Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanillin Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Green Synthesis of Orange Peel-Derived Catalysts (Cu-Fe@OP)
2.3. Catalytic Experiments
2.4. Characterization
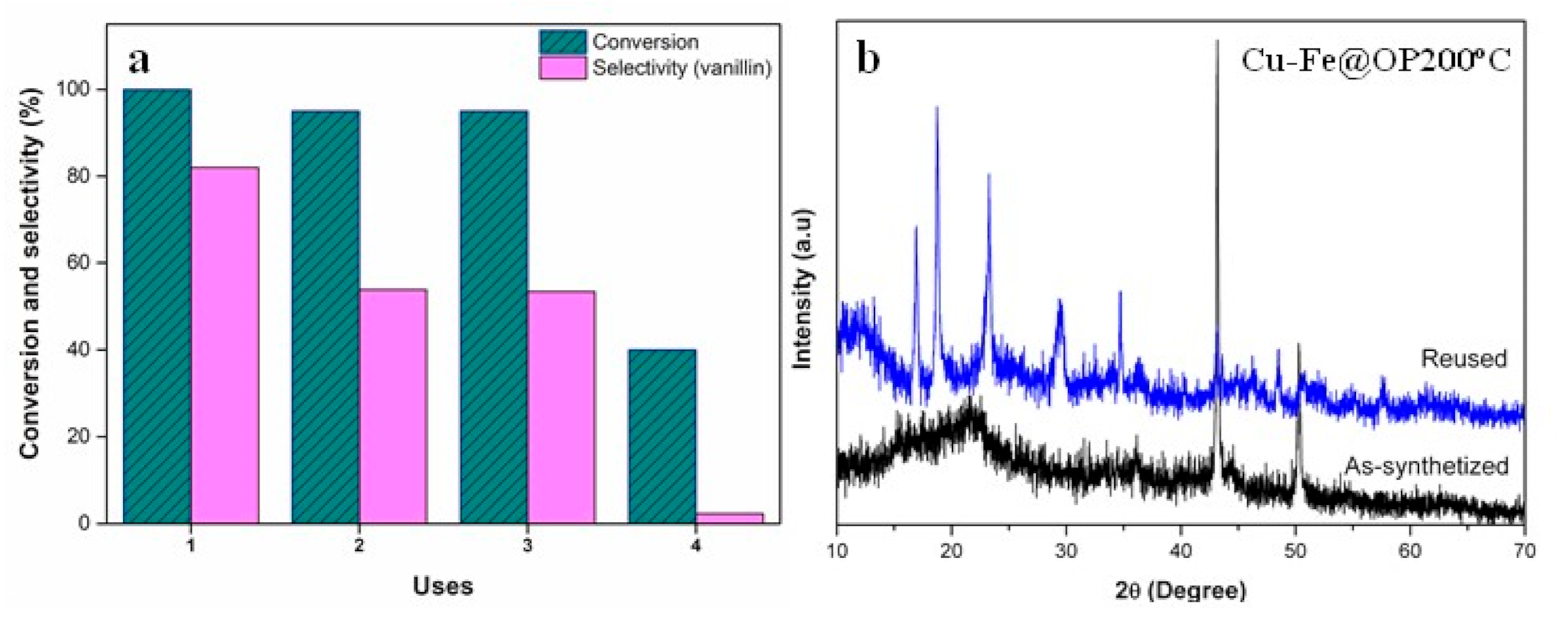
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J. Sci. Food Agric. 2019, 99, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padrón, D.; Luque, R.; Muñoz-Batista, M.J. Waste-derived materials: Opportunities in photocatalysis. In Heterogeneous Photocatalysis, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Farmer, T.J.; Mascal, M. Platform Molecules. In Introduction to Chemicals from Biomass, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 89–155. [Google Scholar]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Appl. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-López, P.; Lázaro, N.; Alvarado-Beltrán, C.G.; Pineda, A.; Balu, A.M.; Luque, R. One-pot Cu/TiO2 nanoparticles synthesis for trans-ferulic acid conversion into vanillin. Molecules 2019, 24, 3985. [Google Scholar] [CrossRef] [Green Version]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 6, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, R.; Chen, C.; Han, M.; Gong, W.; Zhang, H.; Zhang, Y.; Zhao, H.; Wang, G. Highly dispersed copper nanoparticles supported on activated carbon as an efficient catalyst for selective reduction of vanillin. Small 2018, 14, 1801953. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.F. Synthesis, catalysis, surface chemistry and structure of bimetallic nanocatalysts. Chem. Soc. Rev. 2012, 41, 7977–7979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 24, 8075–8098. [Google Scholar] [CrossRef]
- Ling, L.; Wang, Q.; Zhang, R.; Li, D.; Wang, B. Formation of C 2 oxygenates and ethanol from syngas on an Fe-decorated Cu-based catalyst: Insight into the role of Fe as a promoter. Phys. Chem. Chem. Phys. 2017, 19, 30883–30894. [Google Scholar] [CrossRef]
- Xiao, K.; Bao, Z.; Qi, X.; Wang, X.; Zhong, L.; Fang, K.; Lin, M.; Sun, Y. Structural evolution of CuFe bimetallic nanoparticles for higher alcohol synthesis. J. Mol. Cat. A Chem. 2013, 378, 319–325. [Google Scholar]
- Huang, C.; Zhang, M.; Zhu, C.; Mu, X.; Zhang, K.; Zhong, L.; Fan, K.; Wu, M. Fabrication of highly stable SiO2 encapsulated multiple CuFe nanoparticles for higher alcohols synthesis via CO hydrogenation. Catal. Lett. 2018, 4, 1080–1092. [Google Scholar] [CrossRef]
- Gómez-López, P.; Puente-Santiago, A.; Castro-Beltrán, A.; do Nascimento, L.A.S.; Balu, A.M.; Luque, R.; Alvarado-Beltrán, C.G. Nanomaterials and catalysis for green chemistry. Curr. Opin. Green Sustain. Chem. 2020, 24, 48–55. [Google Scholar] [CrossRef]
- Rodriguez-Padron, D.; Munoz-Batista, M.J.; Li, H.; Shih, K.; Balu, A.M.; Pineda, A.; Luque, R. Spent coffee grounds-templated magnetic nanocatalysts for mild oxidations. ACS Sustain. Chem. Eng. 2019, 7, 17030–17038. [Google Scholar] [CrossRef]
- Márquez-Medina, M.D.; Rodríguez-Padrón, D.; Balu, A.M.; Romero, A.A.; Muñoz-Batista, M.J.; Luque, R. Mechanochemically synthesized supported magnetic Fe-nanoparticles as catalysts for efficient vanillin production. Catalysts 2019, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of copper nanoparticles using Ginkgo biloba L. leaf extract and their catalytic activity for the Huisgen [3 + 2] cycloaddition of azides and alkynes at room temperature. J. Colloid Interface Sci. 2015, 457, 141–147. [Google Scholar] [CrossRef]
- Grassmann, J. Terpenoids as plant antioxidants. Vitamin Horm. 2005, 72, 505–535. [Google Scholar]
- Veisi, H.; Dadres, N.; Mohammadi, P.; Hemmati, S. Green synthesis of silver nanoparticles based on oil-water interface method with essential oil of orange peel and its application as nanocatalyst for A3 coupling. Mater. Sci. Eng. C 2019, 105, 110031. [Google Scholar] [CrossRef]
- Siles-López, J.A.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral use of orange peel waste through the biorefinery concept: An experimental, technical, energy, and economic assessment. Biomass Convers. Biorefin. 2021, 11, 645–659. [Google Scholar] [CrossRef]
- Santos, C.M.; Dweck, J.; Viotto, R.S.; Rosa, A.H.; de Morais, L.C. Application of orange peel waste in the production of solid biofuels and biosorbents. Bioresour. Technol. 2015, 196, 469–479. [Google Scholar] [CrossRef]
- Meng, F.; Yang, B.; Wang, B.; Duan, S.; Chen, Z.; Ma, W. Novel dendrimerlike magnetic biosorbent based on modified orange peel waste: Adsorption–reduction behavior of arsenic. ACS Sustain. Chem. Eng. 2017, 5, 9692–9700. [Google Scholar] [CrossRef]
- Xie, Z.; Guan, W.; Ji, F.; Song, Z.; Zhao, Y. Production of biologically activated carbon from orange peel and landfill leachate subsequent treatment technology. J. Chem. 2014, 2014, 491912. [Google Scholar] [CrossRef]
- Rodriguez-Padron, D.; Puente-Santiago, A.R.; Balu, A.M.; Romero, A.A.; Muñoz-Batista, M.J.; Luque, R. Benign-by-design orange peel-templated nanocatalysts for continuous flow conversion of levulinic acid to N-heterocycles. ACS Sustain. Chem. Eng. 2018, 6, 16637–16644. [Google Scholar] [CrossRef]
- Rincon, E.; Balu, A.M.; Luque, R.; Serrano, L. Mechanochemical extraction of antioxidant phenolic compounds from Mediterranean and medicinal Laurus nobilis: A comparative study with other traditional and green novel techniques. Ind. Crop. Prod. 2019, 141, 111805. [Google Scholar] [CrossRef]
- Ci, J.; Cao, C.; Kuga, S.; Shen, J.; Wu, M.; Huang, Y. Improved performance of microbial fuel cell using esterified corncob cellulose nanofibers to fabricate air-cathode gas diffusion layer. ACS Sustain. Chem. Eng. 2017, 5, 9614–9618. [Google Scholar] [CrossRef]
- Wu, K.; Xi, J. Mechanochemical-assisted extraction of platycodin D from the roots of Platycodon grandiflorum with solid alkalis. Ind. Crops Prod. 2020, 145, 112026. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale 2019, 1, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Zhang, Y.; Zhang, Q.; Jia, X.; Zhang, Q.; Sun, X.; Song, L.; Wang, X. Systematic design of superaerophobic nanotube-array electrode comprised of transition-metal sulfides for overall water splitting. Nat. Commun. 2018, 9, 2452. [Google Scholar] [CrossRef] [Green Version]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interf. Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Al-Shihry, S.S.; Halawy, S.A. Unsupported MoO3-Fe2O3 catalysts: Characterization and activity during 2-propanol decomposition. J. Mol. Catal. A Chem. 1996, 113, 479–487. [Google Scholar] [CrossRef]
- Mills, P.; Sullivan, J.L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 1983, 16, 723. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Xu, H.; Jia, H.; Liu, G. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite. Miner. Eng. 2015, 71, 188–193. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Peters, B.; Johnson, J.K.; Gates, B.C.; Scott, S.L. Beyond ordered materials: Understanding catalytic sites on amorphous solids. ACS Catal. 2017, 7, 7543–7557. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Rodríguez-Castellón, E.; van der Waal, J.C.; Luque, R. Benign-by-design preparation of humin-based iron oxide catalytic nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef] [Green Version]
- Bohre, A.; Gupta, D.; Alam, M.I.; Sharma, R.K.; Saha, B. Aerobic oxidation of isoeugenol to vanillin with copper oxide doped reduced graphene oxide. Chem. Select 2017, 2, 3129–3136. [Google Scholar] [CrossRef]
- Yan, L.; Chen, P.; Zhang, S.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci. Rep. 2016, 6, 34644. [Google Scholar] [CrossRef]
- Sánchez-González, E.; López-Olvera, A.; Monroy, O.; Aguilar-Pliego, J.; Flores, J.G.; Islas-Jácome, A.; Rincón-Guevara, M.A.; Gonzalez-Zamora, E.; Rodríguez-Molina, B.; Ibarra, I.A. Synthesis of vanillin via a catalytically active Cu (II)-metal organic polyhedron. CrystEngComm. 2017, 19, 4142–4146. [Google Scholar] [CrossRef]
- Flores, J.G.; Sánchez-González, E.; Gutiérrez-Alejandre, A.; Aguilar-Pliego, J.; Martínez, A.; Jurado-Vázquez, T.; Lima, E.; González-Zamora, E.; Díaz-García, M.; Sánchez-Sánchez, M.; et al. Greener synthesis of Cu-MOF-74 and its catalytic use for the generation of vanillin. Dalton Trans. 2018, 47, 4639–4645. [Google Scholar] [CrossRef]
- Paone, E.; Beneduci, A.; Corrente, G.A.; Malara, A.; Mauriello, F. Hydrogenolysis of aromatic ethers under lignin-first conditions. Mol. Catal. 2020, 497, 111228. [Google Scholar] [CrossRef]
- Gumina, B.; Mauriello, F.; Pietropaolo, R.; Galvagno, S.; Espro, C. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst. Mol. Catal. 2018, 446, 152–160. [Google Scholar] [CrossRef]
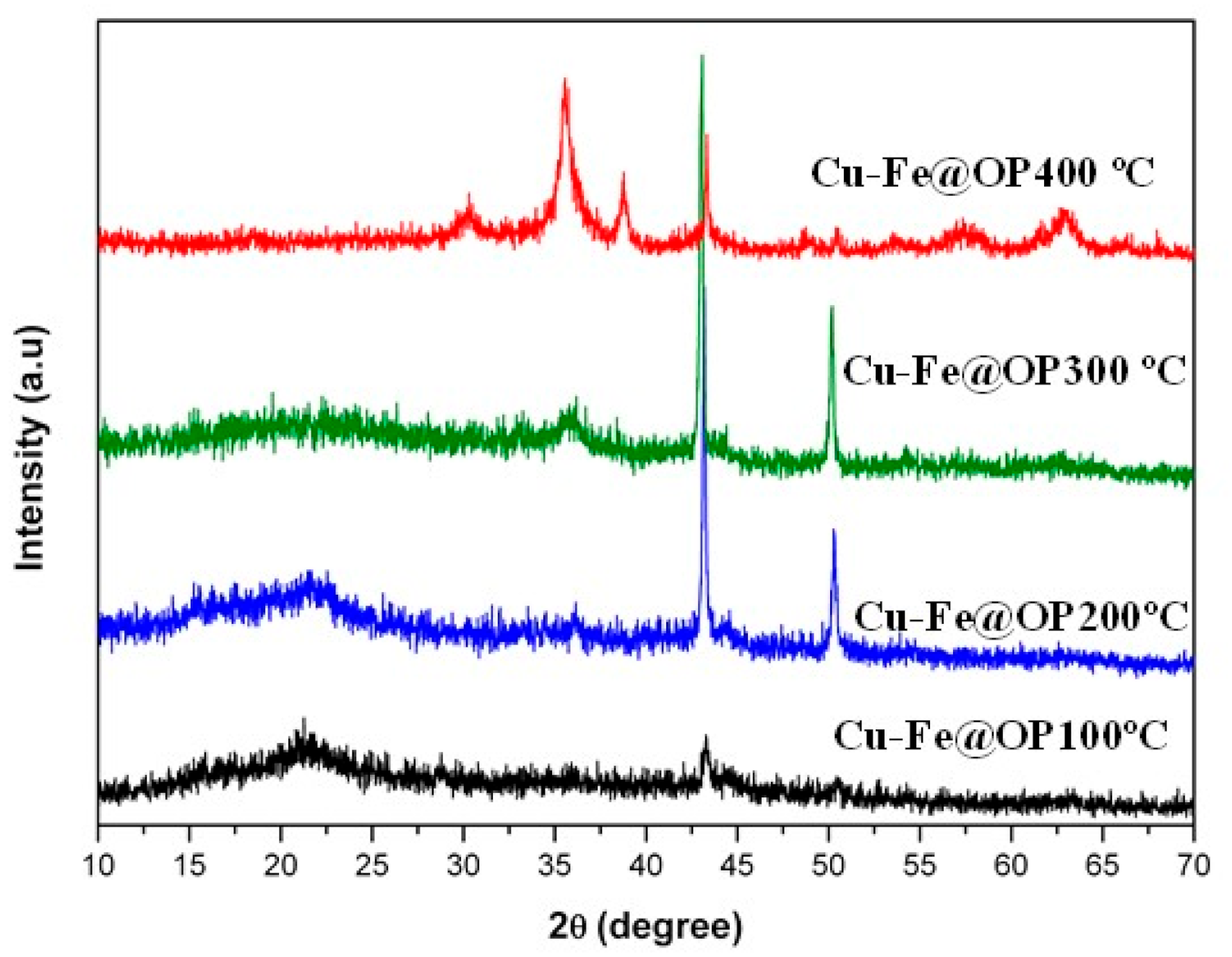

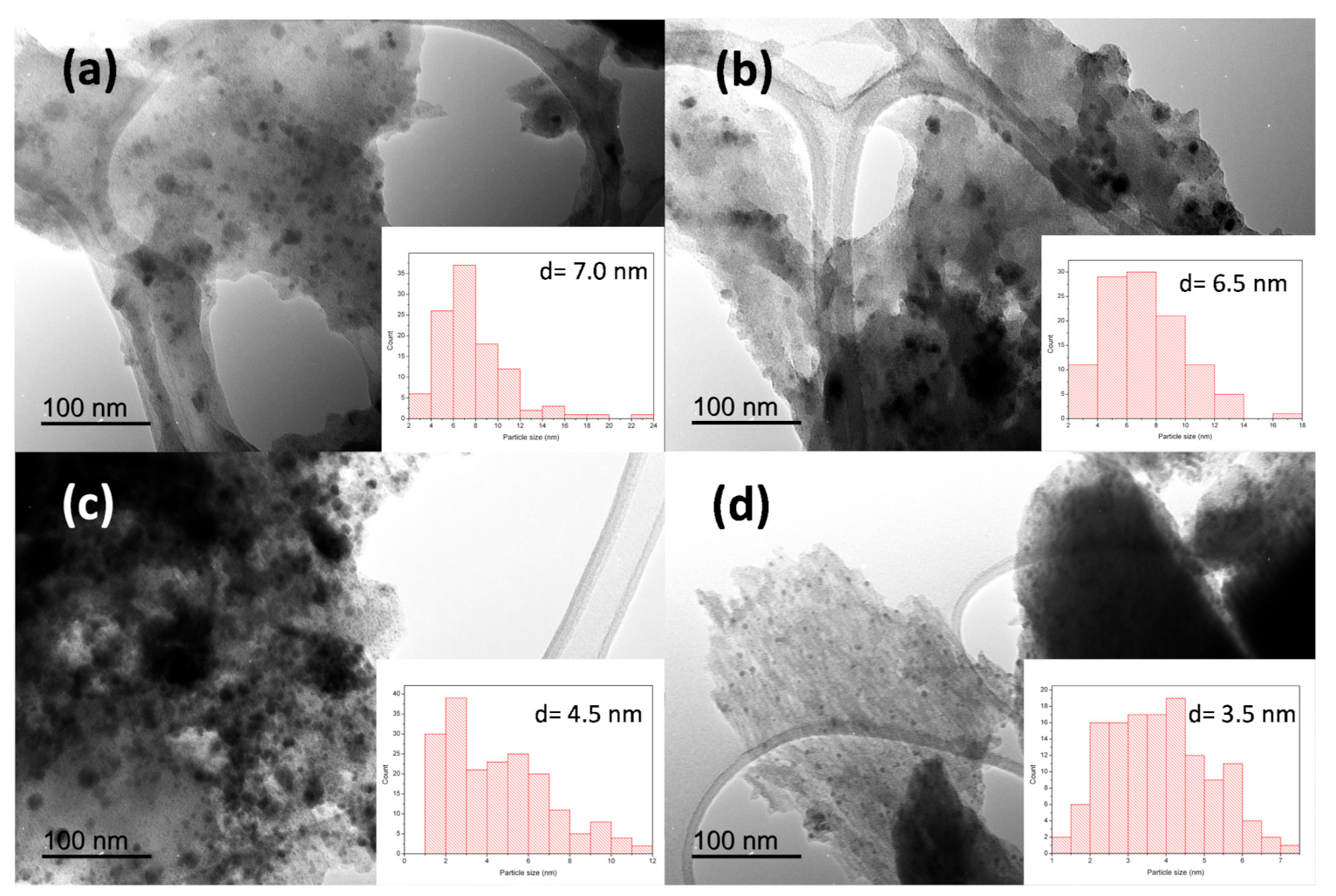
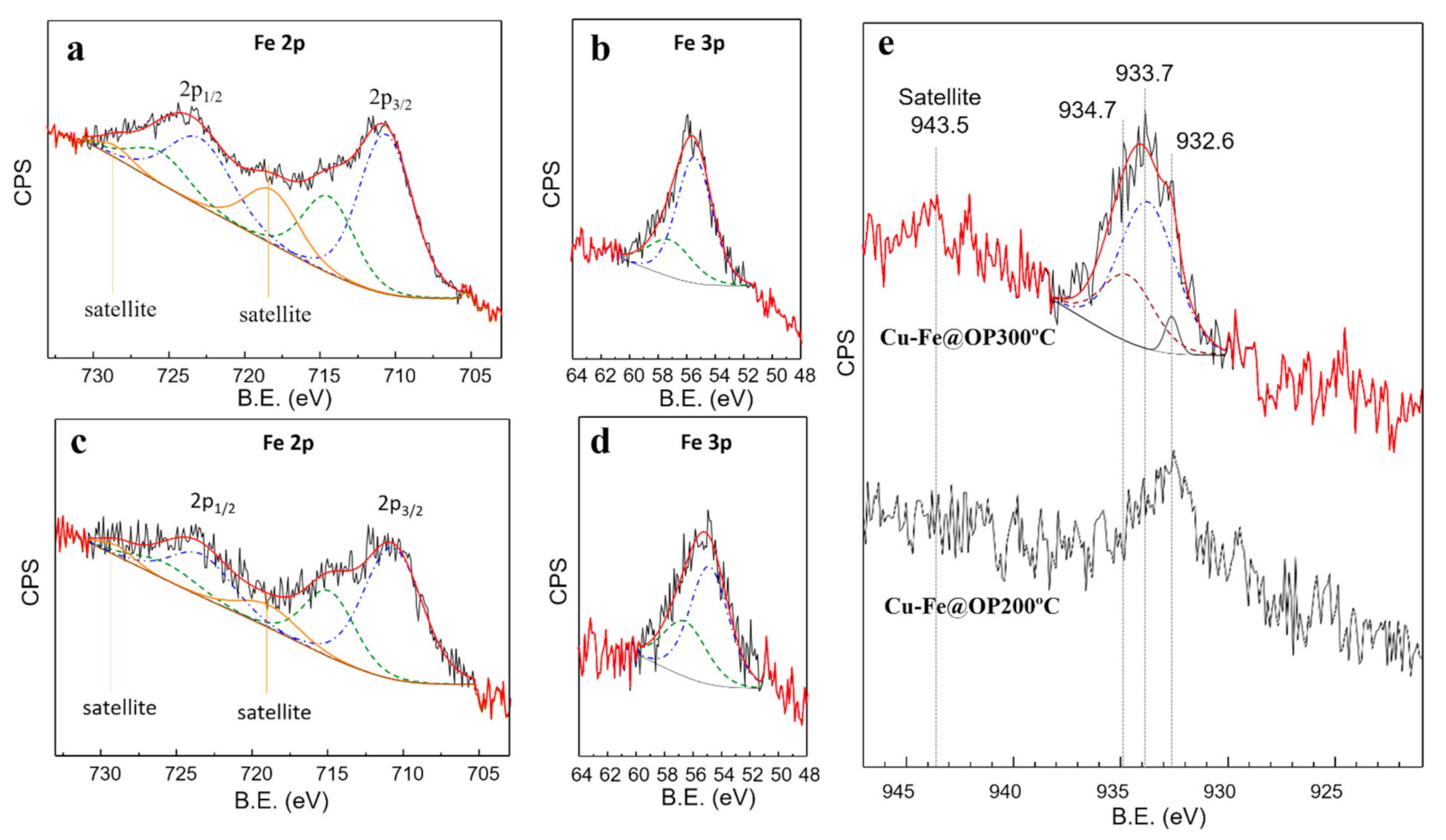
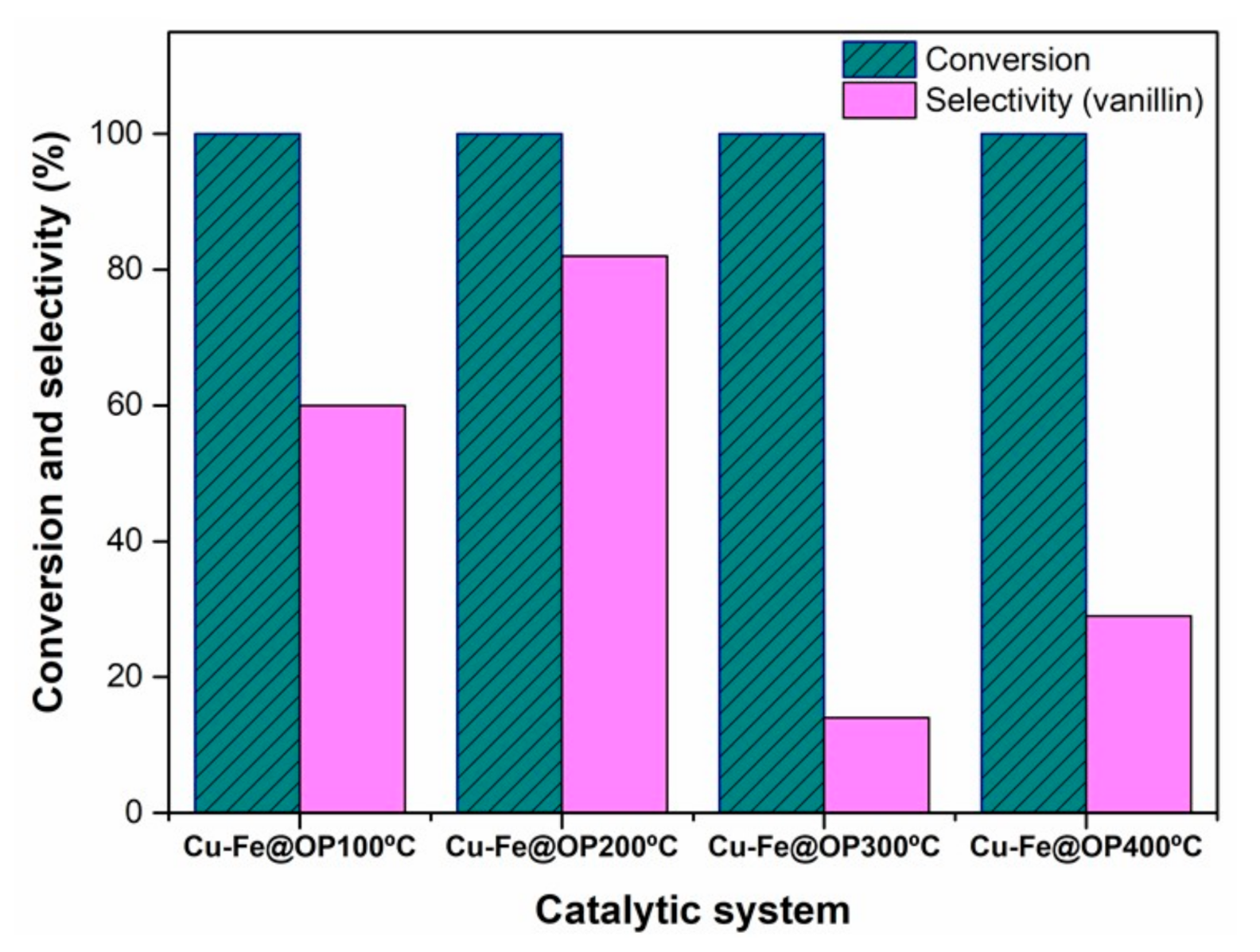
| Catalyst | SBET a (m2/g) | VBJH b (cm3/g) |
|---|---|---|
| Cu-Fe@OP100 °C | <5 | 0.002 |
| Cu-Fe@OP200 °C | <5 | 0.011 |
| Cu-Fe@OP300 °C | 7 | 0.026 |
| Cu-Fe@OP400 °C | 29 | 0.070 |
| Sample | C (%) | O (%) | Cu (%) | Fe (%) | N (%) |
|---|---|---|---|---|---|
| Cu-Fe@OP200 °C | 71.9 | 25.3 | 0.2 | 1.7 | 0.9 |
| Cu-Fe@OP300 °C | 68.0 | 26.0 | 0.7 | 3.5 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-López, P.; Espro, C.; Rodríguez-Padrón, D.; Balu, A.M.; Ivars-Barceló, F.; Moreda, O.I.; Alvarado-Beltrán, C.G.; Luque, R. Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanillin Production. Nanomaterials 2021, 11, 1050. https://doi.org/10.3390/nano11041050
Gómez-López P, Espro C, Rodríguez-Padrón D, Balu AM, Ivars-Barceló F, Moreda OI, Alvarado-Beltrán CG, Luque R. Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanillin Production. Nanomaterials. 2021; 11(4):1050. https://doi.org/10.3390/nano11041050
Chicago/Turabian StyleGómez-López, Paulette, Claudia Espro, Daily Rodríguez-Padrón, Alina M. Balu, Francisco Ivars-Barceló, Olvido Irrazábal Moreda, Clemente G. Alvarado-Beltrán, and Rafael Luque. 2021. "Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanillin Production" Nanomaterials 11, no. 4: 1050. https://doi.org/10.3390/nano11041050
APA StyleGómez-López, P., Espro, C., Rodríguez-Padrón, D., Balu, A. M., Ivars-Barceló, F., Moreda, O. I., Alvarado-Beltrán, C. G., & Luque, R. (2021). Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanillin Production. Nanomaterials, 11(4), 1050. https://doi.org/10.3390/nano11041050










