Co-Doped NdFeO3 Nanoparticles: Synthesis, Optical, and Magnetic Properties Study
Abstract
1. Introduction
2. Materials and Methods
3. Result and Discussion
3.1. Structures and Morphologies of Nanostructured NdFe1−xCoxO3
3.2. Elemental Composition of NdFe1−xCoxO3 Samples
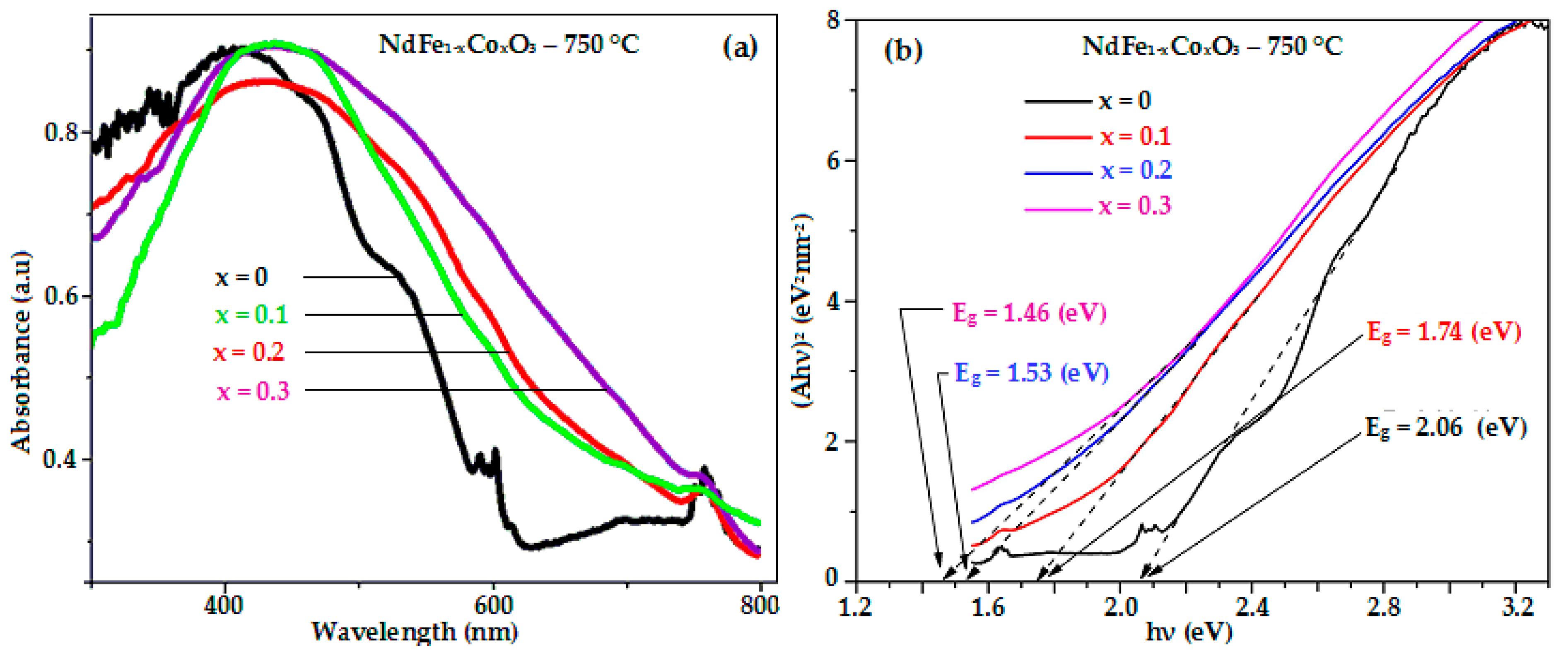
3.3. Optical and Magnetic Properties of Nano-Structured NdFe1−xCoxO3 (x = 0, 0.1, 0.2, and 0.3) Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, I.; Akhtar, M.J.; Siddique, M.; Iqbal, M.; Hasan, M.M. Origin of anomalous octahedral distortions and collapse of magnetic ordering in Nd1−xSrxFeO3 (0 ≤ x ≤ 0.5). Ceram. Int. 2013, 39, 8901–8909. [Google Scholar] [CrossRef]
- Sasikala, C.; Durairaj, N.; Baskaran, I.; Sathyaseelan, B.; Henini, M. Transition metal titanium (Ti) doped LaFeO3 nanoparticles for enhanced optical structure and magnetic properties. J. Alloys Compd. 2017, 712, 870–877. [Google Scholar] [CrossRef]
- Haron, W.; Thaweechai, T.; Wattanathana, W.; Laobuthee, A.; Manaspiya, H.; Veranitisagul, C.; Koonsaeng, N. Structure characteristics and dielectric properties of La1−xCoxFeO3 and LaFe1−xCoxO3 synthesized via metal organic complexes. Energy Procedia 2013, 34, 791–800. [Google Scholar] [CrossRef]
- Xu, J.J.; Xu, D.; Wang, Z.L.; Wang, H.G.; Zhang, L.L.; Zhang, X.B. Synthesis of perovskite-based porous La0.75Sr0.25MnO3 nanotubes as a highly efficient electrocatalyst for rechargeable lithium-oxygen batteries. Angew. Chem. Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef]
- Tijare, S.N.; Bakardjieva, S.; Subrt, J.; Joshi, M.V.; Rayalu, S.S.; Hishita, S.; Labhsetwar, N. Synthesis and visible light photocatalytic activity of nanocrystalline PrFeO3 perovskite for hydrogen generation in ethanol-water system. J. Chem. Sci. 2014, 125, 517–525. [Google Scholar] [CrossRef]
- Megarajan, S.K.; Rayalu, S.; Nishibori, M.; Labhsetwar, N. Improved catalytic activity of PrFeO3 (M = Co and Fe) perovskites: Synthesis of thermally stable nanoparticles by a novel hydrothermal method. N. J. Chem. 2015, 39, 2345–2348. [Google Scholar] [CrossRef]
- Shin, N.; Isao, A.; Yasuhiko, I.; Takeo, H. Preparation of Y(Mn1−xFex)O3 and electrical properties of the sintered bodies. Open J. Inorg. Chem. 2015, 5, 54514589. [Google Scholar]
- Fang, F.; Zhao, P.; Feng, N.; Wan, H.; Guan, G. Surface engineering on popous perovskite-type La0.6Sr0.4CoO3−δ nanotubes for an enhanced performance in diesel soot elimination. J. Hazadous Mater. 2020, 399, 123014. [Google Scholar] [CrossRef]
- Mir, F.A.; Sharma, S.K.; Kumar, R. Magnetizations and magneto-transport properties of Ni-doped PrFeO3 thin films. Chin. Phys. B 2014, 23, 048101. [Google Scholar] [CrossRef]
- Yuan, S.J.; Ren, W.; Hong, F.; Wang, Y.B.; Zhang, J.C.; Bellaiche, L.; Cao, S.X.; Cao, G. Spin switching and magnetization reversal in single-crystal NdFeO3. Phys. Rev. B 2013, 87, 184405. [Google Scholar] [CrossRef]
- Pekinchak, O.; Vasylechko, L.; Lutsyuk, I.; Vakhula, Y.; Prots, Y.; Cabrera, W.C. Sol-gel prepared nanoparticles of mixed praseodymium cobaltites ferrites. Nanoscale Res. Lett. 2016, 11, 75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, R.; Hu, J.; Han, Z.; Zhao, M.; Wu, Z.; Zhang, Y.; Qin, H. Electrical and CO-sensing properties of NdFe1−xCoxO3 perovskite system. J. Rare Earths 2010, 28, 591–595. [Google Scholar] [CrossRef]
- Feng, C.; Ruan, S.; Li, J.; Zou, B.; Luo, J.; Chen, W.; Dong, W.; Wu, F. Ethanol sensing properties of LaCoxFe1−xO3 nanoparticles: Effects of calcination temperature, Co-doping, and carbon nanotube-treatment. Sens. Actuators B Chem. 2011, 155, 232–238. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, L.; Yang, H.; Liu, Q.; Ye, F. Hydrothermal synthesis and magnetic properties of multiferroic rare-earth orthoferrites. J. Alloys Compd. 2014, 583, 21–31. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Mittova, I.Y.; Almjasheva, O.V.; Kirillova, S.A.; Gusarov, V.V. Influence of the preparation conditions on the size and morphology of nanocrystalline lanthanum orthoferrite. Glass Phys. Chem. 2008, 34, 756–761. [Google Scholar]
- Nguyen, A.T.; Almjasheva, O.V.; Mittova, I.Y.; Stognei, O.V.; Soldatenko, S.A. Synthesis and magnetic properties of YFeO3 nanocrystals. Inorg. Mater. 2009, 45, 1304–1308. [Google Scholar]
- Nguyen, A.T.; Pham, V.N.; Nguyen, T.T.L.; Mittova, V.O.; Vo, Q.M.; Berezhnaya, M.V.; Mittova, I.Y.; Do, T.H.; Chau, H.D. Crystal structure and magnetic properties of perovskite YFe1−xMnxO3 nanopowders synthesized by co-precipitation method. Solid State Sci. 2019, 96, 105922. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Chau, D.H.; Nguyen, L.T.T.; Mittova, V.O.; Do, H.T.; Mittova, I.Y. Structural and magnetic properties of YFe1−xCoxO3 (0.1 ≤ x ≤ 0.5) perovskite nanomaterials synthesized by co-precipitation method. Nanosyst. Phys. Chem. Math. 2018, 9, 424–429. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, V.N.T.; Le, H.T.; Chau, D.H.; Mittova, V.O.; Nguyen, L.T.T.; Dinh, D.A.; Hao, T.V.N.; Mittova, I.Y. Crystal structure and magnetic properties of LaFe1−xNixO3 nanomaterials prepared via a simple co-precipitation method. Ceram. Int. 2019, 45, 21768–21772. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, V.; Chau, D.H.; Mittova, V.O.; Mittova, I.Y.; Kopeychenko, E.L.; Nguyen, L.T.T.; Bui, V.X.; Nguyen, A.T.P. Effect of Ni substitution on phase transition, crystal structure and magnetic properties of nanostructured YFeO3 perovskite. J. Mol. Struct. 2020, 1215, 128293. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, V.; Pham, T.L.; Nguyen, L.T.T.; Mittova, I.Y.; Mittova, V.O.; Vo, L.N.; Nguyen, B.T.T.; Bui, V.X.; Viryutina, E.L. Simple synthesis of NdFeO3 by the so-precipitation method based on a study of thermal behaviors of Fe (III) and Nd (III) hydroxides. Crystals 2020, 10, 219. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Pearson, Prentice Hall: Upper Saddle River, NJ, USA, 2005; 950p. [Google Scholar]
- Yousefi, M.; Zeid, S.S.; Motlagh, M.K. Synthesis and characterization of nano-structured perovskite type neodymium orthoferrite NdFeO3. Curr. Chem. Lett. 2017, 6, 23–30. [Google Scholar] [CrossRef]
- Ghobadi, N. Band gap determination using absorption spectrum fitting procedure. Int. Nano Lett. 2013, 3, 2. [Google Scholar] [CrossRef]
- Knurova, M.V.; Mittova, I.Y.; Perov, N.S.; Al’myasheva, O.V.; Nguyen, T.A.; Mittova, V.O.; Bessalova, V.V.; Viryutina, E.L. Effect of the degree of doping on the size and magnetic properties of nanocrystals La1−xZnxFeO3 synthesized by the sol-gel method. Russ. J. Inorg. Chem. 2017, 62, 281–287. [Google Scholar] [CrossRef]
- Habib, Z.; Majid, K.; Ikram, M.; Sultan, K. Influence of Ni substitution at B-site for Fe3+ ions on morphological, optical, and magnetic properties of HoFeO3 ceramics. Appl. Phys. A Mater. Sci. Process. 2016, 12, 550. [Google Scholar] [CrossRef]
- Mir, S.A.; Ikram, M.; Asokan, K. Effect of Ni doping on optical, electrical and magnetic properties of Nd orthoferrite. J. Phys. Conf. Ser. 2014, 534, 012017. [Google Scholar] [CrossRef]
- Bashir, A.; Ikram, M.; Kumar, R.; Lisboa-Filho, P.N. Structural, electronic structure and magnetic studies of GdFe1−xNixO3 (x ≤ 0.5). J. Alloys Compd. 2012, 521, 183–188. [Google Scholar] [CrossRef]
- Somvanshi, A.; Husain, S.; Khan, W. Investigation of structure and physical properties of cobalt doped nano-crystalline neodymium orthoferrite. J. Alloys Compd. 2019, 778, 439. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, L.T.T.; Bui, V.X.; Nguyen, D.H.; Lieu, H.D.; Le, L.M.; Pham, V. Optical and magnetic properties of HoFeO3 nanocrystals prepared by a simple co-precipitation method using ethanol. J. Alloys Compd. 2020, 834, 155098. [Google Scholar] [CrossRef]
- Phokha, S.; Pinitsoontorn, S.; Maensiri, S.; Rujirawat, S. Structure, optical and magnetic properties of LaFeO3 nanoparticles prepared by polymerized complex method. J. Sol Gel Sci. Technol. 2014, 71, 333–341. [Google Scholar] [CrossRef]

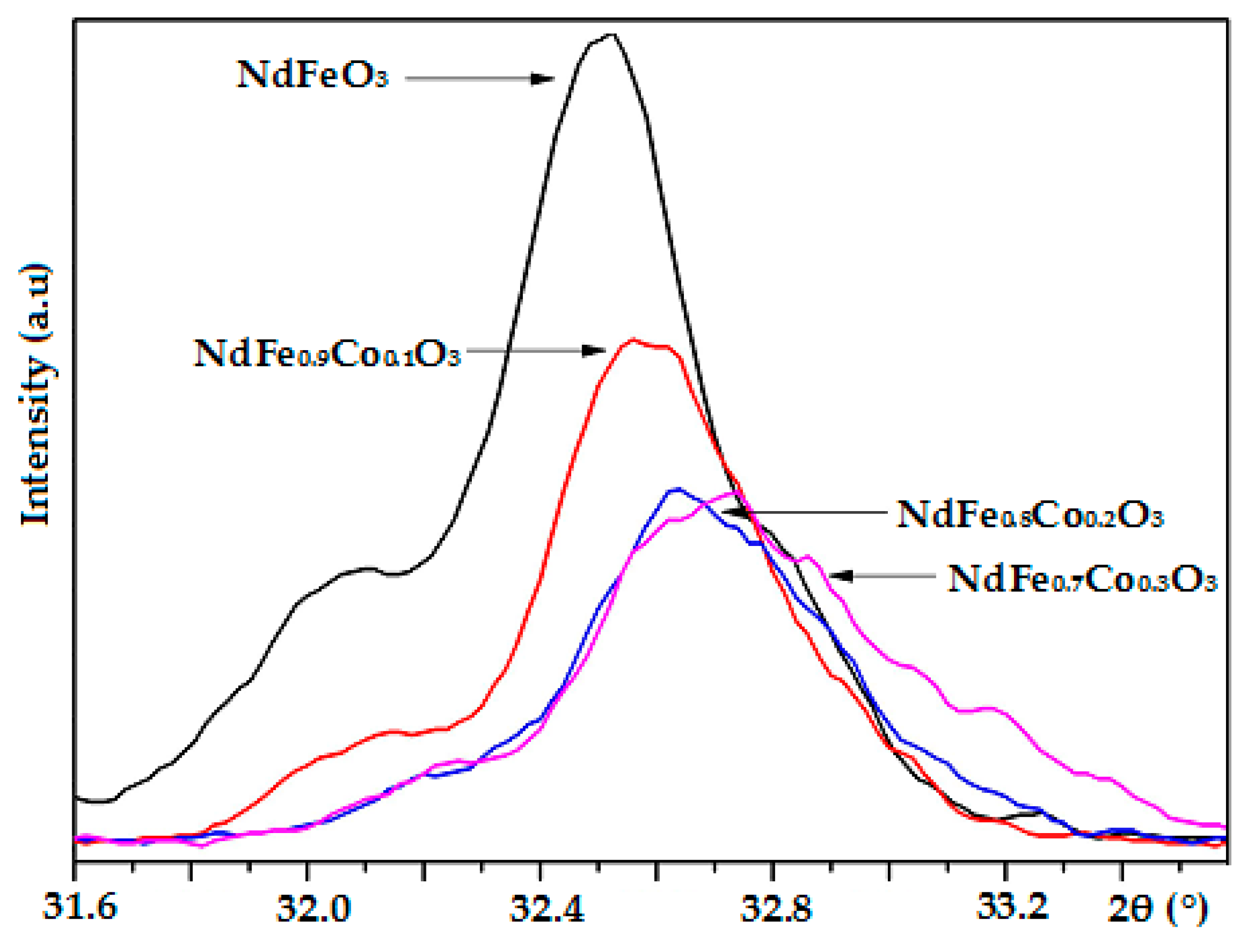


| NdFe1−xCoxO3 | 2θo (112) | Davg, nm | Lattice Constants, Å | V, Å3 | |||
|---|---|---|---|---|---|---|---|
| XRD | TEM | a | b | c | |||
| NdFeO3 [21] | 32.49 | 28 ± 5 | - | 5.4990 | 5.5910 | 7.7592 | 238.56 |
| NdFe0.9Co0.1O3 | 32.54 | 25 ± 3 | 47 ± 5 | 5.4257 | 5.5919 | 7.7638 | 235.55 |
| NdFe0.8Co0.2O3 | 32.57 | 22 ± 2 | 45 ± 6 | 5.4425 | 5.5292 | 7.7616 | 233.57 |
| NdFe0.7Co0.3O3 | 32.61 | 19 ± 3 | 42 ± 3 | 5.4113 | 5.5504 | 7.7673 | 233.29 |
| Nominal Composition of Samples | Elemental Composition (at. %) | Real Composition of Samples | |||
|---|---|---|---|---|---|
| Nd | Fe | Co | O | ||
| NdFeO3 | 18.25 ± 1.57 | 20.45 ± 1.11 | 0.00 | 61.30 ± 2.17 | NdFe1.120O3.359 |
| NdFe0.9Co0.1O3 | 18.95 ± 1.03 | 18.45 ± 1.07 | 1.43 ± 0.17 | 61.17 ± 2.36 | NdFe0.973Co0.075O3.228 |
| NdFe0.8Co0.2O3 | 19.01 ± 1.42 | 16.03 ± 0.89 | 2.13 ± 0.35 | 62.83 ± 3.21 | NdFe0.843Co0.112O3.305 |
| NdFe0.7Co0.3O3 | 19.27 ± 1.35 | 14.31 ± 0.73 | 5.41 ± 0.42 | 61.01 ± 3.08 | NdFe0.743Co0.281O3.166 |
| NdFe1−xCoxO3 | Hc, Oe | Mr, emu/g | Ms, emu/g | Eg, eV |
|---|---|---|---|---|
| NdFeO3 [21] | 136.76 | 0.68 | 0.80 | 2.06 |
| NdFe0.9Co0.1O3 | 258.22 | 0.13 | 0.93 | 1.74 |
| NdFe0.8Co0.2O3 | 395.79 | 0.15 | 0.97 | 1.53 |
| NdFe0.7Co0.3O3 | 416.06 | 0.18 | 0.98 | 1.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.A.; Pham, T.L.; Mittova, I.Y.; Mittova, V.O.; Nguyen, T.L.T.; Nguyen, H.V.; Bui, V.X. Co-Doped NdFeO3 Nanoparticles: Synthesis, Optical, and Magnetic Properties Study. Nanomaterials 2021, 11, 937. https://doi.org/10.3390/nano11040937
Nguyen TA, Pham TL, Mittova IY, Mittova VO, Nguyen TLT, Nguyen HV, Bui VX. Co-Doped NdFeO3 Nanoparticles: Synthesis, Optical, and Magnetic Properties Study. Nanomaterials. 2021; 11(4):937. https://doi.org/10.3390/nano11040937
Chicago/Turabian StyleNguyen, Tien Anh, Thanh Le Pham, Irina Yakovlevna Mittova, Valentina Olegovna Mittova, Truc Linh Thi Nguyen, Hung Van Nguyen, and Vuong Xuan Bui. 2021. "Co-Doped NdFeO3 Nanoparticles: Synthesis, Optical, and Magnetic Properties Study" Nanomaterials 11, no. 4: 937. https://doi.org/10.3390/nano11040937
APA StyleNguyen, T. A., Pham, T. L., Mittova, I. Y., Mittova, V. O., Nguyen, T. L. T., Nguyen, H. V., & Bui, V. X. (2021). Co-Doped NdFeO3 Nanoparticles: Synthesis, Optical, and Magnetic Properties Study. Nanomaterials, 11(4), 937. https://doi.org/10.3390/nano11040937







