmaceutics 2020, 12, 592. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, Y.J.; Shin, H.J.; Park, C.W.; Han, S.B.; Jung, J.K.; Kim, J.S.; Shin, D.H. Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles. Pharmaceutics 2020, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.; Kim, M.G.; Kim, D.; Park, J.Y.; Oh, Y.K. Nanoformulation-Based Sequential Combination Cancer Therapy. Adv. Drug Deliv. Rev. 2017, 115, 57–81. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Zhang, X.; Guo, H.; Gao, H. Nanoparticles in Precision Medicine for Ovarian Cancer: From Chemotherapy to Immunotherapy. Int. J. Pharm. 2020, 591, 119986. [Google Scholar] [CrossRef]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Giovannetti, E.; Peters, G.J. Analysis of Drug Interactions. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Springer Science & Business Media: New York, NY, USA, 2011; pp. 421–434. [Google Scholar] [CrossRef]
- Zhao, W.; Sachsenmeier, K.; Zhang, L.; Sult, E.; Hollingsworth, R.E.; Yang, H. A New Bliss Independence Model to Analyze Drug Combination Data. J. Biomol. Screen. 2014, 19, 817–821. [Google Scholar] [CrossRef]
- Lederer, S.; Dijkstra, T.M.H.; Heskes, T. Additive Dose Response Models: Defining Synergy. Front. Pharmacol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Brietinger, H.-G. Drug Synergy—Mechanisms and Methods of Analysis. In Toxicity and Drug Testing; Acree, W., Ed.; INTECH: Rijeka, Croatia, 2012; pp. 143–166. [Google Scholar]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian Cancer: Current Status and Strategies for Improving Therapeutic Outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Baloch, T.; López-Ozuna, V.M.; Wang, Q.; Matanis, E.; Kessous, R.; Kogan, L.; Yasmeen, A.; Gotlieb, W.H. Sequential Therapeutic Targeting of Ovarian Cancer Harboring Dysfunctional BRCA1. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Jekunen, A.P.; Christen, R.D.; Shalinsky, D.R.; Howell, S.B. Synergistic Interaction between Cisplatin and Taxol in Human Ovarian Carcinoma Cells in Vitro. Br. J. Cancer 1994, 69, 299–306. [Google Scholar] [CrossRef]
- Judson, P.L.; Watson, J.M.; Gehrig, P.A.; Fowler, W.C.; Haskill, J.S. Cisplatin Inhibits Paclitaxel-Induced Apoptosis in Cisplatin-Resistant Ovarian Cancer Cell Lines: Possible Explanation for Failure of Combination Therapy. Cancer Res. 1999, 59, 2425–2432. [Google Scholar]
- Vanhoefer, U.; Harstrick, A.; Wilke, H.; Schleucher, N.; Walles, H.; Schröder, J.; Seeber, S. Schedule-Dependent Antagonism of Paclitaxel and Cisplatin in Human Gastric and Ovarian Carcinoma Cell Lines in Vitro. Eur. J. Cancer 1995, 31, 92–97. [Google Scholar] [CrossRef]
- Ge, L.; Li, N.; Wu, L.Y. Nedaplatin and Paclitaxel Compared with Carboplatin and Paclitaxel for Patients with Platinum-Sensitive Recurrent Ovarian Cancer. Gynecol. Oncol. 2018, 149, 33–34. [Google Scholar] [CrossRef]
- Muggia, F. Relevance of Chemotherapy Dose and Schedule to Outcomes in Ovarian Cancer. Semin. Oncol. 2004, 31, 19–24. [Google Scholar] [CrossRef]
- Rogers, P.; Boxall, F.E.; Allott, C.P.; Stephens, T.C.; Kelland, L.R. Sequence-Dependent Synergism between the New Generation Platinum Agent ZD0473 and Paclitaxel in Cisplatin-Sensitive and -Resistant Human Ovarian Carcinoma Cell Lines. Eur. J. Cancer 2002, 38, 1653–1660. [Google Scholar] [CrossRef]
- Coley, H.M.; Shotton, C.F.; Ajose-Adeogun, A.; Modjtahedi, H.; Thomas, H. Receptor Tyrosine Kinase (RTK) Inhibition Is Effective in Chemosensitising EGFR-Expressing Drug Resistant Human Ovarian Cancer Cell Lines When Used in Combination with Cytotoxic Agents. Biochem. Pharmacol. 2006, 72, 941–948. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, Z.; Lei, J.; Yu, J. Scutellarin Synergistically Enhances Cisplatin Effect against Ovarian Cancer Cells through Enhancing the Ability of Cisplatin Binding to DNA. Eur. J. Pharmacol. 2019, 844, 9–16. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, H.; Wan, T.; Zhang, C.; Tong, C.; Liu, J. Comparison of PARPis with Angiogenesis Inhibitors and Chemotherapy for Maintenance in Ovarian Cancer: A Network Meta-Analysis. Adv. Ther. 2019, 36, 3368–3380. [Google Scholar] [CrossRef] [PubMed]
- Al-Eisawi, Z.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Carboplatin and Oxaliplatin in Sequenced Combination with Bortezomib in Ovarian Tumour Models. J. Ovarian Res. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Qian, W.; Wang, J.; Roginskaya, V.; McDermott, L.A.; Edwards, R.P.; Stolz, D.B.; Llambi, F.; Green, D.R.; Van Houten, B. Novel Combination of Mitochondrial Division Inhibitor 1 (Mdivi-1) and Platinum Agents Produces Synergistic pro-Apoptotic Effect in Drug Resistant Tumor Cells. Oncotarget 2014, 5, 4180–4194. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from Sequenced Combinations of Curcumin and Epigallocatechin-3- Gallate with Cisplatin in the Killing of Human Ovarian Cancer Cells. Anticancer Res. 2011, 31, 1131–1140. [Google Scholar]
- Pistollato, F.; Calderón Iglesias, R.; Ruiz, R.; Aparicio, S.; Crespo, J.; Dzul Lopez, L.; Giampieri, F.; Battino, M. The Use of Natural Compounds for the Targeting and Chemoprevention of Ovarian Cancer. Cancer Lett. 2017, 411, 191–200. [Google Scholar] [CrossRef]
- Xiao, H.; Verdier-Pinard, P.; Fernandez-Fuentes, N.; Burd, B.; Angeletti, R.; Fiser, A.; Horwitz, S.B.; Orr, G.A. Insights into the Mechanism of Microtubule Stabilization by Taxol. Proc. Natl. Acad. Sci. USA 2006, 103, 10166–10173. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, Y.S.; Kim, J.U.; Han, S.M.; Shin, J.W.; Yang, H.O. Mechanism of Taxol-Induced Apoptosis in Human SKOV3 Ovarian Carcinoma Cells. J. Cell. Biochem. 2004, 91, 1043–1052. [Google Scholar] [CrossRef]
- Han, E.S.; Wen, W.; Dellinger, T.H.; Wu, J.; Lu, S.A.; Jove, R.; Yim, J.H. Ruxolitinib Synergistically Enhances the Anti-Tumor Activity of Paclitaxel in Human Ovarian Cancer. Oncotarget 2018, 9, 24304–24319. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shen, J.; Choy, E.; Mankin, H.; Hornicek, F.; Duan, Z. Inhibition of CDK4 Sensitizes Multidrug Resistant Ovarian Cancer Cells to Paclitaxel by Increasing Apoptosiss. Cell. Oncol. 2017, 40, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.J.; Huang, X.X.; Xu, L.N.; Zhang, Y.Y.; Zhao, N.; Ou, R.Y.; Li, W.F.; Zhang, W.J.; Jiang, Q.W.; Yang, Y.; et al. YM155 Enhances Docetaxel Efficacy in Ovarian Cancer. Am. J. Transl. Res. 2018, 10, 696–708. [Google Scholar]
- Rosanò, L.; Cianfrocca, R.; Spinella, F.; Di Castro, V.; Natali, P.G.; Bagnato, A. Combination Therapy of Zibotentan with Cisplatinum and Paclitaxel Is an Effective Regimen for Epithelial Ovarian Cancer. Can. J. Physiol. Pharmacol. 2010, 88, 676–681. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Delie, F.; Cohen, M.; Martin-Sabroso, C.; Mezzanzanica, D.; Figini, M.; Satta, A.; Torres-Suárez, A.I. Enhancing Ovarian Cancer Conventional Chemotherapy through the Combination with Cannabidiol Loaded Microparticles. Eur. J. Pharm. Biopharm. 2020, 154, 246–258. [Google Scholar] [CrossRef]
- Yagi, H.; Yotsumoto, F.; Sonoda, K.; Kuroki, M.; Mekada, E.; Miyamoto, S. Synergistic Anti-Tumor Effect of Paclitaxel with CRM197, an Inhibitor of HB-EGF, in Ovarian Cancer. Int. J. Cancer 2009, 124, 1429–1439. [Google Scholar] [CrossRef]
- Song, Y.; Xin, X.; Zhai, X.; Xia, Z.; Shen, K. Sequential Combination of Flavopiridol with Taxol Synergistically Suppresses Human Ovarian Carcinoma Growth. Arch. Gynecol. Obstet. 2015, 291, 143–150. [Google Scholar] [CrossRef]
- Hirai, H.; Sootome, H.; Nakatsuru, Y.; Miyama, K.; Taguchi, S.; Tsujioka, K.; Ueno, Y.; Hatch, H.; Majumder, P.K.; Pan, B.-S.; et al. MK-2206, an Allosteric Akt Inhibitor, Enhances Antitumor Efficacy by Standard Chemotherapeutic Agents or Molecular Targeted Drugs In Vitro and In Vivo. Mol. Cancer Ther. 2010. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Parsons, S.J. In Vitro and in Vivo Histone Deacetylase Inhibitor Therapy with Vorinostat and Paclitaxel in Ovarian Cancer Models: Does Timing Matter? Gynecol. Oncol. 2010, 119, 351–357. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef]
- Li, W.; Cai, J.H.; Zhang, J.; Tang, Y.X.; Wan, L. Effects of Cyclooxygenase Inhibitors in Combination with Taxol on Expression of Cyclin D1 and Ki-67 in a Xenograft Model of Ovarian Carcinoma. Int. J. Mol. Sci. 2012, 13, 9741–9753. [Google Scholar] [CrossRef]
- McDaid, H.M.; Johnston, P.G. Synergistic Interaction between Paclitaxel and 8-Chloro-Adenosine 3’,5’-Monophosphate in Human Ovarian Carcinoma Cell Lines. Clin. Cancer Res. 1999, 5, 215–220. [Google Scholar] [PubMed]
- Orlandi, L.; Zaffaroni, N.; Bearzatto, A.; Villa, R.; De Marco, C.; Silvestrini, R. Lonidamine as a Modulator of Taxol Activity in Human Ovarian Cancer Cells: Effects on Cell Cycle and Induction of Apoptosis. Int. J. Cancer 1998, 78, 377–384. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Taghipour-Sabzevar, V.; Sharifi, T.; Moghaddam, M.M. Polymeric Nanoparticles as Carrier for Targeted and Controlled Delivery of Anticancer Agents. Ther. Deliv. 2019, 10, 527–550. [Google Scholar] [CrossRef]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs’ Dosage Ratio Effect. Molecules 2019, 24, 1035. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Mo, J.; Wang, L.; Huang, X.; Lu, B.; Zou, C.; Wei, L.; Chu, J.; Eggers, P.K.; Chen, S.; Raston, C.L.; et al. Multifunctional Nanoparticles for Co-Delivery of Paclitaxel and Carboplatin against Ovarian Cancer by Inactivating the JMJD3-HER2 Axis. Nanoscale 2017, 9, 13142–13152. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Luan, J.; Wang, D.; Yu, L.; Ding, J. Sustained Codelivery of Cisplatin and Paclitaxel via an Injectable Prodrug Hydrogel for Ovarian Cancer Treatment. ACS Appl. Mater. Interfaces 2017, 9, 40031–40046. [Google Scholar] [CrossRef]
- Desale, S.S.; Soni, K.S.; Romanova, S.; Cohen, S.M.; Bronich, T.K. Targeted Delivery of Platinum-Taxane Combination Therapy in Ovarian Cancer. J. Control. Release 2015, 220, 651–659. [Google Scholar] [CrossRef]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Jordan, R.; Wang, A.; Sokolsky, M.; Kabanov, A.V. Co-Delivery of Paclitaxel and Cisplatin in Poly(2-Oxazoline) Polymeric Micelles: Implications for Drug Loading, Release, Pharmacokinetics and Outcome of Ovarian and Breast Cancer Treatments. Biomaterials 2019, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, Y.; Wang, Y.; Wei, D.; Wu, Y.; Zheng, L.; Bai, H.; Xiao, H.; Zhang, Z. PH/Redox Sensitive Nanoparticles with Platinum(Iv) Prodrugs and Doxorubicin Enhance Chemotherapy in Ovarian Cancer. RSC Adv. 2019, 9, 20513–20517. [Google Scholar] [CrossRef]
- Zhang, M.; Hagan, C.T.; Min, Y.; Foley, H.; Tian, X.; Yang, F.; Mi, Y.; Au, K.M.; Medik, Y.; Roche, K.; et al. Nanoparticle Co-Delivery of Wortmannin and Cisplatin Synergistically Enhances Chemoradiotherapy and Reverses Platinum Resistance in Ovarian Cancer Models. Biomaterials 2018, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic Acid-Green Tea Catechin Micellar Nanocomplexes: Fail-Safe Cisplatin Nanomedicine for the Treatment of Ovarian Cancer without off-Target Toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.; De Souza, P.; Stenzel, M.H. Dual-Drug Delivery of Curcumin and Platinum Drugs in Polymeric Micelles Enhances the Synergistic Effects: A Double Act for the Treatment of Multidrug-Resistant Cancer. Biomater. Sci. 2015, 3, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A Convergent Synthetic Platform for Single-Nanoparticle Combination Cancer Therapy: Ratiometric Loading and Controlled Release of Cisplatin, Doxorubicin, and Camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of Curcumin: An Emerging Paradigm for Improved Remedial Application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Darvish, M.; Tabatabaeian, J.; Fard, M.R.; Mottaghi, R.; Azadchehr, M.J.; Jahanshahi, M.; Sahebkar, A.; Mirzaei, H. Therapeutic Role of Curcumin and Its Novel Formulations in Gynecological Cancers. J. Ovarian Res. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Boztas, A.O.; Karakuzu, O.; Galante, G.; Ugur, Z.; Kocabas, F.; Altuntas, C.Z.; Yazaydin, A.O. Synergistic Interaction of Paclitaxel and Curcumin with Cyclodextrin Polymer Complexation in Human Cancer Cells. Mol. Pharm. 2013, 10, 2676–2683. [Google Scholar] [CrossRef]
- Zhao, M.D.; Li, J.Q.; Chen, F.Y.; Dong, W.; Wen, L.J.; Fei, W.D.; Zhang, X.; Yang, P.L.; Zhang, X.M.; Zheng, C.H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467. [Google Scholar] [CrossRef]
- Devalapally, H.; Duan, Z.; Seiden, M.V.; Amiji, M.M. Modulation of Drug Resistance in Ovarian Adenocarcinoma by Enhancing Intracellular Ceramide Using Tamoxifen-Loaded Biodegradable Polymeric Nanoparticles. Clin. Cancer Res. 2008, 14, 3193–3203. [Google Scholar] [CrossRef]
- Wang, N.; He, T.; Shen, Y.; Song, L.; Li, L.; Yang, X.; Li, X.; Pang, M.; Su, W.; Liu, X.; et al. Paclitaxel and Tacrolimus Coencapsulated Polymeric Micelles That Enhance the Therapeutic Effect of Drug-Resistant Ovarian Cancer. ACS Appl. Mater. Interfaces 2016, 8, 4368–4377. [Google Scholar] [CrossRef]
- Vergara, D.; Bellomo, C.; Zhang, X.; Vergaro, V.; Tinelli, A.; Lorusso, V.; Rinaldi, R.; Lvov, Y.M.; Leporatti, S.; Maffia, M. Lapatinib/Paclitaxel Polyelectrolyte Nanocapsules for Overcoming Multidrug Resistance in Ovarian Cancer. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Levit, S.L.; Yang, H.; Tang, C. Rapid Self-Assembly of Polymer Nanoparticles for Synergistic Codelivery of Paclitaxel and Lapatinib via Flash Nanoprecipitation. Nanomaterials 2020, 10, 561. [Google Scholar] [CrossRef]
- Milane, L.; Duan, Z.; Amiji, M. Development of EGFR-Targeted Polymer Blend Nanocarriers for Combination Paclitaxel/Lonidamine Delivery to Treat Multi-Drug Resistance in Human Breast and Ovarian Tumor Cells. Mol. Pharm. 2011, 8, 185–203. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Kwon, G.S. Poly(Ethylene Glycol)-Block-Poly(ε-Caprolactone) Micelles for Combination Drug Delivery: Evaluation of Paclitaxel, Cyclopamine and Gossypol in Intraperitoneal Xenograft Models of Ovarian Cancer. J. Control. Release 2013, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Ding, J.; Han, L.; Guo, X. Anti-Tumor Efficacy of Folate Modified PLGA-Based Nanoparticles for the Co-Delivery of Drugs in Ovarian Cancer. Drug Des. Devel. Ther. 2019, 13, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y.; Feng, X.; Deng, M.; Xie, G.; Wang, J.; Zhang, L.; Liu, Q.; Yuan, P. Micellar Nanoparticles Loaded with Gemcitabine and Doxorubicin Showed Synergistic Effect. Colloids Surfaces B Biointerfaces 2014, 113, 158–168. [Google Scholar] [CrossRef]
- Zheng, W.; Li, M.; Lin, Y.; Zhan, X. Encapsulation of Verapamil and Doxorubicin by MPEG-PLA to Reverse Drug Resistance in Ovarian Cancer. Biomed. Pharmacother. 2018, 108, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lv, S.; Li, Y.; He, H.; Ji, Y.; Zheng, M.; Liu, Y.; Yin, L. Co-Delivery of Dual Chemo-Drugs with Precisely Controlled, High Drug Loading Polymeric Micelles for Synergistic Anti-Cancer Therapy. Biomater. Sci. 2020, 8, 949–959. [Google Scholar] [CrossRef]
- Al Fatease, A.; Shah, V.; Nguyen, D.X.; Cote, B.; LeBlanc, N.; Rao, D.A.; Alani, A.W.G. Chemosensitization and Mitigation of Adriamycin-Induced Cardiotoxicity Using Combinational Polymeric Micelles for Co-Delivery of Quercetin/Resveratrol and Resveratrol/Curcumin in Ovarian Cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Doddapaneni, B.S.; AL-Fatease, A.M.; Rao, D.A.; Alani, A.W.G. Dual-Drug Loaded Micelle for Combinatorial Therapy Targeting HIF and MTOR Signaling Pathways for Ovarian Cancer Treatment. J. Control. Release 2019, 307, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, G.; Wang, Z.; Zhang, C.; Wang, F.; Cui, X.; Guo, S.; Huang, W.; Zhang, R.; Yan, D. Floxuridine-Chlorambucil Conjugate Nanodrugs for Ovarian Cancer Combination Chemotherapy. Colloids Surfaces B Biointerfaces 2020, 194, 111164. [Google Scholar] [CrossRef]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and Platinum-Based Combinations for Recurrent Ovarian Cancer: A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J.; et al. Layer-by-layer Nanoparticles for Novel Delivery of Cisplatin and PARP Inhibitors for Platinum-based Drug Resistance Therapy in Ovarian Cancer. Bioeng. Transl. Med. 2019, 4, 1–18. [Google Scholar] [CrossRef]
- Bhise, K.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Nanostructured Lipid Carriers Employing Polyphenols as Promising Anticancer Agents: Quality by Design (QbD) Approach. Int. J. Pharm. 2017, 526, 506–515. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef]
- Ganta, S.; Amiji, M. Coadministration of Paclitaxel and Curcumin in Nanoemulsion Formulations to Overcome Multidrug Resistance in Tumor Cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, M.; Zhao, Z.; Zhu, L.; Yuan, S. A Multicomponent-Based Microemulsion for Boosting Ovarian Cancer Therapy through Dual Modification with Transferrin and SA-R6H4. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Fai Chan, H.; Xie, W.; Chen, S.; He, C.; Wang, Y.; Chen, M. Co-Delivery of Paclitaxel and Tetrandrine via IRGD Peptide Conjugated Lipid-Polymer Hybrid Nanoparticles Overcome Multidrug Resistance in Cancer Cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; O’Halloran, T.V.; Nguyen, S.T. Polymer-Caged Nanobins for Synergistic Cisplatin-Doxorubicin Combination Chemotherapy. J. Am. Chem. Soc. 2010, 132, 17130–17138. [Google Scholar] [CrossRef]
- Wang, J. Combination Treatment of Cervical Cancer Using Folate-Decorated, Ph-Sensitive, Carboplatin and Paclitaxel Co-Loaded Lipid-Polymer Hybrid Nanoparticles. Drug Des. Devel. Ther. 2020, 14, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lu, S.T.; Zhang, L.J.; Zhuo, R.X.; Xu, H.B.; Huang, S.W. Codelivery of Doxorubicin and Triptolide with Reduction-Sensitive Lipid–Polymer Hybrid Nanoparticles for in Vitro and in Vivo Synergistic Cancer Treatment. Int. J. Nanomed. 2017, 12, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Xin, Y.; Li, R.; Ge, Y.; Feng, C.; Xu, X. Targeted Biomimetic Nanoparticles for Synergistic Combination Chemotherapy of Paclitaxel and Doxorubicin. Mol. Pharm. 2017, 14, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-Step Assembly of DOX/ICG Loaded Lipid-Polymer Nanoparticles for Highly Effective Chemo-Photothermal Combination Therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Katragadda, U.; Fan, W.; Wang, Y.; Teng, Q.; Tan, C. Combined Delivery of Paclitaxel and Tanespimycin via Micellar Nanocarriers: Pharmacokinetics, Efficacy and Metabolomic Analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; Duan, X.; Chan, C.; Han, W.; Lin, W. Nanoscale Coordination Polymers Codeliver Carboplatin and Gemcitabine for Highly Effective Treatment of Platinum-Resistant Ovarian Cancer. Mol. Pharm. 2016, 13, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiong, X.; Shen, M.; Ru, D.; Gao, P.; Zhang, X.; Huang, C.; Sun, Y.; Li, H.; Duang, Y. Co-Delivery of Triptolide and Curcumin for Ovarian Caner Targeting Therapy via MPEG-DPPE/CaP Nanoparticle. J. Biomed. Nanotechnol. 2018, 14, 1761–1772. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Kesavan, A.; Pakala, S.B.; Rayala, S.K.; Venkatraman, G. Effective Strategies and Applications of Dendrimers in the Treatment of Ovarian Cancer. Curr. Pharm. Des. 2017, 23, 3099–3104. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer Nanoarchitectures for Cancer Diagnosis and Anticancer Drug Delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; El Seoud, O.; Giarolla, J. Dendrimers in the Context of Nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Cai, L.; Xu, G.; Shi, C.; Guo, D.; Wang, X.; Luo, J. Telodendrimer Nanocarrier for Co-Delivery of Paclitaxel and Cisplatin: A Synergistic Combination Nanotherapy for Ovarian Cancer Treatment. Biomaterials 2015, 37, 456–468. [Google Scholar] [CrossRef]
- Guo, X.L.; Kang, X.X.; Wang, Y.Q.; Zhang, X.J.; Li, C.J.; Liu, Y.; Du, L.B. Co-Delivery of Cisplatin and Doxorubicin by Covalently Conjugating with Polyamidoamine Dendrimer for Enhanced Synergistic Cancer Therapy. Acta Biomater. 2019, 84, 367–377. [Google Scholar] [CrossRef]
- Pathak, R.K.; Dhar, S. A Nanoparticle Cocktail: Temporal Release of Predefined Drug Combinations. J. Am. Chem. Soc. 2015, 137, 8324–8327. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hosseini, E.; Alizadeh Zarei, M.; Babashah, S.; Nakhaei Sistani, R.; Sadeghizadeh, M.; Haddad Kashani, H.; Amini Mahabadi, J.; Izadpanah, F.; Atlasi, M.A.; Nikzad, H. Studies on Combination of Oxaliplatin and Dendrosomal Nanocurcumin on Proliferation, Apoptosis Induction, and Long Non-Coding RNA Expression in Ovarian Cancer Cells. Cell Biol. Toxicol. 2019, 35, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, D.; Hu, Y.; Fu, C.; Li, W.; Dai, L.; Yang, L.; Zhang, L. Drug Resistance Reversal in Ovarian Cancer Cells of Paclitaxel and Borneol Combination Therapy Mediated by PEG-PAMAM Nanoparticles. Oncotarget 2017, 8, 60453–60468. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, C.; Wright, F.A.; Guo, D.; Wang, X.; Wang, D.; Wojcikiewicz, R.J.H.; Luo, J. Multifunctional Telodendrimer Nanocarriers Restore Synergy of Bortezomib and Doxorubicin in Ovarian Cancer Treatment. Cancer Res. 2017, 77, 3293–3305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, D.; Luo, K.; Li, N.; Guo, C.; Zheng, X.; Gu, Z. Dendrimer-Doxorubicin Conjugate as Enzyme-Sensitive and Polymeric Nanoscale Drug Delivery Vehicle for Ovarian Cancer Therapy. Polym. Chem. 2014, 5, 5227–5235. [Google Scholar] [CrossRef]
- Tekade, R.K.; Dutta, T.; Gajbhiye, V.; Jain, N.K. Exploring Dendrimer towards Dual Drug Delivery: PH Responsive Simultaneous Drug-Release Kinetics. J. Microencapsul. 2009, 26, 287–296. [Google Scholar] [CrossRef]
- Zhao, Z.; Lou, S.; Hu, Y.; Zhu, J.; Zhang, C. A Nano-in-Nano Polymer-Dendrimer Nanoparticle-Based Nanosystem for Controlled Multidrug Delivery. Mol. Pharm. 2017, 14, 2697–2710. [Google Scholar] [CrossRef]
- Levit, S.L.; Gade, N.R.; Roper, T.D.; Yang, H.; Tang, C. Self-Assembly of Ph-Labile Polymer Nanoparticles for Paclitaxel Prodrug Delivery: Formulation, Characterization, and Evaluation. Int. J. Mol. Sci. 2020, 21, 9292. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S. Recent Advances in Applied Polymer Science. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Vijayakameswara Rao, N.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef]
- Li, H.; Jin, H.; Wan, W.; Wu, C.; Wei, L. Cancer Nanomedicine: Mechanisms, Obstacles and Strategies. Nanomedicine 2018, 13, 1639–1656. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Jia, Y.; Wu, Y.; Shi, K.; Yang, D.; Li, P.; Qian, Z. Physical-, Chemical-, and Biological-Responsive Nanomedicine for Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1581. [Google Scholar] [CrossRef]
- Levit, S.L. Formulation and Validation of Nanoparticle Controlled Delivery for Chemotherapeutic Drug Products. Ph.D. Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2020. [Google Scholar]
- Zhang, R.; Yang, J.; Sima, M.; Zhou, Y.; Kopeček, J. Sequential Combination Therapy of Ovarian Cancer with Degradable N-(2-Hydroxypropyl)Methacrylamide Copolymer Paclitaxel and Gemcitabine Conjugates. Proc. Natl. Acad. Sci. USA 2014, 111, 12181–12186. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Yang, Y.; Yang, H.; Lu, P. Systematic Synergy Modeling: Understanding Drug Synergy from a Systems Biology Perspective. BMC Syst. Biol. 2015, 9, 1–10. [Google Scholar] [CrossRef]
- Jain, H.V.; Meyer-Hermann, M. The Molecular Basis of Synergism between Carboplatin and ABT-737 Therapy Targeting Ovarian Carcinomas. Cancer Res. 2011, 71, 705–715. [Google Scholar] [CrossRef]
- Pandey, A.; Kulkarni, A.; Roy, B.; Goldman, A.; Sarangi, S.; Sengupta, P.; Phipps, C.; Kopparam, J.; Oh, M.; Basu, S.; et al. Sequential Application of a Cytotoxic Nanoparticle and a PI3K Inhibitor Enhances Antitumor Efficacy. Cancer Res. 2014, 74, 675–685. [Google Scholar] [CrossRef]
- Sun, X.; Bao, J.; Shao, Y. Mathematical Modeling of Therapy-Induced Cancer Drug Resistance: Connecting Cancer Mechanisms to Population Survival Rates. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Dwivedi, S.; Purohit, P.; Misra, R.; Vishnoi, J.R.; Goel, A.; Pareek, P.; Khattri, S.; Sharma, P.; Misra, S.; Pant, K.K. Precision Medicine in Ovarian Carcinoma. In Precision Medicine in Cancers and Non-Communicable Diseases: Prediction, Prevention with Personalization; Barh, D., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 145–171. [Google Scholar]
- Singh, T.; Neal, A.S.; Moatamed, N.A.; Memarzadeh, S. Exploring the Potential of Drug Response Assays for Precision Medicine in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhan, X. Identification of Clinical Trait–Related LncRNA and MRNA Biomarkers with Weighted Gene Co-Expression Network Analysis as Useful Tool for Personalized Medicine in Ovarian Cancer. EPMA J. 2019, 10, 273–290. [Google Scholar] [CrossRef]
- Sharbatoghli, M.; Vafaei, S.; Aboulkheyr Es, H.; Asadi-Lari, M.; Totonchi, M.; Madjd, Z. Prediction of the Treatment Response in Ovarian Cancer: A CtDNA Approach. J. Ovarian Res. 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.R.; Zhang, Z.C.; Zhang, Y.J.; Lou, G.; Jin, W.L. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front. Immunol. 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pemovska, T.; Bigenzahn, J.W.; Superti-Furga, G. Recent Advances in Combinatorial Drug Screening and Synergy Scoring. Curr. Opin. Pharmacol. 2018, 42, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of Active Targeting Nanoparticle Delivery System for Chemotherapeutic Drugs and Traditional/Herbal Medicines in Cancer Therapy: A Systematic Review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjug. Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, J.; Yuan, Z. Strategies and Challenges to Improve the Performance of Tumor-Associated Active Targeting. J. Mater. Chem. B 2020, 8, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Cutler, D.M. Early Returns from the Era of Precision Medicine. JAMA J. Am. Med. Assoc. 2020, 323, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Kasztura, M.; Richard, A.; Bempong, N.E.; Loncar, D.; Flahault, A. Cost-Effectiveness of Precision Medicine: A Scoping Review. Int. J. Public Health 2019, 64, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.S.; Phillips, K.A. Precision Medicine: From Science to Value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef]





| Nanoparticle | Drugs | In Vitro | Key Results In Vitro | In Vivo | Key Results In Vivo | Source |
|---|---|---|---|---|---|---|
| Cyclodextrin nanocarries | paclitaxel/curcumin | A2780, SKOV-3 | Syngeistic (CI ~ 0.65) when compared to free drugs (CI ~ 1) | - | - | [85] |
| PEI-g-stearic acid micelles coated with hyaluronic acid | paclitaxel/curcumin | SKOV-3 and SKOV-3-TR30 (multi-drug resistant) | 17.3-fold lower IC50 in SKOV-3 cells and 115-fold lower in SKOV-3-TR30 cells compared to free paclitaxel | every other day for 5 times via tail vein injection | Reduces tumor volume compared to free drug (t-test, 5%) and PTX only nanoparticles (t-test 10%) | [86] |
| PEO-PCL nanoparticles | paclitaxel/tamoxifen | SKOV-3, SKOV-3TR | 10-fold decrease in IC50 of paclitaxel (SKOV-3), CI ~ 0.4 and (CI ~ 0.7) in SKOV-3TR | SKOV-3, SKOV-3TR xenograft (flank) treated at day 1 and day 24 through tail vein injection | suppressed tumor growth, lowering systemic toxicity, tamoxifen enhanced cytotoxicity of paclitaxel | [87] |
| mPEG-PCL polymer micelles | paclitaxel/tacrolimus (FK506) | A2780/T (PTX resistant) | 5.3-fold decrease in IC50 compared to PTX only micelles; | - | - | [88] |
| Chitosan/alginate nanocapsules | paclitaxel/lapatnib | OVCAR-3 | Increased cytotoxicity compared to PTX | - | - | [89] |
| PS-PEG nanoparticles | paclitaxel/lapatinib | OVCA-432 | 1500-fold decrease in IC compared to free drug; co-loaded formulation 4.4 fold decrease in IC50 concentration compared to PTX only formulation; CI 0.23; co-loaded formulation more potent than two single drug loaded nanoparticle (CI 0.40) | - | - | [90] |
| EGFR-peptide-PCL nanoparticles | paclitaxel/lonidamine | SKOV-3, SKOV-3TR, OVCAR-5 (MDR) | 2-fold decrease in IC50 of paclitaxel in OVCAR-5 cells under hypoxic conditions (no change in IC50 under noroxative conditions or other cell types) | - | - | [91] |
| PEG-b-PCL micelles | paclitaxel/cyclopamine/gossypol | SKOV-3, ES-2 | 2D model: no increased potency compared to paclitaxel micelles; 3D model: disaggregation of the spheroid | ES-2, SKOV-3 xenografts via IP injection once a week for 3 weeks via IP injection | significantly reduced tumor volume and extended survival time compared to free paclitaxel | [92] |
| Nanoparticle | Drugs | In Vitro | Key Results In Vitro | In Vivo | Key Results In Vivo | Source |
|---|---|---|---|---|---|---|
| Folate-PEG-PLGAnanoparticles | docetaxel/gemcitabine | SKOV-3 | 3.59-fold drop in the IC50 and improve cytotoxicity in SKOV-3 cells as compared to free drug combination | SKOV-3 xenograft treated every 2 days for 3 weeks via tail vein injections | Reduced tumor volume and rate of tumor growth compared to free drug combination with no organ toxicity | [93] |
| mPEG-PLA polymer micelles | doxorubicin/gemcitabine | SKOV-3 | drug internalization via endocytosis | - | - | [94] |
| mPEG-PLA nanoparticles | doxorubicin/verapamil | A2780, SKOV-3, A2780/DOX, and SKOV-3/DOXR | micelles increased drug accumulation and enhanced apoptosis | A2780/DOXR and SKOV-3/DOXR xenograft treated every 3 days for 2 weeks via tail vein injection | inhibited tumor growth and increased survival time compared to free doxobucin with reduced side effects | [95] |
| mPEG-b-poly[N-2-hydroxyethyl)-aspartamide]/phenylboronic acid | Doxorubicin/irinotecan | SKOV-3 | Micelles increase IC50 compared to free drug; co-loaded micelles synergistic compared to single drug loaded (CI 0.3) | - | - | [96] |
| Pluronic® F-127 micelles | resveratrol co-loaded with quercetin or curcumin in NPs with free adriamycrin | ES2-Luc, A2780, and A2780ADR, ES2-Luc | Up to 10 –fold reduction in IC50 and synergistic (CI < 0.5) in A2780 and A2780ADR cells | ES2-Luc and A2780ADR xenografts treated with weekly injections for 4 weeks via tail vein injection | Significant tumor reduction and cardioprotective effect compared to ADR alone | [97] |
| mPEG-b-PLA micelles | Chetomin/Everolimus | ES-2, OVCAR-3, TOV-21G | Combination index for co-loaded micelle was <1 compared to single drug loaded micelles | ES-2 treated with weekly injections for 3 weeks via tail vein injection | Significant tumor reduction compared to empty micelles and saline control | [98] |
| Amphiphilic drug-drug conjugate nanopartpices | floxuridine-chlorambucil | OVCAR-3 | Combination index was nanodrugs~0.3 compared to~0.7 for the free drug | - | - | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levit, S.L.; Tang, C. Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer. Nanomaterials 2021, 11, 1048. https://doi.org/10.3390/nano11041048
Levit SL, Tang C. Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer. Nanomaterials. 2021; 11(4):1048. https://doi.org/10.3390/nano11041048
Chicago/Turabian StyleLevit, Shani L., and Christina Tang. 2021. "Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer" Nanomaterials 11, no. 4: 1048. https://doi.org/10.3390/nano11041048
APA StyleLevit, S. L., & Tang, C. (2021). Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer. Nanomaterials, 11(4), 1048. https://doi.org/10.3390/nano11041048