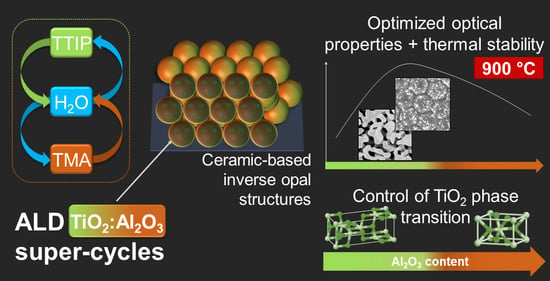
Influence of Alumina Addition on the Optical Properties and the Thermal Stability of Titania Thin Films and Inverse Opals Produced by Atomic Layer Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Refractive Index Evolution
3.2. Phase Transition and Thermal Stability
3.3. Optical Properties of Inverse Opal Photonic Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armstrong, E.; O’Dwyer, C. Artificial opal photonic crystals and inverse opal structures—Fundamentals and applications from optics to energy storage. J. Mater. Chem. C 2015, 3, 6109–6143. [Google Scholar] [CrossRef] [Green Version]
- Shang, G.; Häntsch, Y.; Furlan, K.P.; Janßen, R.; Schneider, G.A.; Petrov, A.; Eich, M. Highly selective photonic glass filter for saturated blue structural color. APL Photonics 2019, 4, 46101. [Google Scholar] [CrossRef]
- Shang, G.; Maiwald, L.; Renner, H.; Jalas, D.; Dosta, M.; Heinrich, S.; Petrov, A.; Eich, M. Photonic glass for high contrast structural color. Sci. Rep. 2018, 8, 7804. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Gu, Z.; Zhao, Y. Structural color materials in evolution. Mater. Today 2016, 19, 420–421. [Google Scholar] [CrossRef]
- Shang, L.; Zhang, W.; Xu, K.; Zhao, Y. Bio-Inspired intelligent structural color materials. Mater. Horiz. 2019, 6, 945–958. [Google Scholar] [CrossRef]
- Häntsch, Y.; Shang, G.; Petrov, A.; Eich, M.; Schneider, G.A. YSZ hollow sphere photonic glasses: Tailoring optical properties for highly saturated non-iridescent structural coloration. Adv. Opt. Mater. 2019, 9, 1900428. [Google Scholar] [CrossRef]
- Do Rosário, J.J.; Häntsch, Y.; Pasquarelli, R.M.; Dyachenko, P.N.; Vriend, E.; Petrov, A.Y.; Furlan, K.P.; Eich, M.; Schneider, G.A. Advancing the fabrication of YSZ-inverse photonic glasses for broadband omnidirectional reflector films. J. Eur. Ceram. Soc. 2019, 39, 3353–3363. [Google Scholar] [CrossRef]
- Arpin, K.A.; Losego, M.D.; Cloud, A.N.; Ning, H.; Mallek, J.; Sergeant, N.P.; Zhu, L.; Yu, Z.; Kalanyan, B.; Parsons, G.N.; et al. Three-Dimensional self-assembled photonic crystals with high temperature stability for thermal emission modification. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Im, E.; Lee, S. Colloidal photonic assemblies for colorful radiative cooling. Langmuir 2020, 36, 6589–6596. [Google Scholar] [CrossRef]
- Zhu, L.; Raman, A.P.; Fan, S. Radiative cooling of solar absorbers using a visibly transparent photonic crystal thermal blackbody. Proc. Natl. Acad. Sci. USA 2015, 112, 12282–12287. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.R.; England, G.T.; Sunny, S.; Shirman, E.; Shirman, T.; Vogel, N.; Aizenberg, J. A colloidoscope of colloid-based porous materials and their uses. Chem. Soc. Rev. 2016, 45, 281–322. [Google Scholar] [CrossRef] [Green Version]
- Vogel, N.; Retsch, M.; Fustin, C.-A.; Del Campo, A.; Jonas, U. Advances in colloidal assembly: The design of structure and hierarchy in two and three dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 21301. [Google Scholar] [CrossRef]
- Graugnard, E.; Chawla, V.; Lorang, D.; Summers, C.J. High filling fraction gallium phosphide inverse opals by atomic layer deposition. Appl. Phys. Lett. 2006, 89, 211102. [Google Scholar] [CrossRef] [Green Version]
- King, J.S.; Neff, C.W.; Summers, C.J.; Park, W.; Blomquist, S.; Forsythe, E.; Morton, D. High-Filling-Fraction inverted ZnS opals fabricated by atomic layer deposition. Appl. Phys. Lett. 2003, 83, 2566–2568. [Google Scholar] [CrossRef] [Green Version]
- Juárez, B.H.; García, P.D.; Golmayo, D.; Blanco, A.; López, C. ZnO inverse opals by chemical vapor deposition. Adv. Mater. 2005, 17, 2761–2765. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.-K.; Noh, H.; Cao, H.; Chang, R.P.H. Photonic bandgap engineering with inverse opal multistacks of different refractive index contrasts. Appl. Phys. Lett. 2009, 95, 91101. [Google Scholar] [CrossRef]
- Sechrist, Z.A.; Schwartz, B.T.; Lee, J.H.; McCormick, J.A.; Piestun, R.; Park, W.; George, S.M. Modification of opal photonic crystals using Al2O3 atomic layer deposition. Chem. Mater. 2006, 18, 3562–3570. [Google Scholar] [CrossRef]
- Furlan, K.P.; Pasquarelli, R.M.; Krekeler, T.; Ritter, M.; Zierold, R.; Nielsch, K.; Schneider, G.A.; Janssen, R. Highly porous α-Al2O3 ceramics obtained by sintering atomic layer deposited inverse opals. Ceram. Int. 2017, 43, 11260–11264. [Google Scholar] [CrossRef]
- Furlan, K.P.; Larsson, E.; Diaz, A.; Holler, M.; Krekeler, T.; Ritter, M.; Petrov, A.Y.; Eich, M.; Blick, R.; Schneider, G.A.; et al. Photonic materials for high-temperature applications: Synthesis and characterization by X-ray ptychographic tomography. Appl. Mater. Today 2018, 13, 359–369. [Google Scholar] [CrossRef]
- Furlan, K.P.; Krekeler, T.; Ritter, M.; Blick, R.; Schneider, G.A.; Nielsch, K.; Zierold, R.; Janßen, R. Low-Temperature mullite formation in ternary oxide coatings deposited by ALD for high-temperature applications. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Pasquarelli, R.M.; Lee, H.S.; Kubrin, R.; Zierold, R.; Petrov, A.Y.; Nielsch, K.; Schneider, G.A.; Eich, M.; Janssen, R. Enhanced structural and phase stability of titania inverse opals. J. Eur. Ceram. Soc. 2015, 35, 3103–3109. [Google Scholar] [CrossRef]
- Graugnard, E.; King, J.S.; Gaillot, D.P.; Summers, C.J. Sacrificial-Layer atomic layer deposition for fabrication of non-close-packed inverse-opal photonic crystals. Adv. Funct. Mater. 2006, 16, 1187–1196. [Google Scholar] [CrossRef]
- King, J.S.; Graugnard, E.; Summers, C.J. TiO2 inverse opals fabricated using low-temperature atomic layer deposition. Adv. Mater. 2005, 17, 1010–1013. [Google Scholar] [CrossRef]
- Piercy, B.D.; Leng, C.Z.; Losego, M.D. Variation in the density, optical polarizabilities, and crystallinity of TiO2 thin films deposited via atomic layer deposition from 38 to 150 °C using the titanium tetrachloride-water reaction. J. Vac. Sci. Technol. A 2017, 35, 03E107. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Hsu, Y.-L.; Su, H.-Y.; Huang, R.-C.; Hou, F.-Y.; Tu, G.-C.; Liu, W.-H. A Comparative study of amorphous, anatase, rutile, and mixed phase TiO2 films by mist chemical vapor deposition and ultraviolet photodetectors applications. IEEE Sens. J. 2018, 18, 4022–4029. [Google Scholar] [CrossRef]
- Hussin, R.; Kwang, L.C.; Hou, X. Deposited TiO2 thin films by atomic layer deposition (ALD) for optical properties. ARPN J. Eng. Appl. Sci. 2016, 11, 7529–7533. [Google Scholar]
- Möls, K.; Aarik, L.; Mändar, H.; Kasikov, A.; Niilisk, A.; Rammula, R.; Aarik, J. Influence of phase composition on optical properties of TiO2: Dependence of refractive index and band gap on formation of TiO2-II phase in thin films. Opt. Mater. 2019, 96, 109335. [Google Scholar] [CrossRef]
- Kasikov, A.; Aarik, J.; Mändar, H.; Moppel, M.; Pärs, M.; Uustare, T. Refractive index gradients in TiO2 thin films grown by atomic layer deposition. J. Phys. D Appl. Phys. 2006, 39, 54–60. [Google Scholar] [CrossRef]
- Dannenberg, R.; Greene, P. Reactive sputter deposition of titanium dioxide. Thin Solid Films 2000, 360, 122–127. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Feltrin, J.; De Noni, A.; Hotza, D.; Frade, J.R. Design guidelines for titania-silica-alumina ceramics with tuned anatase to rutile transformation. Ceram. Int. 2019, 45, 5179–5188. [Google Scholar] [CrossRef]
- Yoshida, H.; Hashimoto, S.; Yamamoto, T. Dopant effect on grain boundary diffusivity in polycrystalline alumina. Acta Mater. 2005, 53, 433–440. [Google Scholar] [CrossRef]
- Bodišová, K.; Galusek, D.; Švančárek, P.; Pouchlý, V.; Maca, K. Grain growth suppression in alumina via doping and two-step sintering. Ceram. Int. 2015, 41, 11975–11983. [Google Scholar] [CrossRef]
- Rodríguez-Talavera, R.; Vargas, S.; Arroyo-Murillo, R.; Montiel-Campos, R.; Haro-Poniatowski, E. Modification of the phase transition temperatures in titania doped with various cations. J. Mater. Res. 1997, 12, 439–443. [Google Scholar] [CrossRef]
- Berghuis, W.J.H.; Melskens, J.; Macco, B.; Basuvalingam, S.B.; Verheijen, M.A.; Kessels, W.M.M. Atomic layer deposition of Nb-doped TiO2: Dopant incorporation and effect of annealing. J. Vac. Sci. Technology A 2020, 38, 22408. [Google Scholar] [CrossRef]
- Depero, L.E.; Sangaletti, L.; Allieri, B.; Bontempi, E.; Salari, R.; Zocchi, M. Niobium-Titanium oxide powders obtained by laser-induced synthesis: Microstructure and structure evolution from diffraction data. J. Mater. Res. 1998, 13, 1644–1649. [Google Scholar] [CrossRef]
- Arbiol, J.; Cerda, J.; Dezanneau, G.; Cirera, A.; Peiro, F.; Cornet, A.; Morante, J.R. Effects of Nb doping on the TiO2 anatase-to-rutile phase transition. J. Appl. Phys. 2002, 92, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.W.; Ma, H.B.; Jin, P.H.; Jin, Y.N.; Jia, N.; Chen, H.; Liu, C.Z. An insight into the low doping efficiency of Al in sol–gel-derived ZnO:Al films: Role of the dopant chemical state. Appl. Phys. A 2020, 126. [Google Scholar] [CrossRef]
- Bueno, P.; Furlan, K.P.; Hotza, D.; Janssen, R. High-Temperature stable inverse opal photonic crystals via mullite-sol-gel infiltration of direct photonic crystals. J. Am. Ceram. Soc. 2018, 24, 13146. [Google Scholar] [CrossRef] [Green Version]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol-gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.B.; Norton, M.G. Sols, gels, and organic chemistry. Ceramic Materials; Springer: New York, NY, USA, 2007; pp. 400–411. ISBN 978-0-387-46270-7. [Google Scholar]
- Kim, S.K.; Choi, G.J.; Kim, J.H.; Hwang, C.S. Growth behavior of al-doped TiO2 thin films by atomic layer deposition. Chem. Mater. 2008, 20, 3723–3727. [Google Scholar] [CrossRef]
- Gorham, C.S.; Gaskins, J.T.; Parsons, G.N.; Losego, M.D.; Hopkins, P.E. Density dependence of the room temperature thermal conductivity of atomic layer deposition-grown amorphous alumina (Al2O3). Appl. Phys. Lett. 2014, 104, 253107. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, U.-J.; Han, C.-H.; Rha, S.-K.; Lee, W.-J.; Park, C.-O. Investigation of silicon oxide thin films prepared by atomic layer deposition using SiH2Cl2 and O3 as the precursors. Jpn. J. Appl. Phys. 2004, 43, L328. [Google Scholar] [CrossRef]
- Wang, H.L.; Lin, C.H.; Hon, M.H. The dependence of hardness on the density of amorphous alumina thin films by PECVD. Thin Solid Films 1997, 310, 260–264. [Google Scholar] [CrossRef]
- Kubrin, R.; Lee, H.S.; Zierold, R.; Petrov, A.Y.; Janssen, R.; Nielsch, K.; Eich, M.; Schneider, G.A. Stacking of ceramic inverse opals with different lattice constants. J. Am. Ceram. Soc. 2012, 95, 2226–2235. [Google Scholar] [CrossRef]
- Kubrin, R.; Lee, H.S.; Petrov, A.; Janssen, R.; Schneider, G.A.; Bachmann, J.; Nielsch, K.; Eich, M. Towards ceramic 3DOM-materials as novel high-temperature reflective coatings and filters for thermophotovoltaics. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 182004. [Google Scholar] [CrossRef] [Green Version]
- Kubrin, R.; Pasquarelli, R.M.; Waleczek, M.; Lee, H.S.; Zierold, R.; Do Rosário, J.J.; Dyachenko, P.N.; Montero Moreno, J.M.; Petrov, A.Y.; Janssen, R.; et al. Bottom-Up fabrication of multilayer stacks of 3D photonic crystals from titanium dioxide. ACS Appl. Mater. Interfaces 2016, 8, 10466–10476. [Google Scholar] [CrossRef] [PubMed]
- Lale, A.; Scheid, E.; Cristiano, F.; Datas, L.; Reig, B.; Launay, J.; Temple-Boyer, P. Study of aluminium oxide thin films deposited by plasma-enhanced atomic layer deposition from tri-methyl-aluminium and dioxygen precursors: Investigation of interfacial and structural properties. Thin Solid Films 2018, 666, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-Y.; Zhang, R.-J.; Lu, H.-L.; Chen, X.; Sun, Y.; Zhang, Y.; Wei, Y.-F.; Xu, J.-P.; Wang, S.-Y.; Zheng, Y.-X.; et al. The impact of thickness and thermal annealing on refractive index for aluminum oxide thin films deposited by atomic layer deposition. Nanoscale Res. Lett. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-Temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Hiller, D.; Zierold, R.; Bachmann, J.; Alexe, M.; Yang, Y.; Gerlach, J.W.; Stesmans, A.; Jivanescu, M.; Müller, U.; Vogt, J.; et al. Low temperature silicon dioxide by thermal atomic layer deposition: Investigation of material properties. J. Appl. Phys. 2010, 107, 64314. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-H.; Chen, K.-T.; Huang, P.-H.; Wu, W.-Y.; Zhang, X.-Y.; Wang, C.; Liang, L.-S.; Gao, P.; Qiu, Y.; Lien, S.-Y.; et al. Effect of annealing temperature on spatial atomic layer deposited titanium oxide and its application in perovskite solar cells. Nanomaterials 2020, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Hegmann, J.; Jahn, R.; Löbmann, P. Adjustable refractive index of titania–alumina thin films prepared from soluble precursor powders. J. Sol Gel Sci. Technol. 2016, 77, 69–77. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Pereira, J.C.; Matos, J.C.; Vasconcelos, H.C. Photonic band gap and bactericide performance of amorphous sol-gel titania: An alternative to crystalline TiO₂. Molecules 2018, 23, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzmil, B.; Gleń, M.; Kic, B.; Lubkowski, K. Study of the anatase to rutile transformation kinetics of the modified TiO2. Pol. J. Chem. Technol. 2013, 15, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Ottermann, C.; Bange, K. Correlation between the density of TiO2 films and their properties. Thin Solid Films 1996, 286, 32–34. [Google Scholar] [CrossRef]
- Sun, Y.; Egawa, T.; Zhang, L.; Yao, X. High anatase-rutile transformation temperature of anatase titania nanoparticles prepared by metalorganic chemical vapor deposition. Jpn. J. Appl. Phys. 2002, 41, L945–L948. [Google Scholar] [CrossRef]
- Li, Y.; Piret, F.; Léonard, T.; Su, B.-L. Rutile TiO2 inverse opal with photonic bandgap in the UV-visible range. J. Colloid Interface Sci. 2010, 348, 43–48. [Google Scholar] [CrossRef]
- Kumar, K.; Keizer, K.; Burggraaf, A.J.; Okubo, T.; Nagamoto, H. Textural evolution and phase transformation in titania membranes: Part 2—Supported membranes. J. Mater. Chem. 1993, 3, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Keizer, K.; Burggraaf, A.J. Textural stability of titania-alumina composite membranes. J. Mater. Chem. 1993, 3, 917–922. [Google Scholar] [CrossRef] [Green Version]
- Gribb, A.A.; Banfield, J.F. Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Am. Mineral. 1997, 82, 717–728. [Google Scholar] [CrossRef]
- Shannon, R.D. Phase transformation studies in TiO2 supporting different defect mechanisms in vacuum-reduced and hydrogen-reduced rutile. J. Appl. Phys. 1964, 35, 3414–3416. [Google Scholar] [CrossRef] [Green Version]
- Gennari, F.C.; Pasquevich, D.M. Kinetics of the anatase–rutile transformation in TiO2 in the presence of Fe2O3. J. Mater. Sci. 1998, 33, 1571–1578. [Google Scholar] [CrossRef]
- Ding, X.-Z.; Liu, X.-H. Correlation between anatase-to-rutile transformation and grain growth in nanocrystalline titania powders. J. Mater. Res. 1998, 13, 2556–2559. [Google Scholar] [CrossRef]
- Habibpanah, A.A.; Pourhashem, S.; Sarpoolaky, H. Preparation and characterization of photocatalytic titania–alumina composite membranes by sol–gel methods. J. Eur. Ceram. Soc. 2011, 31, 2867–2875. [Google Scholar] [CrossRef]
- Vargas, S.; Arroyo, R.; Haro, E.; Rodríguez, R. Effects of cationic dopants on the phase transition temperature of titania prepared by the sol-gel method. J. Mater. Res. 1999, 14, 3932–3937. [Google Scholar] [CrossRef]
- Ding, X.; Liu, L.; Ma, X.; Qi, Z.; He, Y. The influence of alumina dopant on the structural transformation of gel-derived nanometre titania powders. J. Mater. Sci. Lett. 1994, 13, 462–464. [Google Scholar] [CrossRef]
- Edelson, L.; Glaeser, A.M. Role of particle substructure in the sintering of monosized titania. J. Am. Ceram. Soc. 1988, 71, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Rafieian, D.; Ogieglo, W.; Savenije, T.; Lammertink, R.G.H. Controlled formation of anatase and rutile TiO2 thin films by reactive magnetron sputtering. AIP Adv. 2015, 5, 97168. [Google Scholar] [CrossRef]
- Wiegand, C.W.; Faust, R.; Meinhardt, A.; Blick, R.H.; Zierold, R.; Nielsch, K. Understanding the growth mechanisms of multilayered systems in atomic layer deposition process. Chem. Mater. 2018, 30, 1971–1979. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Bouhamed, H.; Calabrese, L.; Proverbio, E.; Bouaziz, J. Properties and microstructural aspects of TiO2-doped sintered alumina-zirconia composite ceramics. Int. J. Appl. Ceram. Technol. 2018, 15, 1532–1541. [Google Scholar] [CrossRef]
- Gao, M.; Liu, B.; Zhao, P.; Yi, X.; Shen, X.; Xu, Y. Mechanical strengths and thermal properties of titania-doped alumina aerogels and the application as high-temperature thermal insulator. J. Sol Gel Sci. Technol. 2019, 91, 514–522. [Google Scholar] [CrossRef]
- Ruckdeschel, P.; Philipp, A.; Retsch, M. Understanding thermal insulation in porous, particulate materials. Adv. Funct. Mater. 2017, 27, 1702256. [Google Scholar] [CrossRef]
- Bauer, J.; Meza, L.R.; Schaedler, T.A.; Schwaiger, R.; Zheng, X.; Valdevit, L. Nanolattices: An emerging class of mechanical metamaterials. Adv. Mater. 2017, 29, 1701850. [Google Scholar] [CrossRef]
- Krautheim, G.; Hecht, T.; Jakschik, S.; Schröder, U.; Zahn, W. Mechanical stress in ALD-Al2O3 films. Appl. Surf. Sci. 2005, 252, 200–204. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Taheri-Nassaj, E. Effects of milling and calcination temperature on the compressibility and sinterability of a nanocrystalline Al2O3-Y 3 Al5O12 composite powder. J. Am. Ceram. Soc. 2008, 91, 3546–3551. [Google Scholar] [CrossRef]
- Seifert, H.J.; Kussmaul, A.; Aldinger, F. Phase equilibria and diffusion paths in the Ti-Al-O-N system. J. Alloys Compd. 2001, 317–318, 19–25. [Google Scholar] [CrossRef]
- Lee, H.S.; Kubrin, R.; Zierold, R.; Petrov, A.Y.; Nielsch, K.; Schneider, G.A.; Eich, M. Thermal radiation transmission and reflection properties of ceramic 3D photonic crystals. J. Opt. Soc. Am. B 2012, 29, 450. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waleczek, M.; Dendooven, J.; Dyachenko, P.; Petrov, A.Y.; Eich, M.; Blick, R.H.; Detavernier, C.; Nielsch, K.; Furlan, K.P.; Zierold, R. Influence of Alumina Addition on the Optical Properties and the Thermal Stability of Titania Thin Films and Inverse Opals Produced by Atomic Layer Deposition. Nanomaterials 2021, 11, 1053. https://doi.org/10.3390/nano11041053
Waleczek M, Dendooven J, Dyachenko P, Petrov AY, Eich M, Blick RH, Detavernier C, Nielsch K, Furlan KP, Zierold R. Influence of Alumina Addition on the Optical Properties and the Thermal Stability of Titania Thin Films and Inverse Opals Produced by Atomic Layer Deposition. Nanomaterials. 2021; 11(4):1053. https://doi.org/10.3390/nano11041053
Chicago/Turabian StyleWaleczek, Martin, Jolien Dendooven, Pavel Dyachenko, Alexander Y. Petrov, Manfred Eich, Robert H. Blick, Christophe Detavernier, Kornelius Nielsch, Kaline P. Furlan, and Robert Zierold. 2021. "Influence of Alumina Addition on the Optical Properties and the Thermal Stability of Titania Thin Films and Inverse Opals Produced by Atomic Layer Deposition" Nanomaterials 11, no. 4: 1053. https://doi.org/10.3390/nano11041053