Branching of Titanium Nanorods
Abstract
1. Introduction
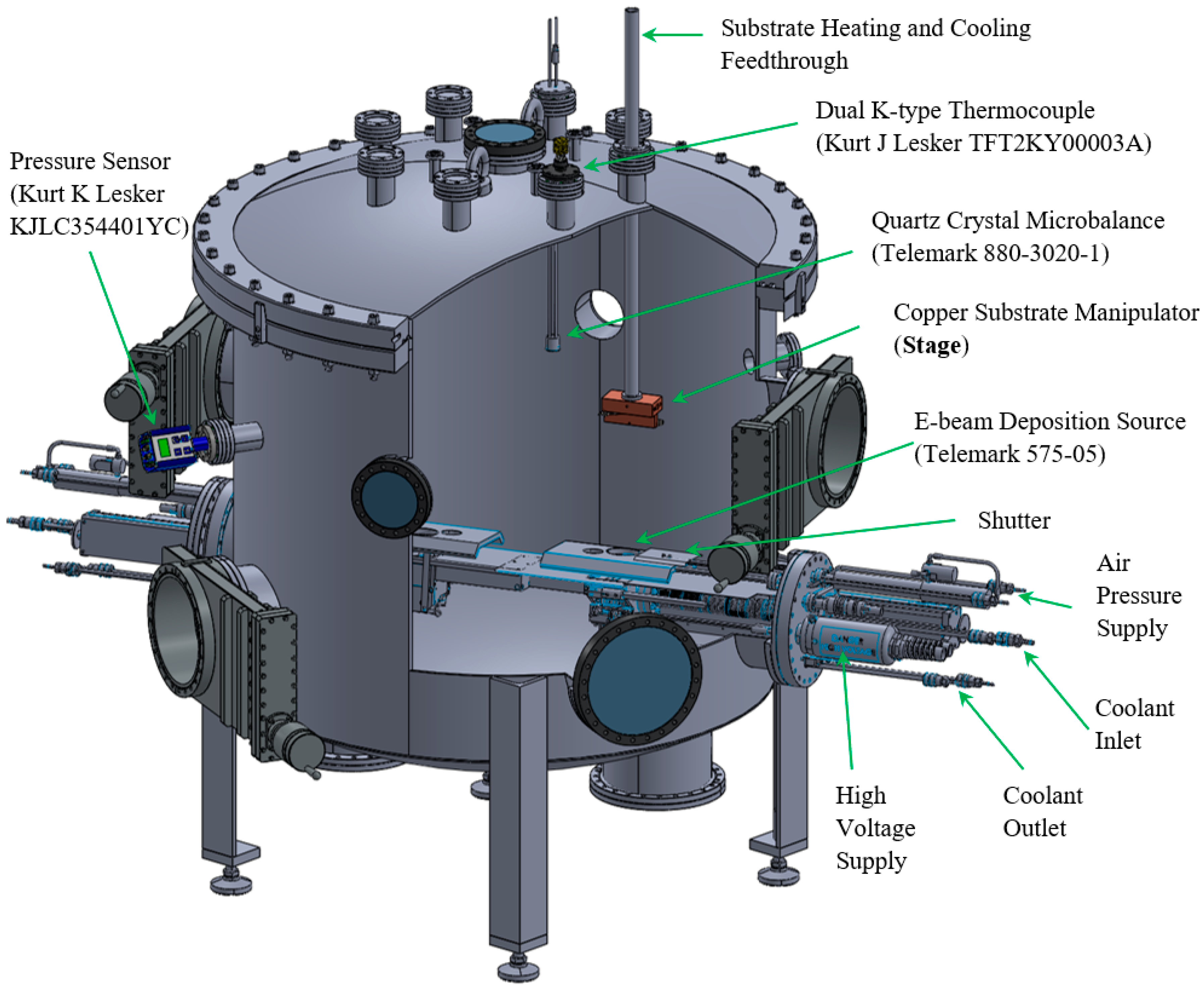
2. Experimental Methods
3. Results and Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Tang, F.; Parker, T.; Wang, G.C.; Lu, T.M. Surface texture evolution of polycrystalline and nanostructured films: RHEED surface pole figure analysis. J. Phys. D Appl. Phys. 2007. [Google Scholar] [CrossRef]
- Sit, J.C.; Vick, D.; Robbie, K.; Brett, M.J. Thin Film Microstructure Control Using Glancing Angle Deposition by Sputtering. J. Mater. Res. 1999, 14, 1197–1199. [Google Scholar] [CrossRef]
- Li, Z.; Xing, L.; Zhang, Z. Photocatalytic Properties of Columnar Nanostructured TiO 2 Films Fabricated by Sputtering Ti and Subsequent Annealing. Adv. Mater. Sci. Eng. 2012, 2012. [Google Scholar] [CrossRef]
- Robbie, K.; Brett, M.J. Sculptured thin films and glancing angle deposition: Growth mechanics and applications. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997. [Google Scholar] [CrossRef]
- Robbie, K. Advanced techniques for glancing angle deposition. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 1998. [Google Scholar] [CrossRef]
- Ai, B.; Zhao, Y. Glancing angle deposition meets colloidal lithography: A new evolution in the design of nanostructures. Nanophotonics 2018. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Huang, H.; Zhang, Z.; Li, X.; Fan, Y. Simultaneous thermal stability and ultrahigh sensitivity of heterojunction SERS substrates. Nanomaterials 2019, 9, 830. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.; Kesapragada, S.V.; Gall, D. Growth of Y-shaped nanorods through physical vapor deposition. Nano Lett. 2005, 5, 2505–2508. [Google Scholar] [CrossRef]
- Liu, S.J.; Huang, H.; Woo, C.H. Schwoebel-Ehrlich barrier: From two to three dimensions. Appl. Phys. Lett. 2002. [Google Scholar] [CrossRef]
- Xiang, S.K.; Huang, H. Ab initio determination of Ehrlich-Schwoebel barriers on Cu{111}. Appl. Phys. Lett. 2008. [Google Scholar] [CrossRef]
- Lagally, M.G.; Zhang, Z. Thin-film cliffhanger. Nature 2002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.G.; Huang, H. Characteristic length scale of nanorod diameter during growth. Phys. Rev. Lett. 2008. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Elliott, P.R.; Huang, H. Generalized theory of smallest diameter of metallic nanorods. Phys. Rev. Mater. 2017. [Google Scholar] [CrossRef]
- Niu, X.; Stagon, S.P.; Huang, H.; Baldwin, J.K.; Misra, A. Smallest Metallic Nanorods Using Physical Vapor Deposition. Phys. Rev. Lett. 2013, 110, 136102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.M.; Gall, D. Branched Ta nanocolumns grown by glancing angle deposition. Appl. Phys. Lett. 2006. [Google Scholar] [CrossRef]
- Khan, S.B.; Wu, H.; Zhang, Z. Omnidirectional SiO2 AR coatings. Coatings 2018, 8, 210. [Google Scholar] [CrossRef]
- Atanasov, P.A.; Nedyalkov, N.N.; Nikov, R.G.; Grüner, C.; Rauschenbach, B.; Fukata, N. SERS analysis of Ag nanostructures produced by ion-beam deposition. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Tajik, N.; Ehsani, M.H.; Moghadam, R.Z.; Dizaji, H.R. Effect of GLAD technique on optical properties of ZnS multilayer antireflection coatings. Mater. Res. Bull. 2018. [Google Scholar] [CrossRef]
- Liedtke, S.; Grüner, C.; Gerlach, J.W.; Rauschenbach, B. Comparative study of sculptured metallic thin films deposited by oblique angle deposition at different temperatures. Beilstein J. Nanotechnol. 2018, 9, 954–962. [Google Scholar] [CrossRef]
- Tao, Y.; Degen, C.L. Growth of magnetic nanowires along freely selectable (hkl) crystal directions. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Hou, M.J.; Zhang, X.; Cui, X.Y.; Liu, C.; Li, Z.C.; Zhang, Z.J. Preparation of SiO2@ Au nanorod array as novel surface enhanced Raman substrate for trace pollutants detection. Chin. Phys. B 2015. [Google Scholar] [CrossRef]
- Chen, L.; Andrea, L.; Timalsina, Y.P.; Wang, G.C.; Lu, T.M. Engineering epitaxial-nanospiral metal films using dynamic oblique angle deposition. Cryst. Growth Des. 2013. [Google Scholar] [CrossRef]
- Alouach, H.; Mankey, G.J. Texture orientation of glancing angle deposited copper nanowire arrays. J. Vac. Sci. Technol. A Vac. Surf. Film. 2004. [Google Scholar] [CrossRef]
- Chen, L.; Lu, T.M.; Wang, G.C. Incident flux angle induced crystal texture transformation in nanostructured molybdenum films. J. Appl. Phys. 2012. [Google Scholar] [CrossRef]
- Tang, F.; Parker, T.; Li, H.-F.; Wang, G.-C.; Lu, T.-M. Unusual Magnesium Crystalline Nanoblades Grown by Oblique Angle Vapor Deposition. J. Nanosci. Nanotechnol. 2007, 7, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, Y. Mg nanostructures tailored by glancing angle deposition. Cryst. Growth Des. 2010, 10, 440–448. [Google Scholar] [CrossRef]
- Zhang, L.J.; Spiridonova, T.I.; Kulkova, S.E.; Yang, R.; Hu, Q.M. Atomic self-diffusion anisotropy of HCP metals from first-principles calculations. Comput. Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Chu, H.; Huang, H.; Wang, J. Clustering on Magnesium Surfaces-Formation and Diffusion Energies. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.G.; Huang, H.; Lu, T.M. Diffusion and formation energies of adatoms and vacancies on magnesium surfaces. Comput. Mater. Sci. 2009. [Google Scholar] [CrossRef]
- Dervaux, J.; Cormier, P.A.; Moskovkin, P.; Douheret, O.; Konstantinidis, S.; Lazzaroni, R.; Lucas, S.; Snyders, R. Synthesis of nanostructured Ti thin films by combining glancing angle deposition and magnetron sputtering: A joint experimental and modeling study. Thin Solid Film. 2017, 636, 644–657. [Google Scholar] [CrossRef]
- Sadeghi-Khosravieh, S.; Robbie, K. Morphology and crystal texture in tilted columnar micro-structured titanium thin film coatings. Thin Solid Film. 2017, 627, 69–76. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007. [Google Scholar] [CrossRef]
- Stagon, S.P.; Huang, H. Controllable growth of Al nanorods using physical vapor deposition. Nanoscale. Res. Lett. 2014, 9, 400. [Google Scholar] [CrossRef]
- Elliott, P.R.; Stagon, S.P.; Huang, H. Control of Separation and Diameter of Ag Nanorods through Self-organized Seeds. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Grüner, C.; Liedtke, S.; Bauer, J.; Mayr, S.G.; Rauschenbach, B. Morphology of Thin Films Formed by Oblique Physical Vapor Deposition. ACS Appl. Nano Mater. 2018. [Google Scholar] [CrossRef]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and characterization of columnar growth in evaporated films. Thin Solid Film. 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Niewehuizen, J.M.; Haanstra, H.B. Microfractography of thin films. Philips Tech. Rev. 1966, 27, 87–91. [Google Scholar]
- Luo, Y.; Qin, R. Surface energy and its anisotropy of hexagonal close-packed metals. Surf. Sci. 2014, 630, 195–201. [Google Scholar] [CrossRef]
- Hilton, H. The Construction of Crystallographic Projections. Mineral. Mag. J. Mineral. Soc. 1905. [Google Scholar] [CrossRef]
- Li, X.Z. SPICA: Stereographic projection for interactive crystallographic analysis. J. Appl. Crystallogr. 2016. [Google Scholar] [CrossRef]
- Mittemeijer, E.J.; Scardi, P. Diffraction Analysis of the Microstructure of Materials; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gall, D. Structure zone model for extreme shadowing conditions. Thin Solid Film. 2013, 527, 158–163. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yussuf, N.A.; Huang, H. Branching of Titanium Nanorods. Nanomaterials 2021, 11, 1070. https://doi.org/10.3390/nano11051070
Yussuf NA, Huang H. Branching of Titanium Nanorods. Nanomaterials. 2021; 11(5):1070. https://doi.org/10.3390/nano11051070
Chicago/Turabian StyleYussuf, Nosirudeen Abayomi, and Hanchen Huang. 2021. "Branching of Titanium Nanorods" Nanomaterials 11, no. 5: 1070. https://doi.org/10.3390/nano11051070
APA StyleYussuf, N. A., & Huang, H. (2021). Branching of Titanium Nanorods. Nanomaterials, 11(5), 1070. https://doi.org/10.3390/nano11051070




