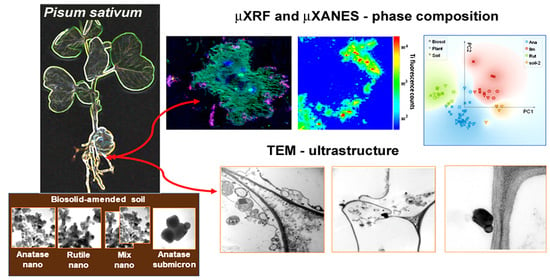
Synchrotron Radiation Spectroscopy and Transmission Electron Microscopy Techniques to Evaluate TiO2 NPs Incorporation, Speciation, and Impact on Root Cells Ultrastructure of Pisum sativum L. Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Growth Matrices
2.2. Titanium Concentration in Plants
2.3. Data Acquisition at Synchrotron Facilities
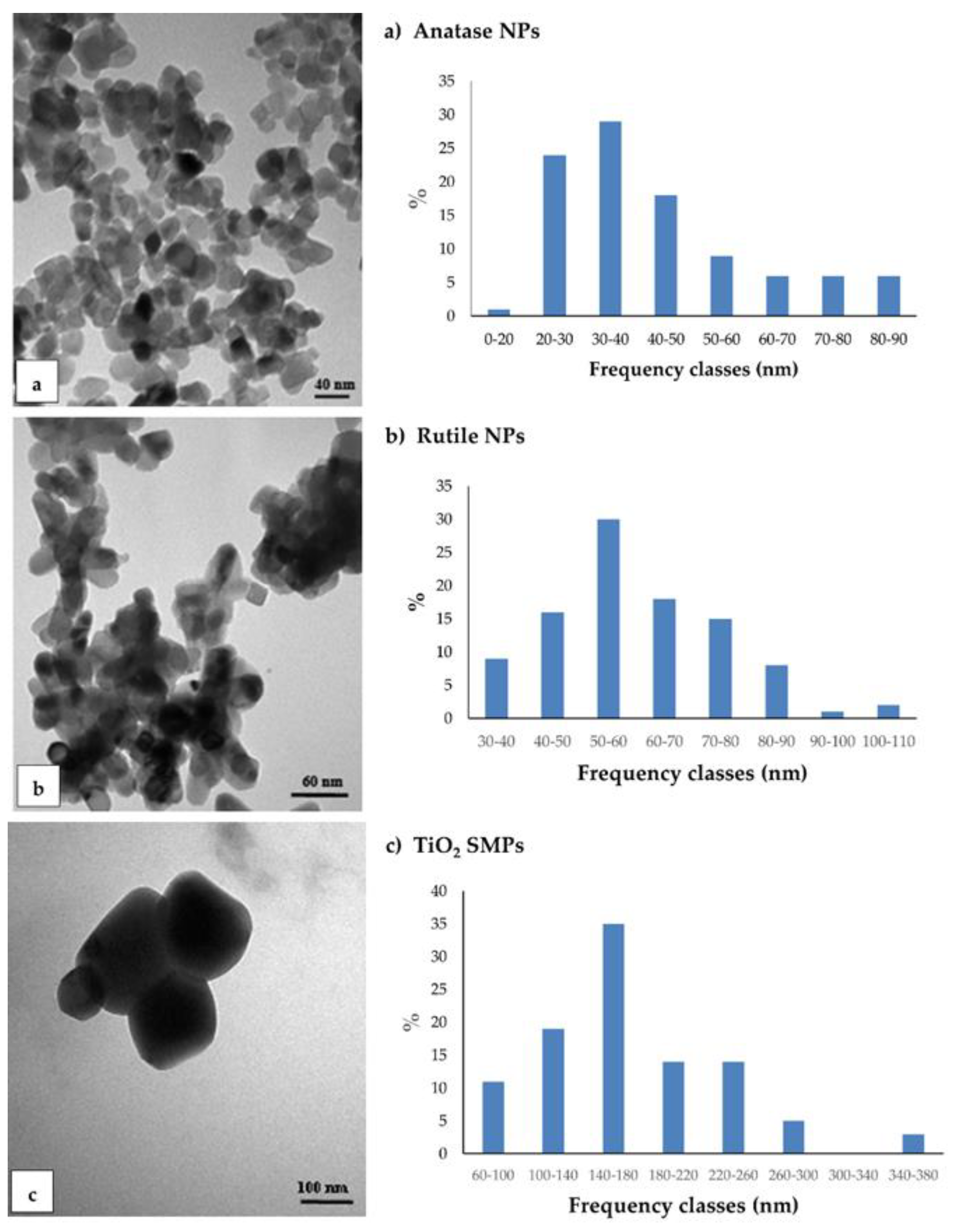
2.4. Transmission Electron Microscopy (TEM)
2.5. Statistical Analysis
3. Results
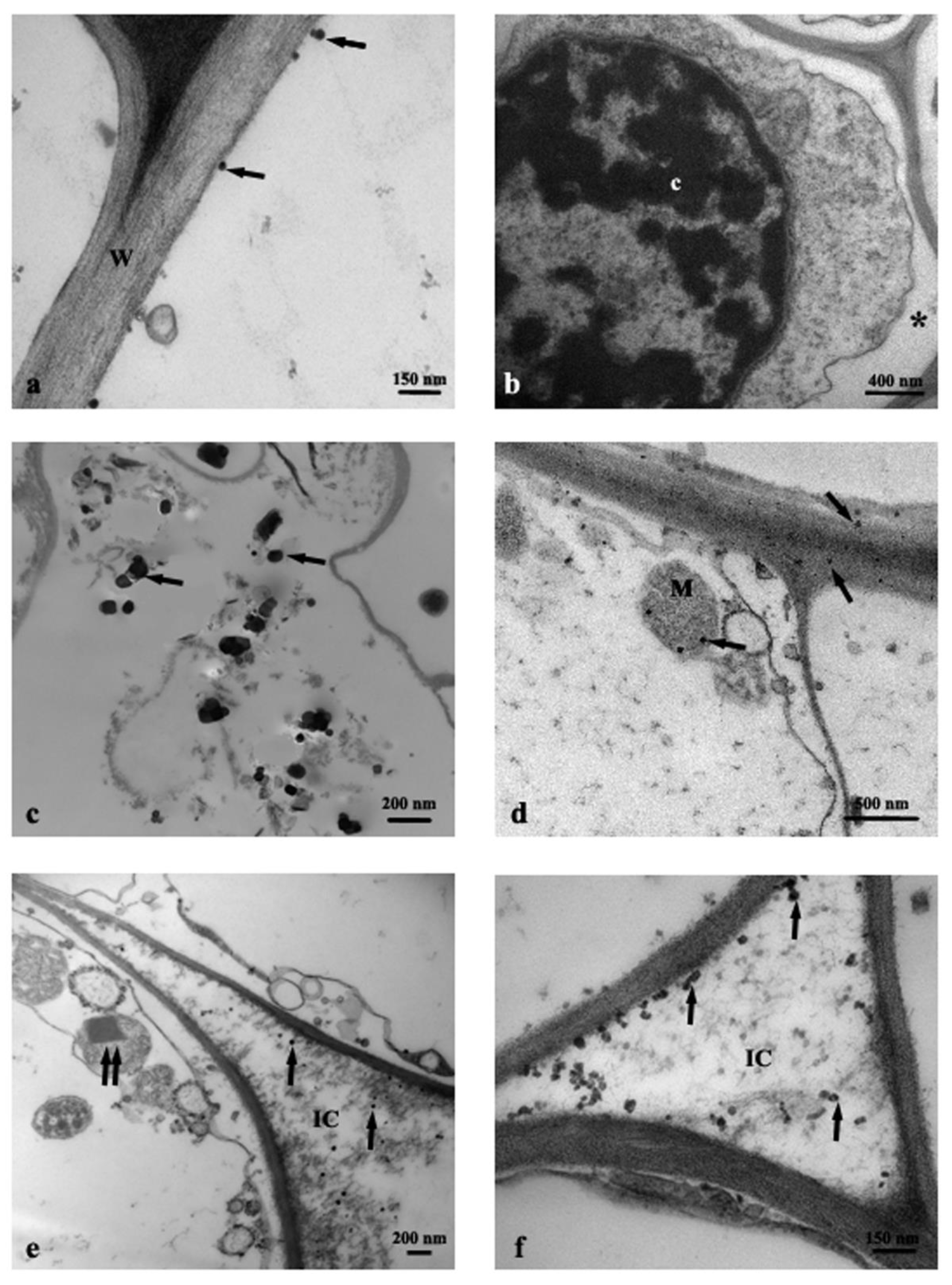
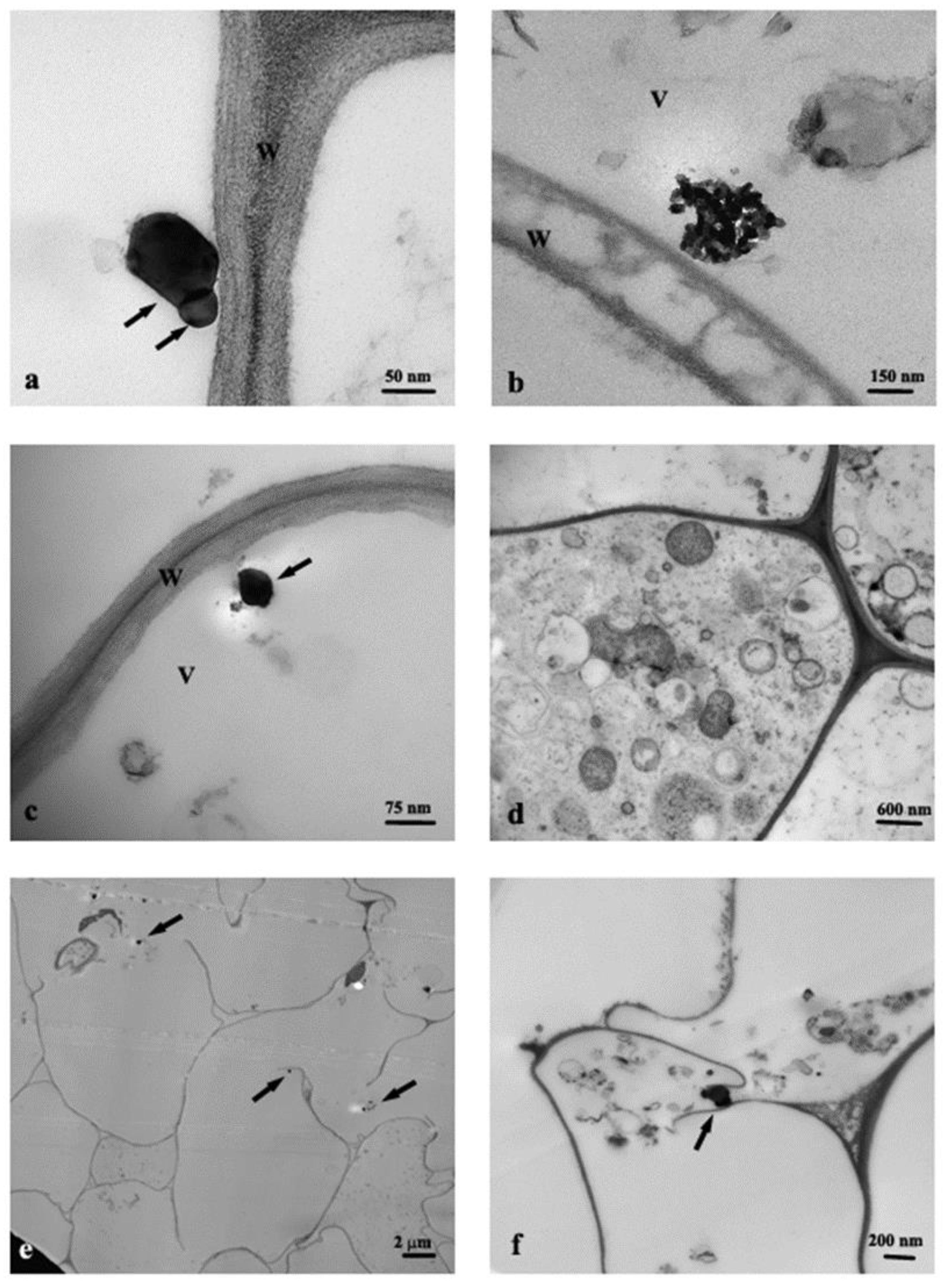
3.1. TEM Observations
3.2. Synchrotron Radiation Based µXRF and µXANES
3.3. Uptake of Ti by Roots and Its Translocation to Shoots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and application of nano-TiO2: A review. Environ. Sci. Poll. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gao, X.; Pan, B. Nanoconfinement-Mediated Water Treatment: From Fundamental to Application. Environ. Sci. Technol. 2020, 54, 8509–8852. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.; Muccifora, S.; Barbieri, F.; Tassi, E.; Castiglione, M.R.; Giorgetti, L. Genotoxicity of the food additive E171, titanium dioxide, in the plants Lens culinaris L. and Allium cepa L. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 849, 503142. [Google Scholar] [CrossRef]
- Pele, L.C.; Thoree, V.; Bruggraber, S.F.; Koller, D.; Thompson, R.; Lomer, M.C.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part Fibre Toxicol. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Hicks, A.L. Estimating Human Exposure to Titanium Dioxide from Personal Care Products Through a Social Survey Approach. Integr. Environ. Assess. Manag. 2019, 16, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Dominguez, A.; Turley, R.S.; Kim, H.; Kazi, A.; Sultana, A.; Shuvo, M.; Alvarado-Tenorio, B.; Montes, M.O.; Lin, Y.; et al. Development of photocatalytic paint based on TiO2 and photopolymer resin for the degradation of organic pollutants in water. Sci. Tot. Environ. 2020, 704, 135406. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.; Borthakur, A.; Srivastava, P.; Srivastava, N.; Tiwary, D.; Mishra, P.K. Effect of nanoscale TiO2-activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energ. Ecol. Environ. 2016, 1, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.; El-Mogazy, S.; Derbalah, A. Fenton reagent and titanium dioxide nanoparticles as antifungal agents to control leaf spot of sugar beet under field conditions. J. Plant Prot. Res. 2016, 56, 270–278. [Google Scholar] [CrossRef]
- Wendt Böhmer-Maas, B.; Martins Fonseca, L.; Murowaniecki Oterob, D.; da Rosa Zavarezea, E.; Zambiazi, R.C. Photocatalytic zein-TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 100508. [Google Scholar] [CrossRef]
- Song, R.; Quin, Y.; Suh, S.; Keller, A.A. Dynamic model for the stocks and release flows of engineered nanomaterials. Environ. Sci. Technol. 2017, 51, 12424–12433. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, Y.; Westerhoff, P.; Hristovski, K.; Jin, V.L.; Johnson, M.V.V.; Arnold, J.G. Metal and nanoparticle occurrence in biosolid-amended soils. Sci. Total Environ. 2014, 485, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Vriens, B.; Voegelin, A.; Hug, S.J.; Kaegi, R.; Winkel, L.H.E.; Buser, A.M.; Berg, M. Quantification of Element Fluxes in Wastewaters: A Nationwide Survey in Switzerland. Environ. Sci. Technol. 2017, 51, 10943–10953. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, H.; Nabavi, M.; Kashefi, H. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornam. Hortic. Plants 2013, 3, 25–32. [Google Scholar]
- Timmusk, S.; Seisenbaeva, G.; Behers, L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci. Rep. 2018, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef]
- Liu, H.; Ma, C.; Chen, G.; White, J.C.; Wang, Z.; Xing, B.; Dhankher, O.P. Titanium dioxide nanoparticles alleviate tetracycline toxicity to Arabidopsis thaliana (L.). ACS Sustain. Chem. Eng. 2017, 5, 3204–3213. [Google Scholar] [CrossRef]
- Bakshi, M.; Linéa, C.; Bedolla, D.E.; Stein, R.J.; Kaegi, R.; Sarret, G.; Pradas del Real, A.E.; Castillo-Michel, H.; Abhilash, P.C.; Larue, C. Assessing the impacts of sewage sludge amendment containing nano-TiO2 on tomato plants: A life cycle study. J. Hazard. Mater. 2019, 369, 191–198. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bellani, L.; Tassi, E.L.; Bottega, S.; Di Gregorio, S.; Siracusa, G.; Sanità di Toppi, L.; Ruffini Castiglione, M. An integrated approach to highlight biological responses of Pisum sativum root to nano-TiO2 exposure in a biosolid-amended agricultural soil. Sci. Tot. Environ. 2019, 650, 2705–2716. [Google Scholar] [CrossRef]
- Khan, Z.; Shahwar, D.; Ansari, M.K.Y.; Chandel, R. Toxicity assessment of anatase (TiO2) nanoparticles: A pilot study on stressresponse alterations and DNA damage studies in Lens culinaris Medik. Heliyon 2019, 5, e02069. [Google Scholar] [CrossRef] [Green Version]
- Bellani, L.; Siracusa, G.; Giorgetti, L.; Di Gregorio, S.; Ruffini Castiglione, M.; Spanò, L.; Muccifora, S.; Bottega, S.; Pini, R.; Tassi, E.L. TiO2 nanoparticles in a biosolid-amended soil and their implication in soil nutrients, microorganisms and Pisum sativum nutrition. Ecotox. Environ. Safe. 2020, 190, 110095. [Google Scholar] [CrossRef]
- Silva, S.; Ribeiro, T.P.; Santos, C.; Pinto, D.; Silva, A.M.S. TiO2 nanoparticles induced sugar impairments and metabolic pathway shift towards amino acid metabolism in wheat. J. Hazard. Mater. 2020, 399, 122982. [Google Scholar] [CrossRef]
- Mattiello, A.; Marchiol, L. Application of Nanotechnology in Agriculture: Assessment of TiO2 Nanoparticle Effects on Barley. In Application of Titanium Dioxide; Janus, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Bellani, L.; Muccifora, S.; Bottega, S.; Spanò, C. Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant model system Vicia faba L. Environ. Exp. Bot. 2016, 130, 11–21. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, N.C.; Fleischmann, P.; Burbage, J.; Venkatachalam, P.; Sahi, S.V. Nanotitania Exposure Causes Alterations in Physiological, Nutritional and Stress Responses in Tomato (Solanum lycopersicum). Front. Plant Sci. 2017, 8, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.; Oliveira, H.; Silva, A.M.S.; Santos, C. The cytotoxic targets of anatase or rutile + anatase nanoparticles depend on the plant species. Biol. Plant. 2017, 61, 717–725. [Google Scholar] [CrossRef]
- Larue, C.; Baratange, C.; Vantelon, D.; Khodja, H.; Sublé, S.; Elger, A.; Carrière, M. Influence of soil type on TiO2 nanoparticle fate in an agro-ecosystem. Sci. Tot. Environ. 2018, 630, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassi, E.L.; Pini, R.; Gorini, F.; Valadao, I.; de Castro, J.A. Chemical and Physical Soil Properties Influencing TiO2 Nanoparticles Availability in Terrestrial Ecosystems. J. Environ. Res. Dev. 2012, 6, 1034–1038. [Google Scholar]
- Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of titanium dioxide nanoparticles with soil components and plants: Current knowledge and future research needs—A critical review. Environ. Sci. Nano 2018, 5, 257–278. [Google Scholar] [CrossRef]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef]
- He, X.; Sanders, S.; Aker, W.G.; Lin, Y.; Douglas, J.; Hwang, H. Assessing the effects of surface-bound humic acid on the phototoxicity of anatase and rutile TiO2 nanoparticles in vitro. J. Environ. Sci. 2016, 42, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Brevik, E.C.; Burgess, L.C. (Eds.) Soils and Human Health, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- European Commission. Summary Report of the Standing Committee on Plants, Animals, Food and Feed, Held in Brussels on 13 May 2019. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/reg-com_toxic_20190513_sum.pdf (accessed on 22 December 2020).
- Lv, J.; Zhang, S.; Luo, L.; Zhang, J.; Yang, K.; Christie, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68. [Google Scholar] [CrossRef]
- Castillo-Michel, H.; Larue, C.; Pradas del Real, A.E.; Cotte, M.; Sarret, G. Practical review on the use of synchrotron based micro- and nano- X-ray fluorescence mapping and X-ray absorption spectroscopy to investigate the interactions between plants and engineered nanomaterials. Plant Physiol. Biochem. 2017, 110, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Shan, X.; Wen, B.; Lin, J.; Owens, G. Stability of titania nanoparticles in soil suspensions and transport in saturated homogeneous soil columns. Environ. Pollut. 2009, 157, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, S.; Atashpaz, B.; Moghaddam, S.S.; Kalavrouziotis, I.K.; Damalas, C.A. Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 2019, 231, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cotte, M.; Pouyet, E.; Salome, M.; Rivard, C.; De Nolf, W.; Castillo-Michel, H.; Fabris, T.; Monico, L.; Janssens, K.; Wang, T.; et al. The ID21 X-ray and infrared microscopy beamline at the ESRF: Status and recent applications to artistic materials. J. Anal. At. Spectrom. 2017, 32, 477–493. [Google Scholar] [CrossRef]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, Č.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Toplak, M.; Birarda, G.; Read, S.; Sandt, C.; Rosendahl, S.M.; Vaccari, L.; Demšar, J.; Borondics, F. Infrared orange: Connecting hyperspectral data with machine learning. Synchrotron Radiat. News 2017, 30, 40–45. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Mathew, K.; Zheng, C.; Winston, D.; Chen, C.; Dozier, A.; Rehr, J.J.; Ong, S.P.; Persson, K.A. High-throughput computational X-ray absorption spectroscopy. Sci. Data 2018, 5, 180151. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Phoohinkong, W.; Boonyarattanakalin, K.; Mekprasart, W.; Pavasupree, S.; Pecharapa, W. Nonlocal XANES pre-edge feature of FeTiO3 ilmenite-type at Ti and Fe K-edge. Radiat. Phys. Chem. 2020, 174, 108919. [Google Scholar] [CrossRef]
- Farges, F.; Brown, G.E.; Rehr, J.J. Ti K-edge XANES studies of Ti coordination and disorder in oxide compounds: Comparison between theory and experiment. Phys. Rev. B 1997, 56, 1809–1819. [Google Scholar] [CrossRef]
- Carvajal, M.; Alcaaraz, C.F. Why Titanium Is a Beneficial Element for Plants. J. Plant. Nutr. 1998, 21, 655–664. [Google Scholar] [CrossRef]
- Ramírez, W.A.; Domene, X.; Ortiz, O.; Alcañiz, J.M. Toxic effects of digested, composted and thermally-dried sewage sludge on three plants. Bioresour. Technol. 2008, 99, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Veronesi, G.; Flank, A.; Surble, S.; Herlin-Boime, N.; Carrière, M. Comparative Uptake and Impact of TiO2 Nanoparticles in Wheat and Rapeseed. J. Toxicol. Environ. Health Part A 2012, 75, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Roppolo, D.; De Rybel, B.; Tendon, V.D.; Pfister, A.; Alassimone, J.; Vermeer, J.E.; Yamazaki, M.; Stierhof, Y.D.; Beeckman, T.; Geldner, N. A novel protein family mediates Casparian strip formation in the endodermis. Nature 2011, 473, 380–384. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Niltharach, A.; Kityakarn, S.; Worayingyong, S.; Thienprasert, J.T.; Klysubun, W.; Songsiriritthigul, P.; Limpijumnong, S. Structural characterizations of sol–gel synthesized TiO2 and Ce/TiO2 nanostructures. Phys. B Condens. Matter 2012, 407, 2915–2918. [Google Scholar] [CrossRef]
- Geochemical Atlas of Europe; Salminen, R. (Ed.). Available online: http://weppi.gtk.fi/publ/foregsatlas/ (accessed on 22 December 2020).
- Kiser, M.A.; Westerhoff, P.; Benn, T.; Wang, Y.; Pérez-Rivera, J.; Hristovski, K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Technol. 2009, 43, 6757–6763. [Google Scholar] [CrossRef]
- Philippe, A.; Campos, D.A.; Guigner, J.; Buchmann, C.; Diehl, D.; Schaumann, G.E. Characterization of the Natural Colloidal TiO2 Background in Soil. Separations 2018, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Pradas del Real, A.E.; Castillo-Michel, H.; Kaegi, R.; Larue, C.; De Nolf, V.; Reyes-Herrera, J.; Tucoulou, R.; Findling, N.; Salas-Colera, E.; Sarret, G. Searching for relevant criteria to distinguish natural vs. anthropogenic TiO2 nanoparticles in soils. Environ. Sci. Nano 2018, 5, 2853–2863. [Google Scholar] [CrossRef] [Green Version]
- Tong, T.; Hill, A.N.; Alsina, M.A.; Wu, J.; Shang, K.Y.; Kelly, J.J.; Gray, K.A.; Gaillard, J.F. Spectroscopic characterization of TiO2 polymorphs in wastewater treatment and sediment samples. Environ. Sci. Technol. Lett. 2014, 2, 12–18. [Google Scholar] [CrossRef]
- Lazareva, A.; Keller, A.A. Estimating Potential Life Cycle Releases of Engineered Nanomaterials from Wastewater Treatment Plants. ACS Sustain. Chem. Eng. 2014, 2, 1656–1665. [Google Scholar] [CrossRef]
- Registration-Dossier of the European Chemicals Agency (ECHA). Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/15560/5/1# (accessed on 7 January 2021).
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron Verification of TiO2 Accumulation in Cucumber Fruit: A Possible Pathway of TiO2 Nanoparticle Transfer from Soil into the Food Chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef]
- Larue, C.; Castillo-Michel, C.H.; Stein, R.J.; Fayard, B.; Pouyet, E.; Villanova, J.; Magnin, V.; Pradas del Real, A.E.; Trcera, N.; Legros, S.; et al. Innovative combination of spectroscopic techniques to reveal nanoparticle fate in a crop plant. Spectrochim. Acta Part B 2016, 119, 17–24. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Munir, M.A.M.; Cheema, A.I.; Hussain, I.; Rinklebe, J. Biochar-mediated transformation of titanium dioxide nanoparticles concerning TiO2NPs-biochar interactions, plant traits and tissue accumulation to cell translocation. Environ. Pollut. 2021, 270, 116077. [Google Scholar] [CrossRef] [PubMed]
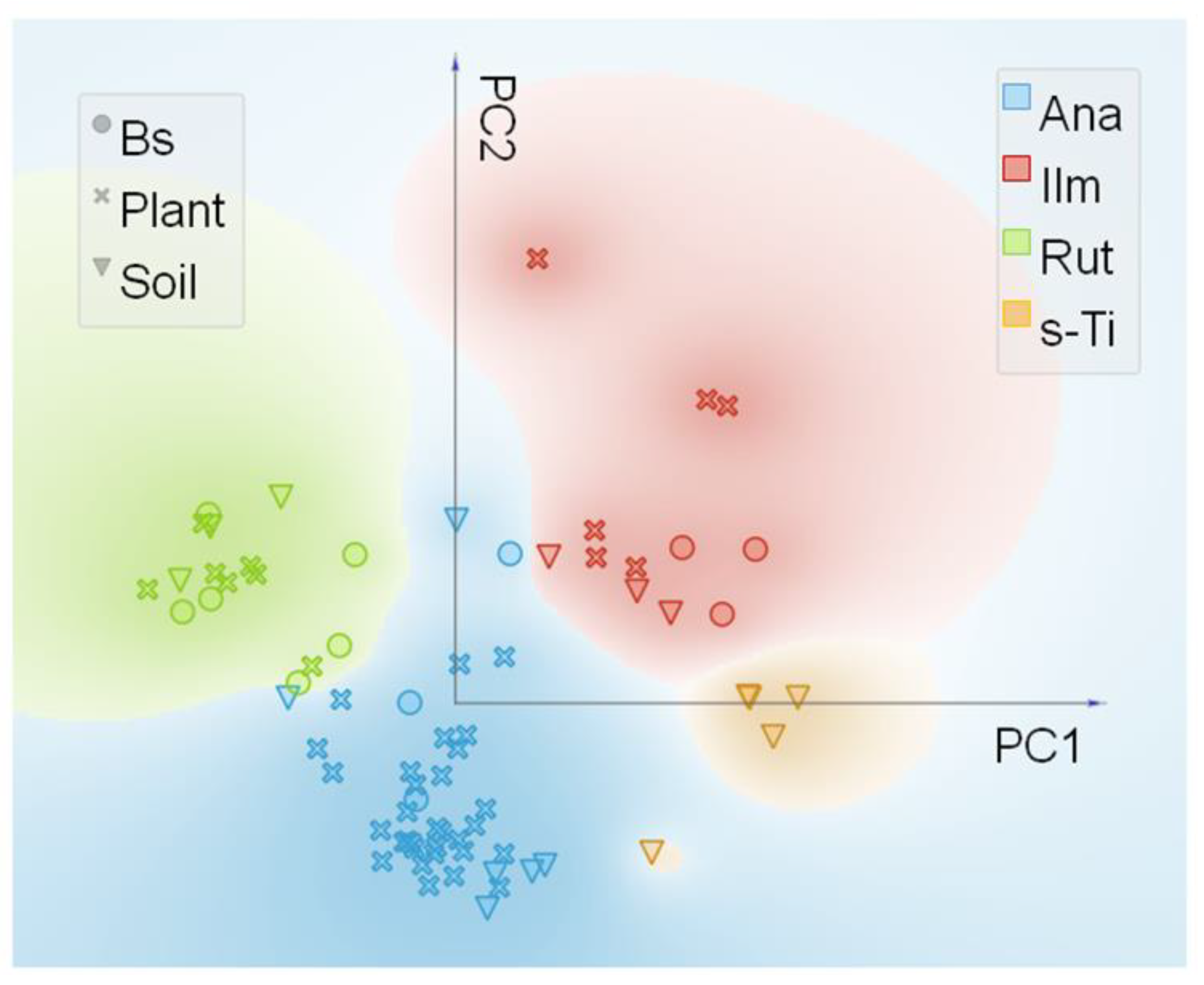

| % Frequency of Ti Species Identified | |||||
|---|---|---|---|---|---|
| Treatments | Ilm-Group | Rut-Group | Ana-Group | s-Ti | Total n. Spectra |
| SOLIDS | |||||
| soil | 17.6 | 17.6 | 35.3 | 29.4 | 17 |
| Bs | 25.0 | 50.0 | 25.0 | 0.0 | 12 |
| ROOTS | |||||
| C | 16.6 | 0.0 | 83.3 | 0.0 | 6 |
| Ana800 | 22.7 | 13.6 | 63.6 | 0.0 | 22 |
| Rut800 | 0.0 | 57.1 | 42.8 | 0.0 | 7 |
| Mix800 | 0.0 | 0.0 | 100 | 0.0 | 4 |
| SMP800 | 0.0 | 0.0 | 100 | 0.0 | 5 |
| Soil Treatments | Ti Roots (mg kg−1) | TF (Ti shoot/Ti root) × 10−3 |
|---|---|---|
| C | 420 ± 60.5 a | 13 ± 2 a |
| Ana800 | 591 ± 9.66 bc | 47 ± 8 d |
| Rut800 | 655 ± 44.5 c | 54 ± 9 d |
| Mix800 | 580 ± 70.8 bc | 38 ± 6 c |
| SMP800 | 657 ± 92.0 c | 24 ± 3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muccifora, S.; Castillo-Michel, H.; Barbieri, F.; Bellani, L.; Ruffini Castiglione, M.; Spanò, C.; Pradas del Real, A.E.; Giorgetti, L.; Tassi, E.L. Synchrotron Radiation Spectroscopy and Transmission Electron Microscopy Techniques to Evaluate TiO2 NPs Incorporation, Speciation, and Impact on Root Cells Ultrastructure of Pisum sativum L. Plants. Nanomaterials 2021, 11, 921. https://doi.org/10.3390/nano11040921
Muccifora S, Castillo-Michel H, Barbieri F, Bellani L, Ruffini Castiglione M, Spanò C, Pradas del Real AE, Giorgetti L, Tassi EL. Synchrotron Radiation Spectroscopy and Transmission Electron Microscopy Techniques to Evaluate TiO2 NPs Incorporation, Speciation, and Impact on Root Cells Ultrastructure of Pisum sativum L. Plants. Nanomaterials. 2021; 11(4):921. https://doi.org/10.3390/nano11040921
Chicago/Turabian StyleMuccifora, Simonetta, Hiram Castillo-Michel, Francesco Barbieri, Lorenza Bellani, Monica Ruffini Castiglione, Carmelina Spanò, Ana E. Pradas del Real, Lucia Giorgetti, and Eliana L. Tassi. 2021. "Synchrotron Radiation Spectroscopy and Transmission Electron Microscopy Techniques to Evaluate TiO2 NPs Incorporation, Speciation, and Impact on Root Cells Ultrastructure of Pisum sativum L. Plants" Nanomaterials 11, no. 4: 921. https://doi.org/10.3390/nano11040921
APA StyleMuccifora, S., Castillo-Michel, H., Barbieri, F., Bellani, L., Ruffini Castiglione, M., Spanò, C., Pradas del Real, A. E., Giorgetti, L., & Tassi, E. L. (2021). Synchrotron Radiation Spectroscopy and Transmission Electron Microscopy Techniques to Evaluate TiO2 NPs Incorporation, Speciation, and Impact on Root Cells Ultrastructure of Pisum sativum L. Plants. Nanomaterials, 11(4), 921. https://doi.org/10.3390/nano11040921