Spreading and Drying Dynamics of Water Drop on Hot Surface of Superwicking Ti-6Al-4V Alloy Material Fabricated by Femtosecond Laser
Abstract
1. Introduction
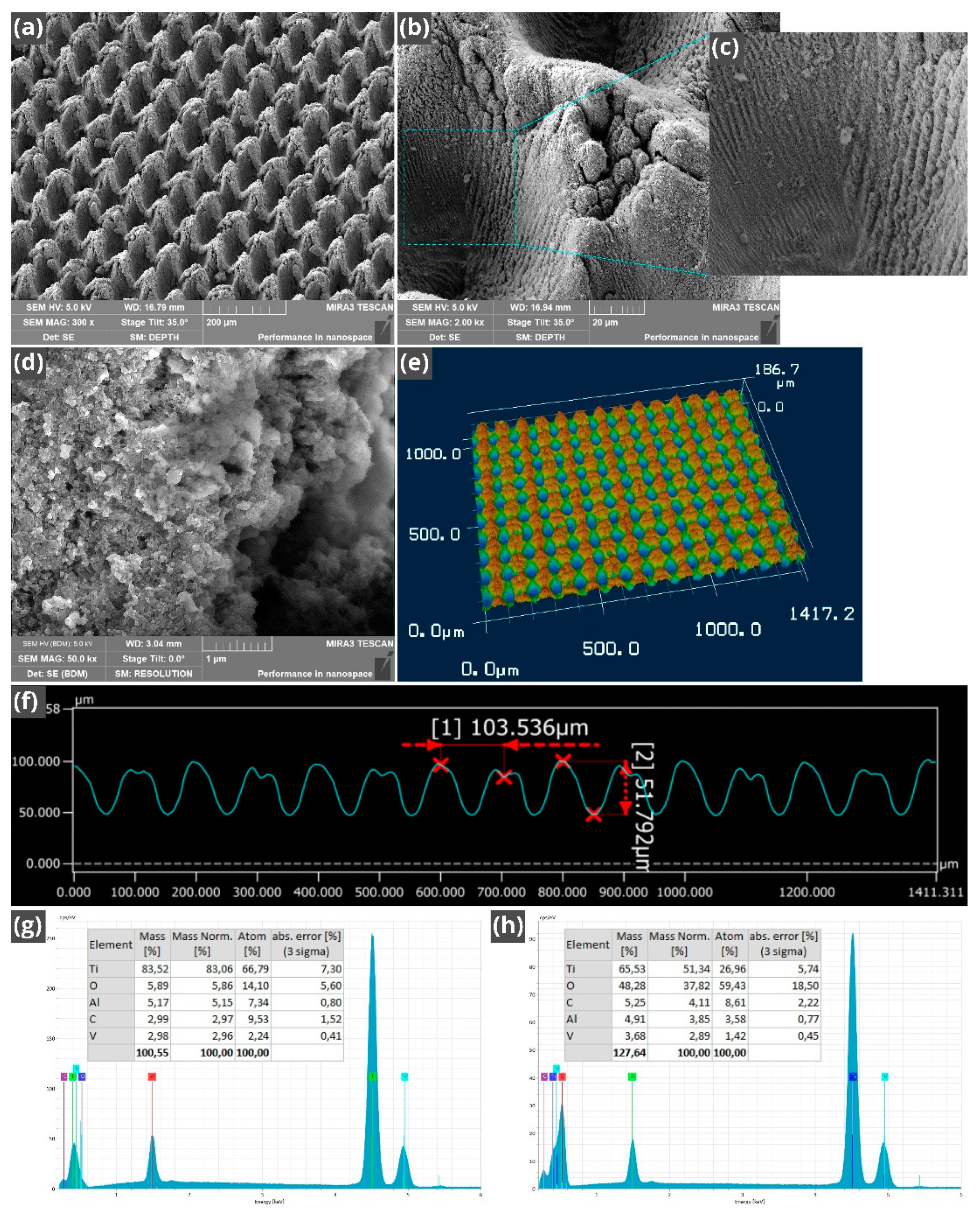
2. Experimental: Fabrication and Characterization
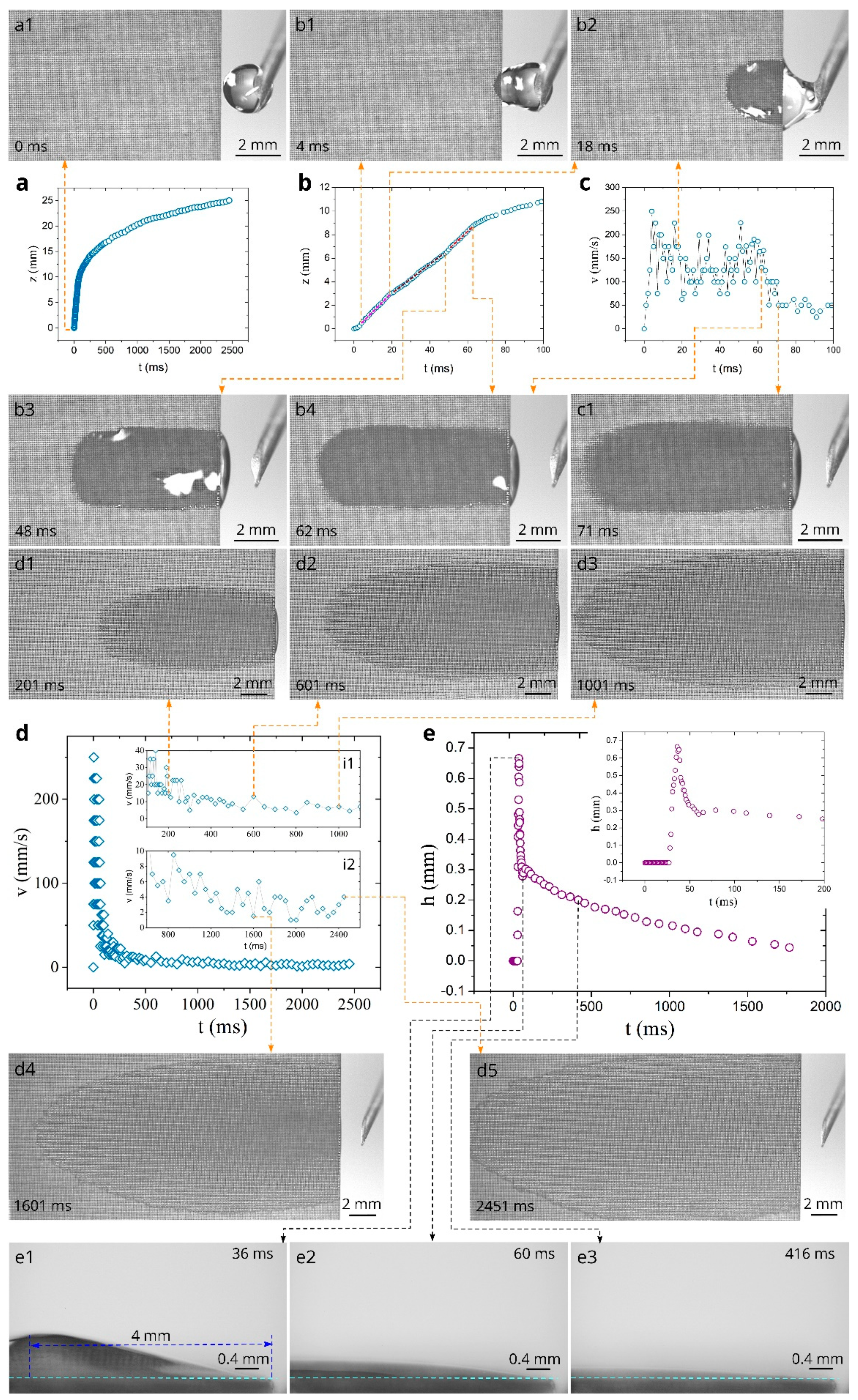
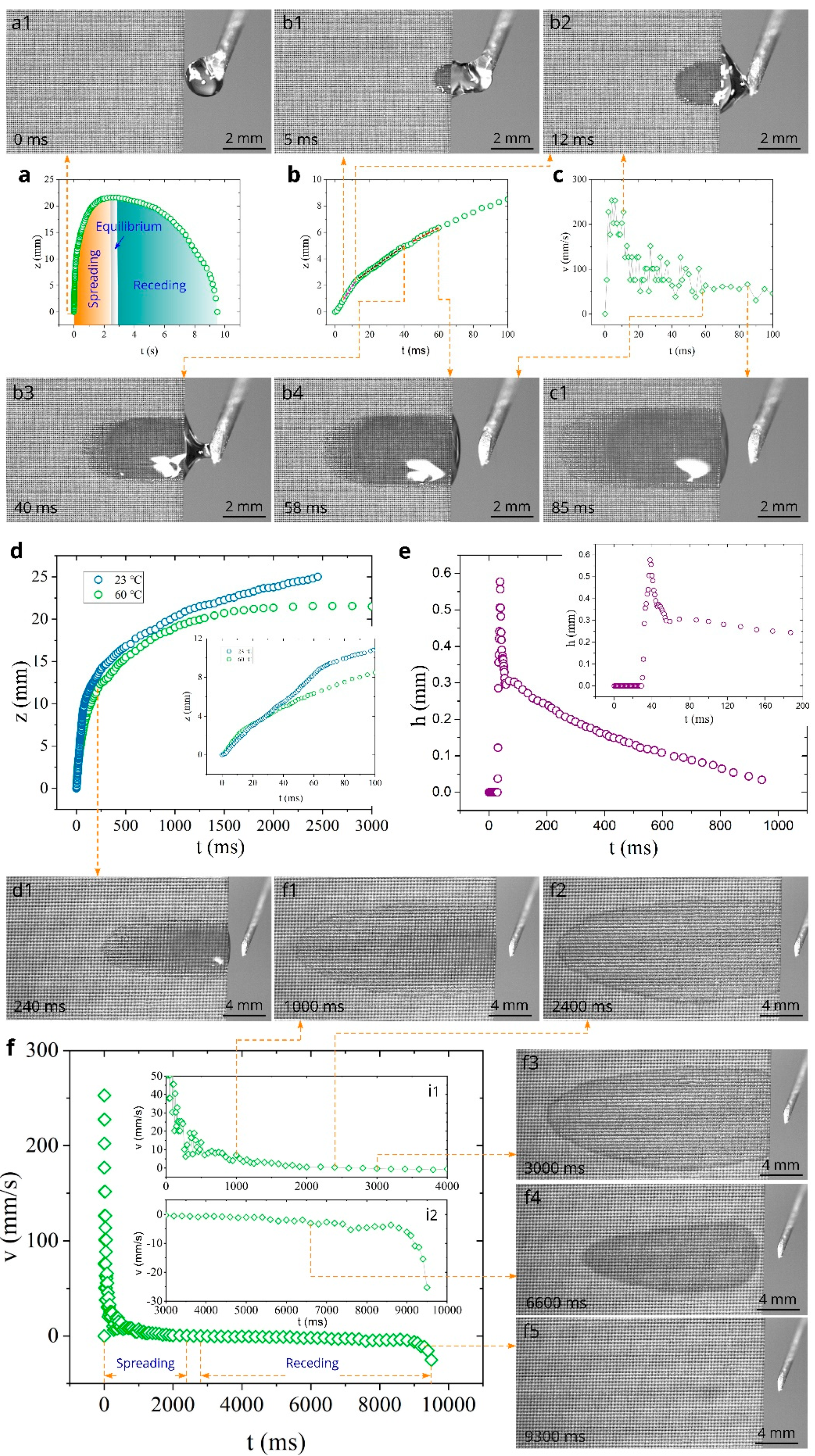
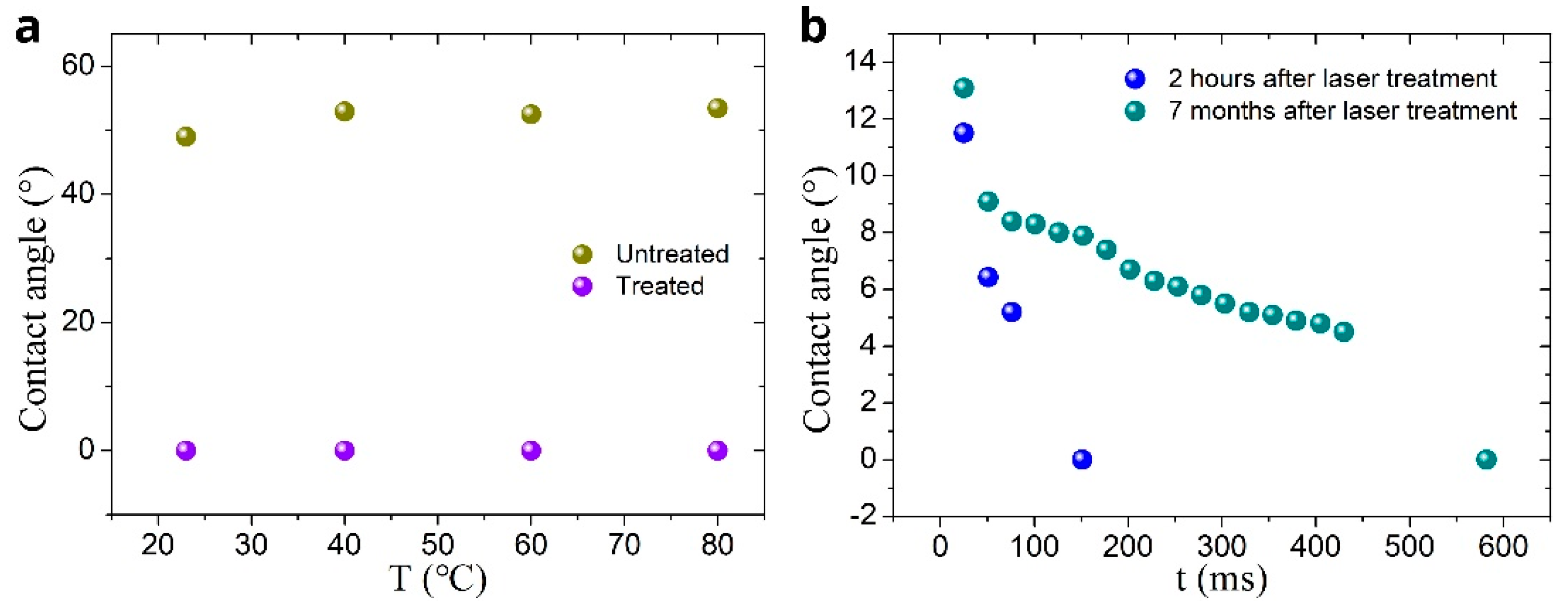
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nadjahi, C.; Louahlia, H.; Lemasson, S. A review of thermal management and innovative cooling strategies for data center. Sustain. Comput. Inform. 2018, 19, 14–28. [Google Scholar] [CrossRef]
- Han, Z.W.; Sun, X.Q.; Wei, H.T.; Ji, Q.; Xue, D. Energy saving analysis of evaporative cooling composite air conditioning system for data centers. Appl. Therm. Eng. 2021, 186, 116506. [Google Scholar] [CrossRef]
- Izadi, A.; Siavashi, M.; Rasam, H.; Xiong, Q.G. MHD enhanced nanofluid mediated heat transfer in porous metal for CPU cooling. Appl. Therm. Eng. 2019, 168, 114843. [Google Scholar] [CrossRef]
- Chernysheva, M.A.; Yushakova, S.I.; Maydanik, Y.F. Copper–water loop heat pipes for energy-efficient cooling systems of supercomputers. Energy 2014, 69, 534–542. [Google Scholar] [CrossRef]
- Zhang, H.X.; Li, G.G.; Chen, L.; Man, G.L.; Miao, J.Y.; Ren, X.Z.; He, J.; Huo, Y.H. Development of flat-plate loop heat pipes for spacecraft thermal control. Microgravity Sci. Technol. 2019, 31, 435–443. [Google Scholar] [CrossRef]
- Shukla, K.N. Heat pipe for aerospace applications—An overview. J. Electron. Cool. Therm. Control. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Ku, J.T.; Ottenstein, L.; Douglas, D.; Hoang, T. Technology overview of a multi-evaporator miniature loop heat pipe for spacecraft applications. J. Spacecr. Rocket. 2012, 49, 999–1007. [Google Scholar] [CrossRef]
- Zhang, X.T.; Liu, Y.X.; Wen, X.Y.; Li, C.Z.; Hu, X.J. Low-grade waste heat driven desalination with an open loop heat pipe. Energy 2018, 163, 221–228. [Google Scholar] [CrossRef]
- Tariq, R.; Sheikh, N.A.; Xamán, J.; Bassam, A. An innovative air saturator for humidification-dehumidification desalination application. Appl. Energy 2018, 228, 789–807. [Google Scholar] [CrossRef]
- Singh, S.C.; Elkabbash, M.; Li, Z.L.; Li, X.H.; Regmi, B.; Madsen, M.; Jalil, S.A.; Zhan, Z.B.; Zhang, J.H.; Guo, C.L. Solar-trackable super-wicking black metal panel for photothermal water sanitation. Nat. Sustain. 2020, 3, 938–946. [Google Scholar] [CrossRef]
- Aono, Y.; Watanabe, N.; Ueno, A.; Nagano, H. Development of a loop heat pipe with kW-class heat transport capability. Appl. Therm. Eng. 2021, 183, 116169. [Google Scholar] [CrossRef]
- Ouyang, T.C.; Su, Z.X.; Yang, R.; Li, C.Z.; Huang, H.Z.; Wei, Q.F. A framework for evaluating and optimizing the cascade utilization of medium-low grade waste heat in marine dual-fuel engines. J. Clean. Prod. 2020, 276, 123289. [Google Scholar] [CrossRef]
- Brough, D.; Ramos, J.; Delpech, B.; Jouhara, H. Development and validation of a TRNSYS type to simulate heat pipe heat exchangers in transient applications of waste heat recovery. Int. J. Thermofluids 2021, 9, 100056. [Google Scholar] [CrossRef]
- Jalil, S.A.; Elkabbash, M.; Li, Z.H.; Zhang, J.H.; Singh, S.; Zhan, Z.B.; Guo, C.L. Multipronged heat-exchanger based on femtosecond laser-nano/microstructured aluminum for thermoelectric heat scavengers. Nano Energy 2020, 75, 104987. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.D.; Ye, F.; Zhu, Y.; Li, K.; Shen, J.L.; Su, L. Experimental investigation on system performances and transient response of a pumped two-phase battery cooling system using R1233zd. Energy Rep. 2020, 6, 238–247. [Google Scholar] [CrossRef]
- Liao, Z.R.; Xu, C.; Ren, Y.X.; Gao, F.; Ju, X.; Du, X.Z. Thermal analysis of a conceptual loop heat pipe for solar central receivers. Energy 2018, 158, 709–718. [Google Scholar] [CrossRef]
- Shafieian, A.; Khiadani, M.; Nosrati, A. Strategies to improve the thermal performance of heat pipe solar collectors in solar systems: A review. Energy Convers. Manag. 2019, 183, 307–331. [Google Scholar] [CrossRef]
- Erickson, D.; Sinton, D.; Psaltis, D. Optofluidics for energy applications. Nat. Photonics 2011, 5, 583–590. [Google Scholar] [CrossRef]
- Li, N.B.; Yang, D.J.; Shao, Y.; Liu, Y.T.; Tang, J.B.; Yang, L.P.; Sun, T.Y.; Zhou, W.J.; Liu, H.; Xue, G.B. Nanostructured black aluminum prepared by laser direct writing as a high-performance plasmonic absorber for photothermal/electric conversion. ACS Appl. Mater. Interfaces 2021, 13, 4305–4315. [Google Scholar] [CrossRef]
- Su, Q.; Chang, S.N.; Zhao, Y.Y.; Zheng, H.K.; Dang, C.B. A review of loop heat pipes for aircraft anti-icing applications. Appl. Therm. Eng. 2018, 130, 528–540. [Google Scholar] [CrossRef]
- Mahmood, M.H.; Sultan, M.; Miyazaki, T.; Koyama, S.; Maisotsenko, V.S. Overview of the Maisotsenko cycle—A way towards dew point evaporative cooling. Renew. Sustain. Energy Rev. 2016, 66, 537–555. [Google Scholar] [CrossRef]
- Dizaji, H.S.; Hu, E.J.; Chen, L.; Pourhedayat, S. Comprehensive exergetic study of regenerative Maisotsenko air cooler; formulation and sensitivity analysis. Appl. Therm. Eng. 2019, 152, 455–467. [Google Scholar] [CrossRef]
- Katramiz, E.; Al Jebaei, H.; Alotaibi, S.; Chakroun, W.; Ghaddar, N.; Ghali, K. Sustainable cooling system for Kuwait hot climate combining diurnal radiative cooling and indirect evaporative cooling system. Energy 2020, 213, 119045. [Google Scholar] [CrossRef]
- Duan, Z.Y.; Zhao, X.D.; Zhan, C.H.; Dong, X.L.; Chen, H.B. Energy saving potential of a counter-flow regenerative evaporative cooler for various climates of China: Experiment-based evaluation. Energy Build. 2017, 148, 199–210. [Google Scholar] [CrossRef]
- Zanchini, E.; Naldi, C. Energy saving obtainable by applying a commercially available M-cycle evaporative cooling system to the air conditioning of an office building in North Italy. Energy 2019, 179, 975–988. [Google Scholar] [CrossRef]
- Duan, Z.Y.; Zhan, C.H.; Zhang, X.X.; Mustafa, M.; Zhao, X.D.; Alimohammadisagvand, B.; Hasan, A. Indirect evaporative cooling: Past, present and future potentials. Renew. Sustain. Energy Rev. 2012, 16, 6823–6850. [Google Scholar] [CrossRef]
- Caliskan, H.; Hepbasli, A.; Dincer, I.; Maisotsenko, V. Thermodynamic performance assessment of a novel air cooling cycle: Maisotsenko cycle. Int. J. Refrig. 2011, 34, 980–990. [Google Scholar] [CrossRef]
- Anisimov, S.; Pandelidis, D. Numerical study of the Maisotsenko cycle heat and mass exchanger. Int. J. Heat Mass Transf. 2014, 75, 75–96. [Google Scholar] [CrossRef]
- Xu, P.; Ma, X.L.; Zhao, X.D.; Xiong, Y.X.; Sun, Y.B. Feasibility analysis for a novel dew point air cooler applied in warm and humid climate: A case study in Beijing. Energy Procedia 2019, 158, 2126–2131. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.C.; Chen, B.B.; Liu, W.; Li, C.; Yang, J.G. Development of biporous wicks for flat-plate loop heat pipe. Exp. Therm. Fluid Sci. 2012, 37, 91–97. [Google Scholar] [CrossRef]
- Guo, H.; Ji, X.B.; Xu, J.L. Enhancement of loop heat pipe heat transfer performance with superhydrophilic porous wick. Int. J. Therm. Sci. 2020, 156, 106466. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhao, R.Z.; Liu, Z.C.; Liu, W. Application of biporous wick in flat-plate loop heat pipe with long heat transfer distance. Appl. Therm. Eng. 2021, 184, 116283. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C.L. Metal pumps liquid uphill. Appl. Phys. Lett. 2009, 94, 224102. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C.L. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Cheng, L.; Tan, B.; Chen, Z.F.; Zhang, W.; Liu, Z.L.; Peng, J. Directional liquid spreading on laser textured aluminum surface. Microsyst. Technol. 2020, 26, 2767–2776. [Google Scholar] [CrossRef]
- Fang, R.R.; Zhu, H.B.; Li, Z.K.; Zhu, X.H.; Zhang, X.H.; Huang, Z.Y.; Li, K.; Yan, W.S.; Huang, Y.; Maisotsenko, V.S.; et al. Temperature effect on capillary flow dynamics in 1D array of open nanotextured microchannels produced by femtosecond laser on silicon. Nanomaterials 2020, 796, 796. [Google Scholar] [CrossRef]
- Xie, F.; Yang, J.J.; Ngo, C.V. The effect of femtosecond laser fluence and pitches between V-shaped microgrooves on the dynamics of capillary flow. Results Phys. 2020, 19, 103606. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.R.; Zhu, H.B.; Li, Z.K.; Yan, W.S.; Zhang, X.H.; Zhu, X.H.; Maisotsenko, V.S.; Vorobyev, A.Y. Capillary Nylon 6 polymer material produced by femtosecond laser processing. Opt. Express 2019, 27, 36066–36074. [Google Scholar] [CrossRef]
- Kim, D.E.; Yu, D.I.; Park, S.C.; Kwak, H.J.; Ahn, H.S. Critical heat flux triggering mechanism on micro-structured surfaces: Coalesced bubble departure frequency and liquid furnishing capability. Int. J. Heat Mass Transf. 2015, 91, 1237–1247. [Google Scholar] [CrossRef]
- Vaartstra, G.; Lu, Z.M.; Wang, E.N. Simultaneous prediction of dryout heat flux and local temperature for thin film evaporation in micropillar wicks. Int. J. Heat Mass Transf. 2019, 136, 170–177. [Google Scholar] [CrossRef]
- Chu, K.H.; Joung, Y.S.; Enright, R.; Buie, C.R.; Wang, E.N. Hierarchically structured surfaces for boiling critical heat flux enhancement. Appl. Phys. Lett. 2013, 102, 151602. [Google Scholar] [CrossRef]
- Dhillon, N.S.; Buongiorno, J.; Varanasi, K.K. Critical heat flux maxima during boiling crisis on textured surfaces. Nat. Commun. 2015, 6, 8247. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; He, B.; Liang, Q.; Somasundaram, S.; Tan, C.S.; Wang, E.N. Optimization and thermal characterization of uniform silicon micropillar based evaporators. Int. J. Heat Mass Transf. 2018, 127, 51–60. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Antao, D.S.; Lu, Z.M.; Somasundaram, S.; Zhang, T.J.; Wang, E.N. Prediction and characterization of dry-out heat flux in micropillar wick structures. Langmuir 2016, 32, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, K.M.T.; Grambow, C.; Kietzig, A.M. Fabrication of micro/nano structures on metals by femtosecond laser micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Gamaly, E.G.; Rode, A.V. Physics of ultra-short laser interaction with matter: From phonon excitation to ultimate transformations. Prog. Quant. Electron. 2013, 37, 215–323. [Google Scholar] [CrossRef]
- Shugaev, M.V.; Wu, C.P.; Armbruster, O.; Naghilou, A.; Brouwer, N.; Ivanov, D.S.; Derrien, T.J.Y.; Bulgakova, N.M.; Kautek, W.; Rethfeld, B.; et al. Fundamentals of ultrafast laser-material interaction. MRS Bull. 2016, 41, 960–968. [Google Scholar] [CrossRef]
- Inogamov, N.A.; Zhakhovskii, V.V.; Ashitkov, S.I.; Petrov, Y.V.; Agranat, M.B.; Anisimov, S.I.; Nishihara, K.; Fortov, V.E. Nanospallation induced by an ultrashort laser pulse. J. Exp. Theor. Phys. 2008, 107, 1–19. [Google Scholar] [CrossRef]
- Povarnitsyn, M.E.; Fokin, V.B.; Levashov, P.R.; Itina, T.E. Molecular dynamics simulation of subpicosecond double-pulse laser ablation of metals. Phys. Rev. B 2015, 92, 174104. [Google Scholar] [CrossRef]
- Fang, R.R.; Vorobyev, A.Y.; Guo, C.L. Direct visualization of the complete evolution of femtosecond laser-induced surface structural dynamics of metals. Light Sci. Appl. 2017, 6, e16256. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C.L. Laser turns silicon superwicking. Opt. Express 2010, 18, 6455–6460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Q.; Li, G.Q.; Li, J.W.; Xie, H.; Hu, Y.L.; Chu, J.R.; Huang, W.H. Self-driven flow in surface grooves fabricated by femtosecond laser. Surf. Coat. Technol. 2014, 242, 246–250. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C.L. Superwicking glass produced by femtosecond laser. J. Appl. Phys. 2010, 108, 123512. [Google Scholar] [CrossRef]
- Yin, K.; Duan, J.A.; Sun, X.Y.; Wang, C.; Luo, Z. Formation of superwetting surface with line-patterned nanostructure on sapphire induced by femtosecond laser. Appl. Phys. A 2015, 119, 69–74. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell meets Marangoni—A review of theories on laser-induced periodic surface structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Huerta-Murillo, D.; Garcia-Giron, A.; Romano, J.M.; Cardoso, J.T.; Cordovilla, F.; Walker, M.; Dimov, S.S.; Ocana, J.L. Wettability modification of laser-fabricated hierarchical surface structures in Ti-6Al-4V titanium alloy. Appl. Surf. Sci. 2019, 463, 838–846. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E. Superhydrophilic and superwetting surfaces: Definition and mechanisms of control. Langmuir 2010, 26, 18621–18623. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C.L. Multifunctional surfaces produced by femtosecond laser. J. Appl. Phys. 2015, 117, 033103. [Google Scholar] [CrossRef]
- Amer, O.; Boukhanouf, R.; Ibrahim, H.G. A review of evaporative cooling technologies. Int. J. Environ. Sci. Dev. 2015, 6, 111–117. [Google Scholar] [CrossRef]
- NSIDC Data Center: Energy Reduction Strategies. United States: U.S. Department of Energy, Energy Efficiency & Renewable Energy, Federal Energy Management Program (FEMP), Report No. DOE/GO-102012-3509. 2012. Available online: https://www.energy.gov/sites/prod/files/2013/10/f3/nsidc_dcstrategies.pdf (accessed on 31 March 2021).
- David, S.; Sefiane, K.; Tadrist, L. Experimental investigation of the effect of thermal properties of the substrate in the wetting and evaporation of sessile drops. Colloids Surf. A 2007, 298, 108–114. [Google Scholar] [CrossRef]
- Chandramohan, A.; Dash, S.; Weibel, J.A.; Chen, X.M.; Garimella, S.V. Marangoni convection in evaporating organic liquid droplets on a nonwetting substrate. Langmuir 2016, 32, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Makin, V.S.; Guo, C.L. Periodic ordering of random surface nanostructures induced by femtosecond laser pulses on metals. J. Appl. Phys. 2007, 101, 034903. [Google Scholar] [CrossRef]
- Makin, V.S.; Makin, R.S.; Vorobyev, A.Y.; Guo, C.L. Dissipative nanostructures and Feigenbaum’s universality in the “Metal-high-power ultrashort-pulsed polarized radiation” nonequilibrium nonlinear dynamical system. Tech. Phys. Lett. 2008, 34, 387–390. [Google Scholar] [CrossRef]
- Hwang, T.Y.; Guo, C.L. Angular effects of nanostructure-covered femtosecond laser induced periodic surface structures on metals. J. Appl. Phys. 2010, 108, 073523. [Google Scholar] [CrossRef]
- Ionin, A.A.; Kudryashov, S.I.; Rudenko, A.A.; Seleznev, L.V.; Sinitsyn, D.V.; Makarov, S.V. Nonlinear optical feedback for nano- and micropatterning of silicon surface under femtosecond laser irradiation. Opt. Mater. Express 2017, 7, 2793–2807. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C.L. Nanochemical effects in femtosecond laser ablation of metals. Appl. Phys. Lett. 2013, 102, 074107. [Google Scholar] [CrossRef]
- Kolobov, Y.R.; Zhidkov, M.V.; Golosov, E.V.; Vershinina, T.N.; Kudryashov, S.I.; Makarov, S.V.; Ionin, A.A.; Ligachev, A.E. Phase composition and structure of femtosecond laser-produced oxide layer on VT6 alloy surface. Laser Phys. Lett. 2016, 13, 076103. [Google Scholar] [CrossRef]
- Ionin, A.A.; Kudryashov, S.I.; Makarov, S.V.; Saltuganov, P.N.; Seleznev, L.V.; Sinitsyn, D.V.; Golosov, E.V.; Goryainov, A.A.; Kolobov, Y.R.; Kornieieva, K.A.; et al. Femtosecond laser modification of titanium surfaces: Direct imprinting of hydroxylapatite nanopowder and wettability tuning via surface microstructuring. Laser Phys. Lett. 2013, 10, 045605. [Google Scholar] [CrossRef]
- Stange, M.; Dreyer, M.E.; Rath, H.J. Capillary driven flow in circular cylindrical tubes. Phys. Fluids 2003, 15, 2587–2601. [Google Scholar] [CrossRef]
- Huang, W.F.; Liu, Q.S.; Li, Y. Capillary filling flows inside patterned-surface microchannels. Chem. Eng. Technol. 2006, 29, 716–723. [Google Scholar] [CrossRef]
- Das, S.; Waghmare, P.R.; Mitra, S.K. Early regimes of capillary filling. Phys. Rev. E 2012, 86, 067301. [Google Scholar] [CrossRef] [PubMed]
- Quere, D. Inertial capillarity. Europhys. Lett. 1997, 39, 533–538. [Google Scholar] [CrossRef]
- Siebold, A.; Nardin, M.; Schultz, J.; Walliser, A.; Oppliger, M. Effect of dynamic contact angle on capillary rise phenomena. Colloids Surf. A 2000, 161, 81–87. [Google Scholar] [CrossRef]
- Andrukh, T.; Monaenkova, D.; Rubin, B.; Lee, W.K.; Kornev, K.G. Meniscus formation in a capillary and the role of contact line friction. Soft Matter 2014, 10, 609–615. [Google Scholar] [CrossRef]
- Fries, N.; Dreyer, M. The transition from inertial to viscous flow in capillary rise. J. Colloid Interface Sci. 2008, 327, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Deng, D.X.; Tang, Y.; Zeng, J.; Yang, S.; Shao, H.R. Characterization of capillary rise dynamics in parallel micro V-grooves. Int. J. Heat Mass Transf. 2014, 77, 311–320. [Google Scholar] [CrossRef]
- O’Loughlin, M.; Wilk, K.; Priest, C.; Ralston, J.; Popescu, M.N. Capillary rise dynamics of aqueous glycerol solutions in glass capillaries: A critical examination of the Washburn equation. J. Colloid Interface Sci. 2013, 411, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Courbin, L.; Denieul, E.; Dressaire, E.; Roper, M.; Ajdari, A.; Stone, H.A. Imbibition by polygonal spreading on microdecorated surfaces. Nat. Mater. 2007, 6, 661–664. [Google Scholar] [CrossRef]
- Xing, H.T.; Cheng, J.; Zhou, C.L. Effect of gradient wettability on capillary imbibition in open semicircular copper channel. Phys. Fluids 2020, 32, 112004. [Google Scholar] [CrossRef]
- Fu, R.; Hu, X.G.; Zhang, H.J.; Yan, Y.Y.; Zhou, W.B.; Wang, J.H. Investigation of the influence of Fe3O4-water nanofluids on capillary performance in microgrooves wick. Appl. Therm. Eng. 2021, 182, 115899. [Google Scholar] [CrossRef]
- Abdulshaheed, A.A.; Wang, P.T.; Huang, G.H.; Li, C. High performance copper-water heat pipes with nanoengineered evaporator sections. Int. J. Heat Mass Transf. 2019, 133, 474–486. [Google Scholar] [CrossRef]
- Zhang, S.W.; Lin, L.; Chen, G.; Tang, H.; Zeng, J.; Yuan, W.; Tang, Y. Experimental study on the capillary performance of aluminum micro-grooved wicks with reentrant cavity array. Int. J. Heat Mass Transf. 2019, 139, 917–927. [Google Scholar] [CrossRef]
- Siddique, J.I.; Anderson, D.M.; Bondarev, A. Capillary rise of a liquid into a deformable porous material. Phys. Fluids 2009, 21, 013106. [Google Scholar] [CrossRef]
- Tang, J.C.; Hu, X.G. Evaluation of capillary wetting performance of micro-nano hybrid structures for open microgrooves heat sink. Exp. Therm. Fluid Sci. 2020, 112, 109948. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, M.W.; Lee, K.R.; Lee, D.Y.; Chang, Y.S.; Kim, H.Y. Liquid spreading on superhydrophilic micropillar arrays. J. Fluid Mech. 2011, 680, 477–487. [Google Scholar] [CrossRef]
- Tian, J.F.; Kannangara, D.; Li, X.; Shen, W. Capillary driven low-cost V-groove microfluidic device with high sample transport efficiency. Lab. Chip 2010, 10, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Bico, J.; Tordeux, C.; Quere, D. Rough wetting. Europhys. Lett. 2001, 55, 214–220. [Google Scholar] [CrossRef]
- Rye, R.R.; Mann, J.A.; Yost, F.G. The flow of liquids in surface grooves. Langmuir 1996, 12, 555–565. [Google Scholar] [CrossRef]
- Romero, L.A.; Yost, F.G. Flow in an open channel capillary. J. Fluid Mech. 1996, 322, 109–129. [Google Scholar] [CrossRef]
- Yang, M.; Cao, B.Y.; Wang, W.; Yun, H.M.; Chen, B.M. Experimental study on capillary filling in nanochannels. Chem. Phys. Lett. 2016, 662, 137–140. [Google Scholar] [CrossRef]
- Haneveld, J.; Tas, N.R.; Brunets, N.; Jansen, H.V.; Elwenspoek, M. Capillary filling of sub-10 nm nanochannels. J. Appl. Phys. 2008, 104, 014309. [Google Scholar] [CrossRef]
- Fisher, L.R.; Lark, P.D. An experimental study of the Washburn equation for liquid flow in very fine capillaries. J. Colloid Interface Sci. 1979, 69, 486–492. [Google Scholar] [CrossRef]
- Dimitrov, D.I.; Milchev, A.; Binder, K. Capillary rise in nanopores: Molecular dynamics evidence for the Lucas-Washburn equation. Phys. Rev. Lett. 2007, 99, 054501. [Google Scholar] [CrossRef]
- Bar-Cohen, A.; Geisler, K.J.L. Cooling the electronic brain. Mech. Eng. 2011, 133, 38–41. [Google Scholar] [CrossRef]
- Ebadian, M.A.; Lin, C.X. A review of high-heat-flux heat removal technologies. J. Heat Transf. 2011, 133, 110801. [Google Scholar] [CrossRef]
- Chen, L.G.; Shen, J.F.; Ge, Y.L.; Wu, Z.X.; Wang, W.H.; Zhu, F.L.; Feng, H.J. Power and efficiency optimization of open Maisotsenko-Brayton cycle and performance comparison with traditional open regenerated Brayton cycle. Energy Convers. Manag. 2020, 217, 113001. [Google Scholar] [CrossRef]
- Zhu, F.L.; Chen, L.G.; Wang, W.H. Thermodynamic analysis of an irreversible Maisotsenko reciprocating Brayton cycle. Entropy 2018, 20, 167. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Chow, T.T.; Fong, K.F.; Lee, C.K. Investigation on humidified gas turbine cycles with Maisotsenko-cycle-based air saturator. Energy Procedia 2019, 158, 5195–5200. [Google Scholar] [CrossRef]
- Saghafifar, M.; Gadalla, M. Analysis of Maisotsenko open gas turbine power cycle with a detailed air saturator model. Appl. Energy 2015, 149, 338–353. [Google Scholar] [CrossRef]
- Abgrall, P.; Gue, A.M. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15–R49. [Google Scholar] [CrossRef]
- Marmur, A. Penetration of a small drop into a capillary. J. Colloid Interface Sci. 1988, 122, 209–219. [Google Scholar] [CrossRef]
- Radiom, M.; Chan, W.K.; Yang, C. A study of capillary flow from a pendant droplet. Microfluid. Nanofluid. 2009, 7, 697–707. [Google Scholar] [CrossRef]
- Lade, R.K.; Hippchen, E.J.; Macosko, C.W.; Francis, L.F. Dynamics of capillary-driven flow in 3D printed open microchannels. Langmuir 2017, 33, 2949–2964. [Google Scholar] [CrossRef] [PubMed]
- Alhosani, M.H.; Zhang, T.J. Dynamics of microscale liquid propagation in micropillar arrays. Langmuir 2017, 33, 6620–6629. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.S.; Meinhart, C.D.; MacDonald, N.C. Surface modifications of bulk micromachined titanium pillar arrays: A wick material for thin flat heat pipes. In Proceedings of the ASME 2009 Second International Conference on Micro/Nanoscale Heat and Mass Transfer, Shanghai, China, 18–21 December 2009; pp. 415–419. [Google Scholar] [CrossRef]
- Srivastava, N.; Din, C.S.; Judson, A.; MacDonald, N.C.; Meinhart, C.D. A unified scaling model for flow through a lattice of microfabricated posts. Lab. Chip 2010, 10, 1148–1152. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, E.N. Microscale liquid dynamics and the effect on macroscale propagation in pillar arrays. Langmuir 2011, 27, 10360–10364. [Google Scholar] [CrossRef]
- Rao, Z.H.; Wang, S.F. A review of power battery thermal energy management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Mudawar, I.; Bharathan, D.; Kelly, K.; Narumanchi, S. Two-phase spray cooling of hybrid vehicle electronics. IEEE Trans. Compon. Packag. Technol. 2009, 32, 501–512. [Google Scholar] [CrossRef]
- Vaartstra, G.; Zhang, L.N.; Lu, Z.M.; Díaz-Marín, C.D.; Grossman, J.C.; Wang, E.N. Capillary-fed, thin film evaporation devices. J. Appl. Phys. 2020, 128, 130901. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z.C.; Cai, Z.R.; Ganapathy, T.; Fang, N.X.; Li, C.X.; Yu, C.L.; Zhang, Y.; Song, Y.L. Highly efficient three-dimensional solar evaporator for high salinity desalination by localized crystallization. Nat. Commun. 2020, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.K.; Haechler, I.; Kaur, S.; Lubner, S.; Preasher, R.S. Enhanced solar evaporation using a photo-thermal umbrella for wastewater management. Nat. Sustain. 2020, 3, 144–151. [Google Scholar] [CrossRef]
- Pandelidis, D. Numerical study and performance evaluation of the Maisotsenko cycle cooling tower. Energy Convers. Manag. 2020, 210, 112735. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, R.; Li, Z.; Zhang, X.; Zhu, X.; Zhang, H.; Li, J.; Pan, Z.; Huang, Z.; Yang, C.; Zheng, J.; et al. Spreading and Drying Dynamics of Water Drop on Hot Surface of Superwicking Ti-6Al-4V Alloy Material Fabricated by Femtosecond Laser. Nanomaterials 2021, 11, 899. https://doi.org/10.3390/nano11040899
Fang R, Li Z, Zhang X, Zhu X, Zhang H, Li J, Pan Z, Huang Z, Yang C, Zheng J, et al. Spreading and Drying Dynamics of Water Drop on Hot Surface of Superwicking Ti-6Al-4V Alloy Material Fabricated by Femtosecond Laser. Nanomaterials. 2021; 11(4):899. https://doi.org/10.3390/nano11040899
Chicago/Turabian StyleFang, Ranran, Zekai Li, Xianhang Zhang, Xiaohui Zhu, Hanlin Zhang, Junchang Li, Zhonglin Pan, Zhiyu Huang, Chen Yang, Jiangen Zheng, and et al. 2021. "Spreading and Drying Dynamics of Water Drop on Hot Surface of Superwicking Ti-6Al-4V Alloy Material Fabricated by Femtosecond Laser" Nanomaterials 11, no. 4: 899. https://doi.org/10.3390/nano11040899
APA StyleFang, R., Li, Z., Zhang, X., Zhu, X., Zhang, H., Li, J., Pan, Z., Huang, Z., Yang, C., Zheng, J., Yan, W., Huang, Y., Maisotsenko, V. S., & Vorobyev, A. Y. (2021). Spreading and Drying Dynamics of Water Drop on Hot Surface of Superwicking Ti-6Al-4V Alloy Material Fabricated by Femtosecond Laser. Nanomaterials, 11(4), 899. https://doi.org/10.3390/nano11040899





