Integration of an Aerosol-Assisted Deposition Technique for the Deposition of Functional Biomaterials Applied to the Fabrication of Miniaturised Ion Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
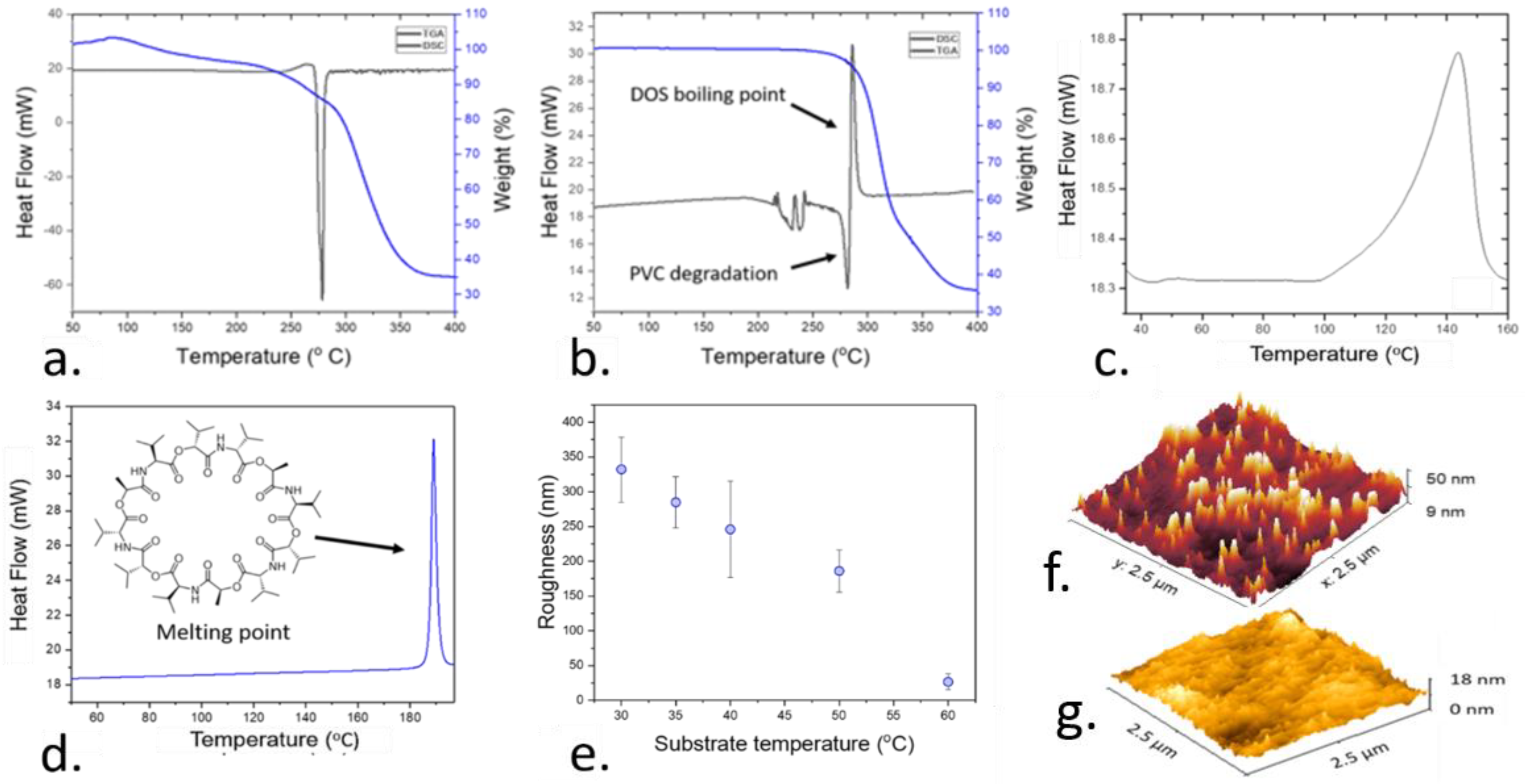
2.3. Thermal Characterization of the Plasticized Polymer Films
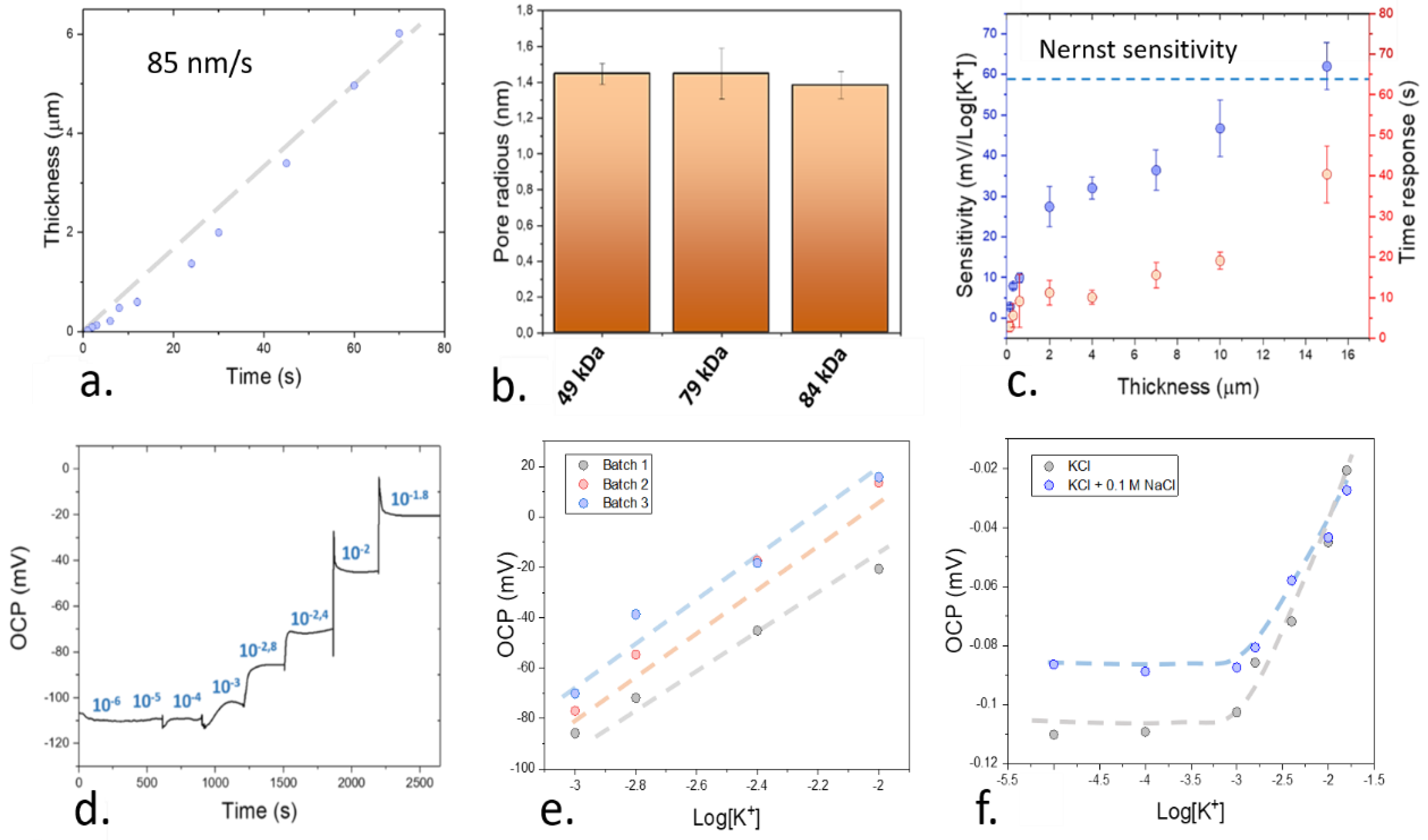
2.4. Study of the Sensitivity of the Sensing Films
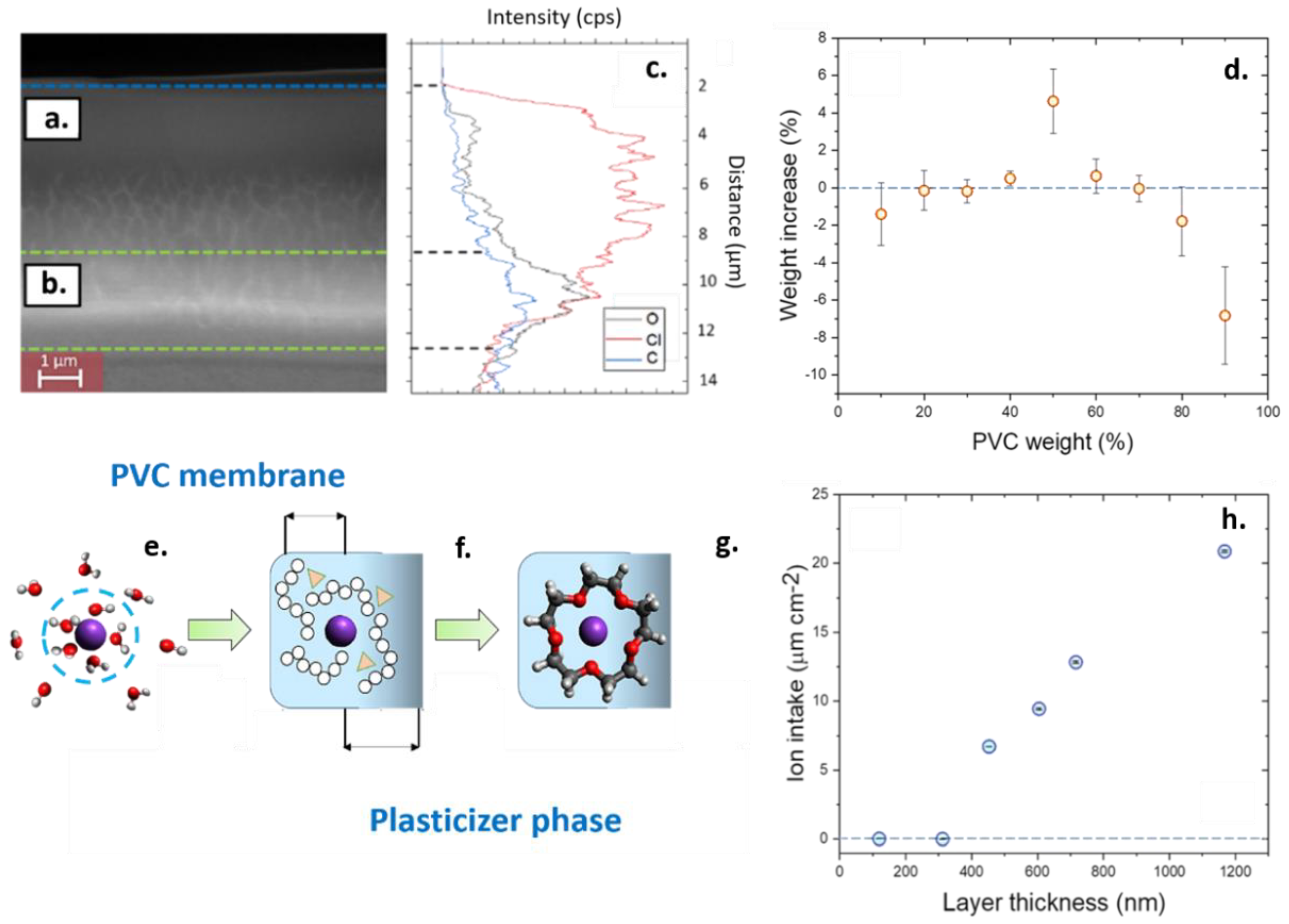
2.5. Determination of the Ion Intake by Quartz Microbalance and Scanning Electron Microscopy (SEM) Morphology
2.6. Deposition of the Upper Protective Films
2.7. Characterization of the Protective Films
3. Results and Discussion
3.1. Optimization of the Deposition Temperature and Roughness of the Sensitive Films
3.2. Comparison of the Aerosol-Assisted Chemical Deposition (AACD) and Drop Casting Results for the Development of Homogeneous Sensing Films
3.3. Systematic Study of the Effects of Thickness on the Performance of the Plasticized Sensing Films
3.4. Systematic Study of the Effects of Thickness on the Performance of the Plasticized Sensing Films
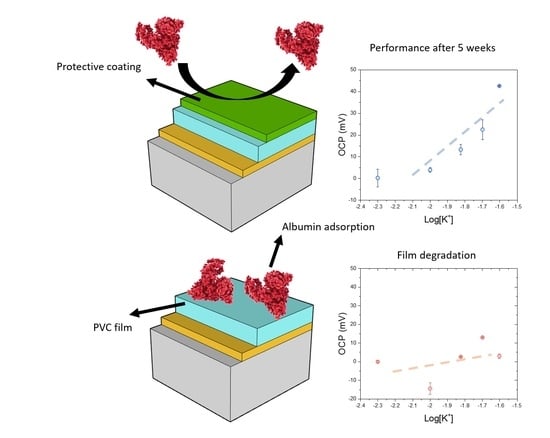
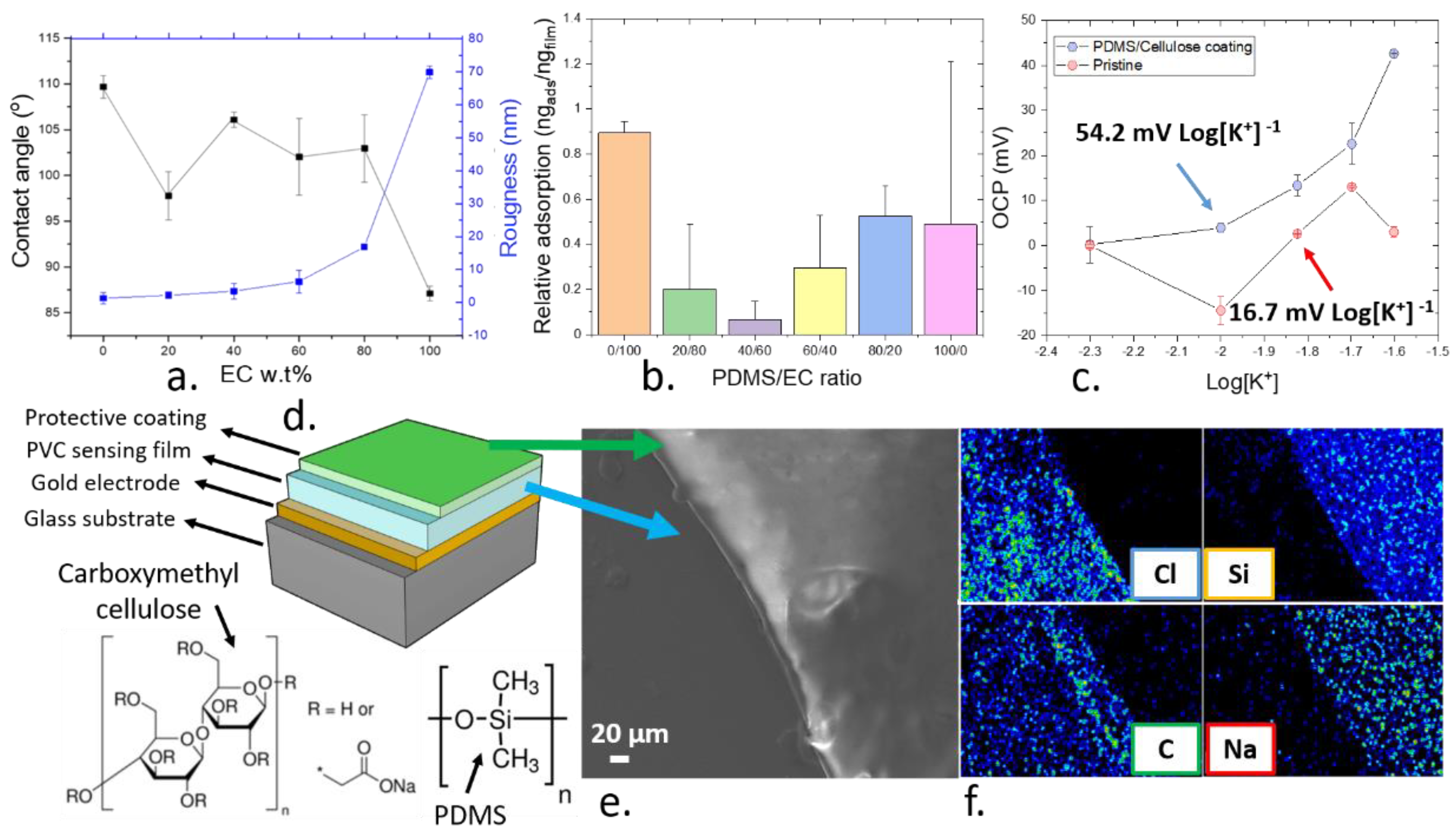
3.5. Development of a Biocompatible Protective Coating for the Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siswoyo, S.; Zulfikar, Z.; Asnawati, A. Ion Selective Electrodes as Analytical Tools for Rapid Analysis of Soil Nutrients. In Proceedings of the International Seminar on New Paradigm and Innovation on Natural Sciences and Its Application, Central Java, Indonesia, 22–22 October 2012. [Google Scholar]
- Dhatt, G.; Talor, Z.; Kazory, A. Direct ion-selective electrode method is useful in diagnosis of pseudohyponatremia. J. Emerg. Med. 2012, 43, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Neri, G. Miniaturized Bio-and Chemical-Sensors for Point-of-Care Monitoring of Chronic Kidney Diseases. Sensors 2018, 18, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liamis, G.; Liberopoulos, E.; Barkas, F.; Elisaf, M. Diabetes mellitus and electrolyte disorders. World J. Clin. Cases 2014, 2, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Alwazeer, D.; Cachon, R. Ion-selective electrode integrated in small-scale bioreactor for continuous intracellular pH determination in Lactobacillus plantarum. Folia Microbiol. 2020, 65, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Ogawara, S.; Carey, J.L., III; Zou, X.U.; Bühlmann, P. Donnan Failure of Ion-Selective Electrodes with Hydrophilic High-Capacity Ion-Exchanger Membranes. ACS Sens. 2016, 1, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Morf, W.E.; Pretsch, E.; De Rooij, N.F. Computer Simulation of Ion-Selective Membrane Electrodes and Related Systems by Finite-Element Procedures. J. Electroanal. Chem. 2007, 602, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Heng, L.Y.; Toth, K.; Hall, E.A. Ion-transport and diffusion coefficients of non-plasticised methacrylic–acrylic ion-selective membranes. Talanta 2004, 63, 73–87. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]
- Blass, C.R. PVC as a biomedical polymer--plasticizer and stabilizer toxicity. Med. Device Technol. 1992, 3, 32–40. [Google Scholar] [PubMed]
- Yin, J.; Luan, S. Opportunities and challenges for the development of polymer-based biomaterials and medical devices. Regen. Biomater. 2016, 3, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R.D.; Horvai, G. Properties of PVC based membranes used in ion-selective electrodes. Electrochim. Acta 1990, 35, 1–7. [Google Scholar] [CrossRef]
- Zahran, E.M.; New, A.; Gavalas, V.; Bachas, L.G. Polymeric plasticizer extends the lifetime of PVC-membrane ion-selective electrodes. Analyst 2014, 139, 757–763. [Google Scholar] [CrossRef]
- Platzer, N. The Technology of Plasticizers; Sears, J.K., Darby, J.R., Eds.; SPE Monograph Series; Wiley: New York, NY, USA, 1982; p. 459. [Google Scholar]
- Attiyat, A.S.; Christian, G.D.; Hallman, J.L.; Bartsch, R.A. A comparative study of the effect of o-nitrophenyl octyl ether and o-nitrophenyl pentyl ether as plasticizers on the response and selectivity of carrier-based liquid membrane ion-selective electrodes. Talanta 1988, 35, 789–794. [Google Scholar] [CrossRef]
- Hassan, S.K.A.G.; Moody, G.J.; Thomas, J.D.R. Divalent (water hardness) ion-selective electrodes based on poly(vinyl chloride) and poly(methyl acrylate) matrix membranes. Analyst 1980, 105, 147–153. [Google Scholar] [CrossRef]
- Craggs, A.; Moody, G.J.; Thomas, J.D.R. Thomas, Evaluation of calcium ion-selective electrodes based on di(n-alkylphenyl)-phosphate sensors and their calibration with ion buffers. Analyst 1979, 104, 412–418. [Google Scholar] [CrossRef]
- Ishimatsu, R.; Izadyar, A.; Kabagambe, B.; Kim, Y.; Kim, J.; Amemiya, S. Electrochemical Mechanism of Ion–Ionophore Recognition at Plasticized Polymer Membrane/Water Interfaces. J. Am. Chem. Soc. 2011, 133, 16300–16308. [Google Scholar] [CrossRef]
- Josowicz, M.; Janata, J. Organic polymer films for solid-state sensor applications. Solid State Ion. 1988, 28, 1625–1630. [Google Scholar] [CrossRef]
- Ufer, S.; Mundt, C.; Ash, B.; Buck, R.P. Stencil printing of ion-selective membranes on flexible planar sensor arrays for cardiovascular applications. In Proceedings of the 16th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Baltimore, MA, USA, 3–6 November 1994. [Google Scholar]
- Ali, T.A.; Mohamed, G.G.; Al-Sabagh, A.M.; Migahed, M.A. A New Screen-printed Ion Selective Electrode for Determination of Citalopram Hydrobromide in Pharmaceutical Formulation. Chin. J. Anal. Chem. 2014, 42, 565–572. [Google Scholar] [CrossRef]
- Frag, E.Y.; Mohamed, G.G.; El-Dien, F.N.; Mohamed, M.E. Construction and performance characterization of screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in pharmaceutical preparations. Analyst 2011, 136, 332–339. [Google Scholar] [CrossRef]
- Koncki, R.; Tymecki, Ł.; Zwierkowska, E.; Głąb, S. Screen-printed copper ion-selective electrodes. Fresenius J. Anal. Chem. 2000, 367, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Kabagambe, B.; Garada, M.B.; Ishimatsu, R.; Amemiya, S. Sub-Nanomolar Detection Limit of Stripping Voltammetric Ca2+-Selective Electrode: Effects of Analyte Charge and Sample Contamination. Anal. Chem. 2014, 86, 7939–7946. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Cao, J.; Ding, H.; Zhao, X.; Li, Z. Electronic devices based on solution-processed two-dimensional materials. In Synthesis, Modeling, and Characterization of 2D Materials, and Their Heterostructures; He, P., Cao, J., Ding, H., Zhao, X., Li, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–384. [Google Scholar]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H. Chapter 3-Surfaces for Self-Cleaning. In Self-Cleaning of Surfaces and Water Droplet Mobility; Yilbas, B.S., Al-Sharafi, A., Ali, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–98. [Google Scholar]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Ali, M. CHAPTER 9-Pulmonary Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 209–246. [Google Scholar]
- Jarolímová, Z.; Han, T.; Mattinen, U.; Bobacka, J.; Bakker, E. Capacitive Model for Coulometric Readout of Ion-Selective Electrodes. Anal. Chem. 2018, 90, 8700–8707. [Google Scholar] [CrossRef] [PubMed]
- Morf, W.E.; Pretsch, E.; De Rooij, N.F. Memory Effects of Ion-Selective Electrodes: Theory and Computer Simulation of the Time-Dependent Potential Response to Multiple Sample Changes. J. Electroanal. Chem. 2009, 633, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Hulanicki, A.; Lewenstam, A. Interpretation of selectivity coefficients of solid-state ion-selective electrodes by means of the diffusion-layer model. Talanta 1977, 24, 171–175. [Google Scholar] [CrossRef]
- Veder, J.P.; De Marco, R.; Patel, K.; Si, P.; Grygolowicz-Pawlak, E.; James, M.; Alam, M.T.; Sohail, M.; Lee, J.; Pretsch, E.; et al. Evidence for a Surface Confined Ion-to-Electron Transduction Reaction in Solid-Contact Ion-Selective Electrodes Based on Poly(3-octylthiophene). Anal. Chem. 2013, 85, 10495–10502. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Enhancing ion-selective polymeric membrane electrodes by instrumental control. TrAC Trends Anal. Chem. 2014, 53, 98–105. [Google Scholar] [CrossRef]
- Cassidy, J.; Mullen, D.; Casey, K.; Cullen, P. Rotating disk ion selective electrodes. Electroanalysis 1996, 8, 918–921. [Google Scholar] [CrossRef] [Green Version]
- Buck, R.P. Electrochemistry of Ion-Selective Electrodes. In Comprehensive Treatise of Electrochemistry: Volume 8 Experimental Methods in Electrochemistry; Horsman, P., Conway, B.E., Yeager, E., Eds.; Springer: Boston, MA, USA, 1984; pp. 137–248. [Google Scholar]
- Piao, M.H.; Yoon, J.H.; Jeon, G.; Shim, Y.B. Characterization of All Solid State Hydrogen Ion Selective Electrode Based on PVC-SR Hybrid Membranes. Sensors 2003, 3, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Tran TN, T.; Qiu, S.; Chung, H.J. Potassium Ion Selective Electrode Using Polyaniline and Matrix-Supported Ion-Selective PVC Membrane. IEEE Sens. J. 2018, 18, 9081–9087. [Google Scholar] [CrossRef]
- Dan-Asabe, B. Thermo-mechanical characterization of banana particulate reinforced PVC composite as piping material. J. King Saud Univ. Eng. Sci. 2018, 30, 296–304. [Google Scholar] [CrossRef]
- Sankaram, M.B.; Easwaran, K.R.K. Location of valinomycin in lipid vesicles. J. Biosci. 1984, 6, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256. [Google Scholar] [CrossRef] [Green Version]
- Senichev, V.Y.; Tereshatov, V.V. 6–Theories of compatibility. In Handbook of Plasticizers, 3rd ed.; Wypych, G., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017; pp. 135–164. [Google Scholar]
- De Los Angeles Arada Pérez, M.; Zobeiri-Pedram, M. Potentiometric response of polymeric liquid membranes based on quaternary ammonium salts for nitrate ion determination. J. Chil. Chem. Soc. 2004, 49, 227–230. [Google Scholar]
- González-Bellavista, A.; Macanás, J.; Muñoz, M.; Fàbregas, E. Polysulfone as an alternative material to PVC in the design of ion-selective electrodes. Sens. Actuators B Chem. 2006, 115, 691–696. [Google Scholar] [CrossRef]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Buss, F.; Göcke, J.; Scharfer, P.; Schabel, W. From Micro to Nano Thin Polymer Layers: Thickness and Concentration Dependence of Sorption and the Solvent Diffusion Coefficient. Macromolecules 2015, 48, 8285–8293. [Google Scholar] [CrossRef]
- Greer, A.L. Diffusion and reactions in thin films. Appl. Surf. Sci. 1995, 86, 329–337. [Google Scholar] [CrossRef]
- Thomas, J.D.R. Aspects of the optimization of poly(vinyl chloride) matrix membrane ion-selective electrodes. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 1135–1143. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Petrović, S.; Harrison, D.J. Dual-Sorption Model of Water Uptake in Poly(vinyl chloride)-Based Ion-Selective Membranes: Experimental Water Concentration and Transport Parameters. Anal. Chem. 1996, 68, 1717–1725. [Google Scholar] [CrossRef]
- Lewenstam, A.; Hulanicki, A.; Sokalski, T. Response mechanism of solid-state ion-selective electrodes in the presence of interfering ions. Anal. Chem. 1987, 59, 1539–1544. [Google Scholar] [CrossRef]
- Gemene, K.L.; Meyerhoff, M.E. Selectivity Enhancement for Chloride Ion by In(III)-Porphyrin-Based Polymeric Membrane Electrode Operated in Pulsed Chronopotentiometric Mode. Electroanalysis 2012, 24, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemene, K.L.; Shvarev, A.; Bakker, E. Selectivity enhancement of anion-responsive electrodes by pulsed chronopotentiometry. Anal. Chim. Acta 2007, 583, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, E. Ion-Selective electrodes|Liquid Membrane. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 509–515. [Google Scholar]
- Lindfors, T.; Sundfors, F.; Höfler, L.; Gyurcsányi, R.E. FTIR-ATR Study of Water Uptake and Diffusion Through Ion-Selective Membranes Based on Plasticized Poly(vinyl chloride). Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1914–1922. [Google Scholar] [CrossRef]
- Maksymiuk, K.; Stelmach, E.; Michalska, A. Unintended Changes of Ion-Selective Membranes Composition—Origin and Effect on Analytical Performance. Membranes 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Miura, N.; Ushiro, M.; Kawaguchi, M.; Nakamura, H.; Iguchi, H.; Ogino, J.; Oishi, M.; Wakui, N.; Iwasaki, Y.; et al. Effect of gamma-ray irradiation on degradation of di(2-ethylhexyl)phthalate in polyvinyl chloride sheet. Int. J. Pharm. 2009, 376, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Haishima, Y.; Isama, K.; Hasegawa, C.; Yuba, T.; Matsuoka, A. A development and biological safety evaluation of novel PVC medical devices with surface structures modified by UV irradiation to suppress plasticizer migration. J. Biomed. Mater. Res. Part A 2013, 101, 2630–2643. [Google Scholar] [CrossRef]
- Joon, N.K.; He, N.; Ruzgas, T.; Bobacka, J.; Lisak, G. PVC-Based Ion-Selective Electrodes with a Silicone Rubber Outer Coating with Improved Analytical Performance. Anal. Chem. 2019, 91, 10524–10531. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R. Chapter 5-Molecular Events at Tissue–Biomaterial Interface. In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Oxford, UK, 2015; pp. 81–116. [Google Scholar]
- Jiwakanon, S.; Mehrotra, R. Chapter 33-Nutritional Management of End-Stage Renal Disease Patients Treated with Peritoneal Dialysis. In Nutritional Management of Renal Disease; Kopple, J.D., Massry, S.G., Kalantar-Zadeh, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 539–561. [Google Scholar]

| Sample | AACD Roughness (nm) | Drop Casting Roughness (nm) |
|---|---|---|
| High molecular weight PVC | 26.5 ± 11.1 | 943 ± 624 |
| Medium molecular weight PVC | 92.3 ± 5.3 | 309 ± 153 |
| Low molecular weight PVC | 122 ± 17 | 458 ± 216 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Gonzalez, A.; Choy, K.-L. Integration of an Aerosol-Assisted Deposition Technique for the Deposition of Functional Biomaterials Applied to the Fabrication of Miniaturised Ion Sensors. Nanomaterials 2021, 11, 938. https://doi.org/10.3390/nano11040938
Ruiz-Gonzalez A, Choy K-L. Integration of an Aerosol-Assisted Deposition Technique for the Deposition of Functional Biomaterials Applied to the Fabrication of Miniaturised Ion Sensors. Nanomaterials. 2021; 11(4):938. https://doi.org/10.3390/nano11040938
Chicago/Turabian StyleRuiz-Gonzalez, Antonio, and Kwang-Leong Choy. 2021. "Integration of an Aerosol-Assisted Deposition Technique for the Deposition of Functional Biomaterials Applied to the Fabrication of Miniaturised Ion Sensors" Nanomaterials 11, no. 4: 938. https://doi.org/10.3390/nano11040938