Enhancement of Volumetric Capacitance of Binder-Free Single-Walled Carbon Nanotube Film via Fluorination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purification of SWNTs
2.2. Preparation and Fluorination of Films
2.3. Characterization Methods
2.4. Electrochemical Measurements
3. Results
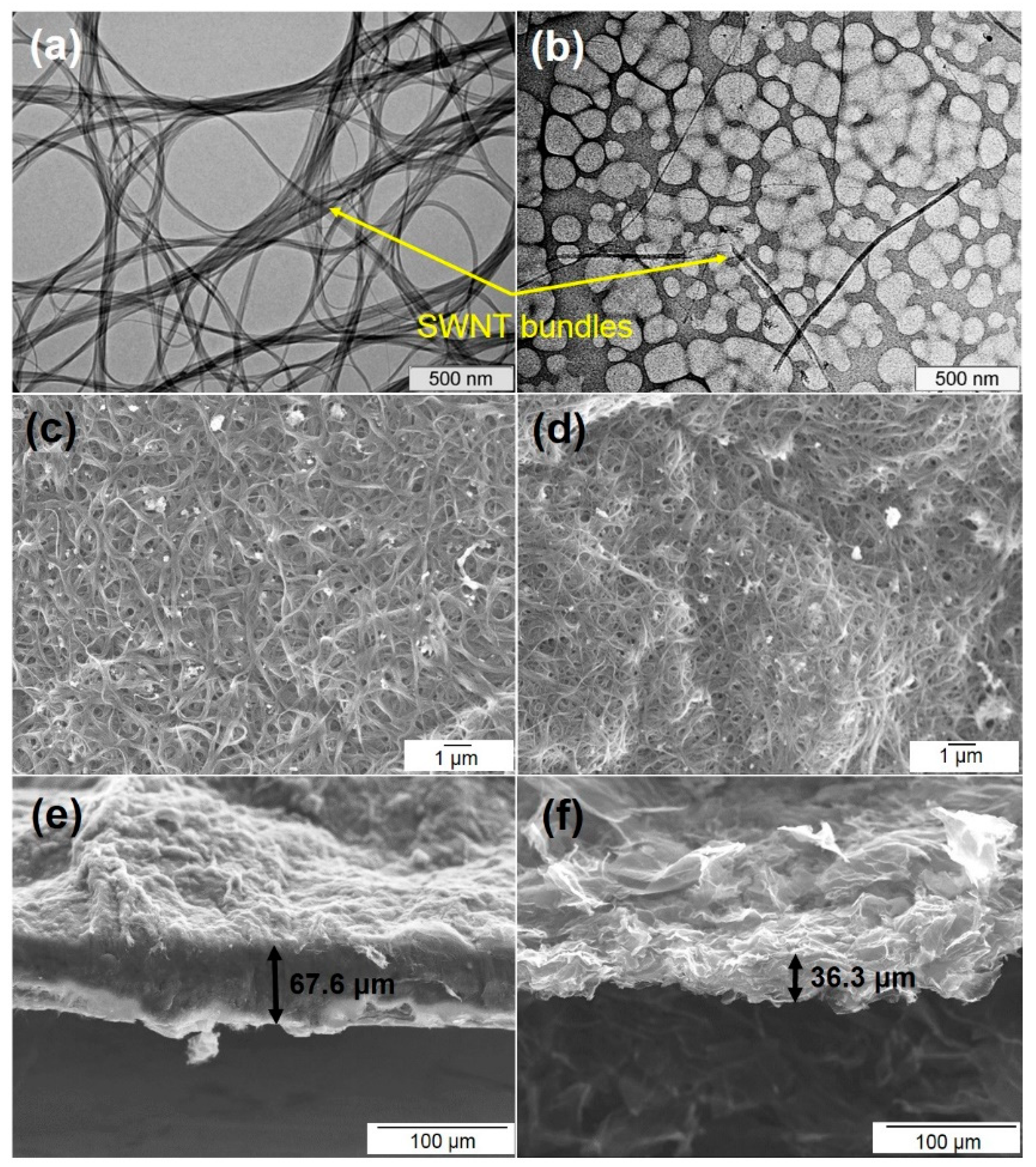
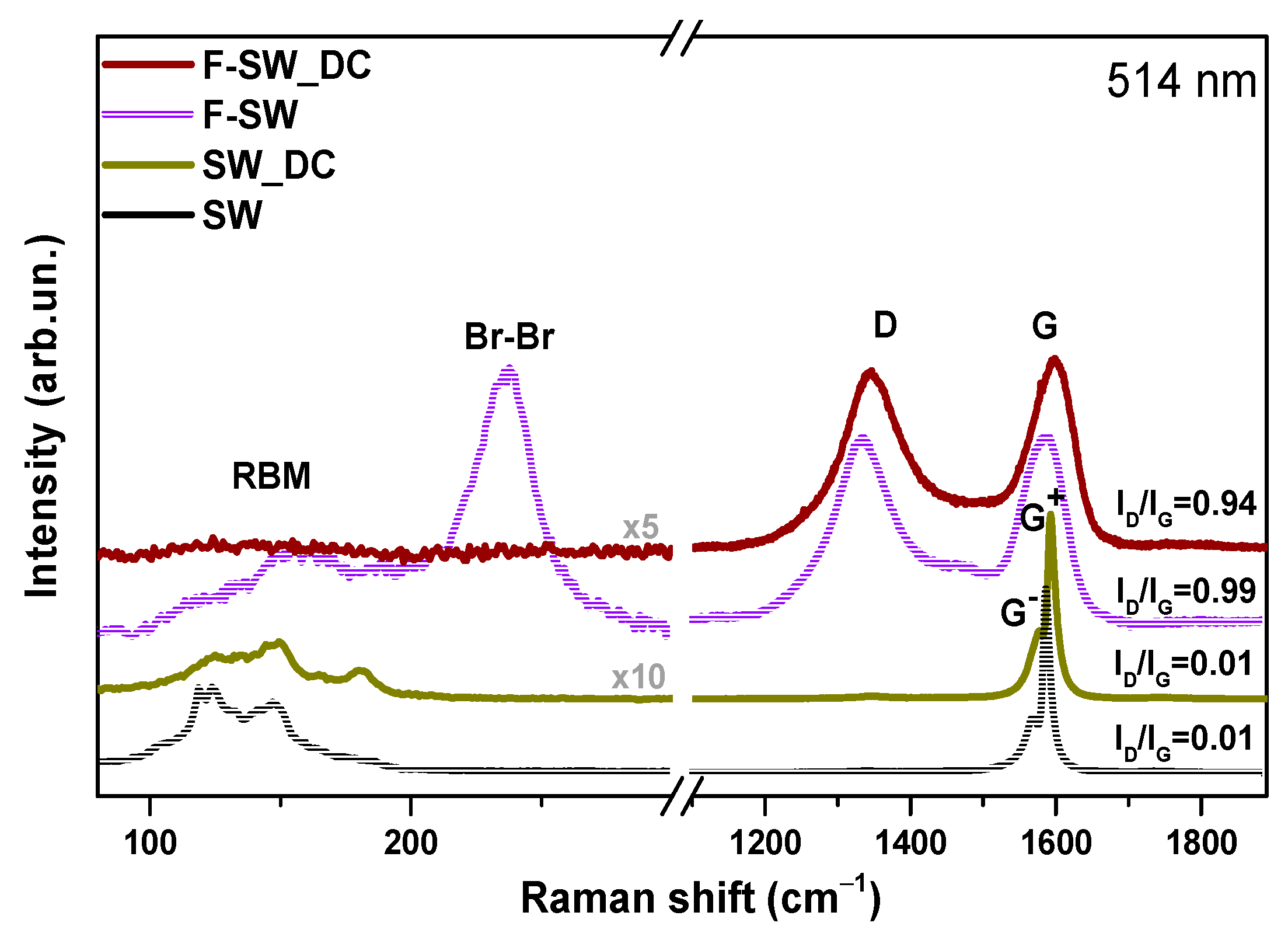
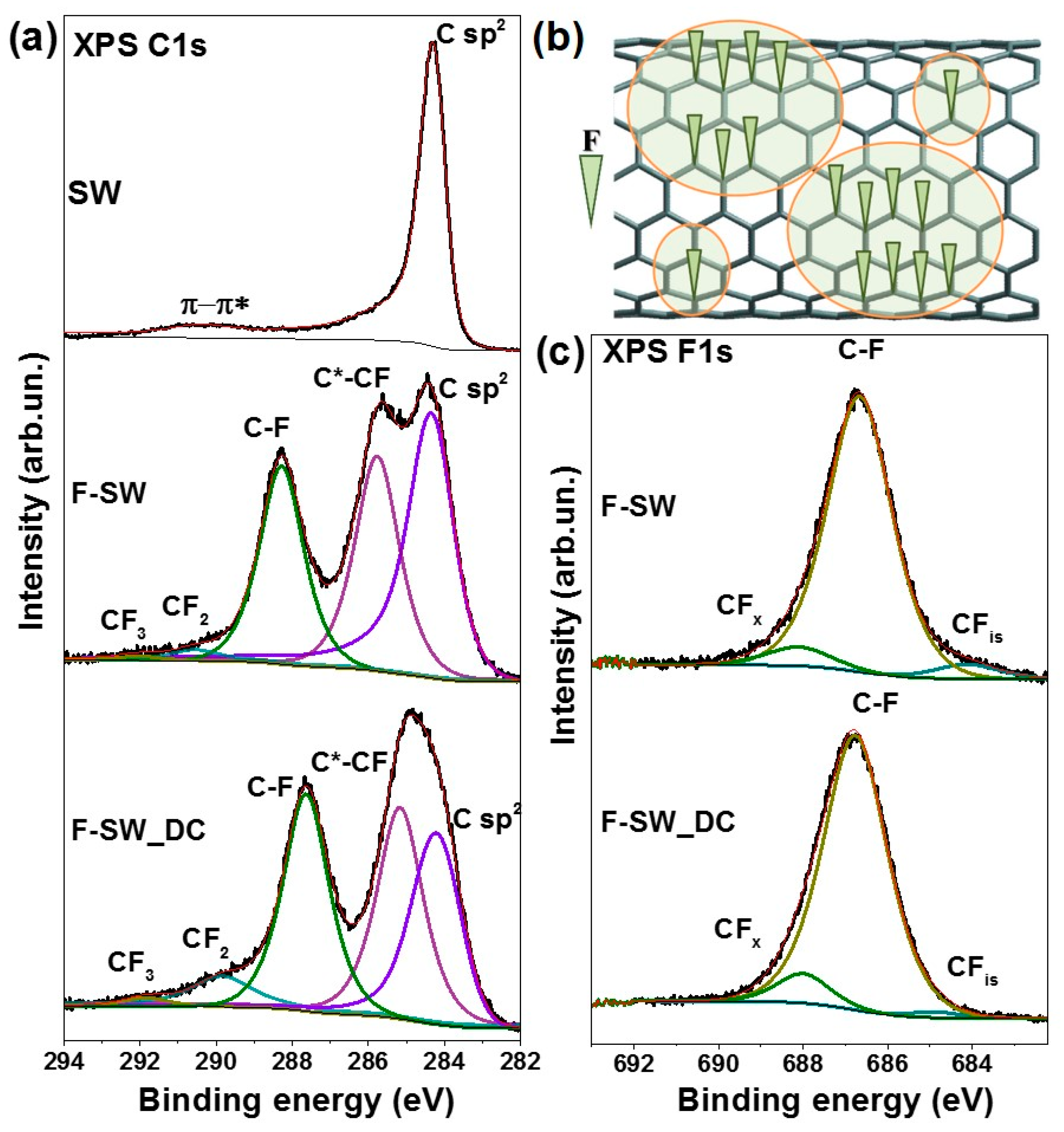
3.1. Structural Aspects
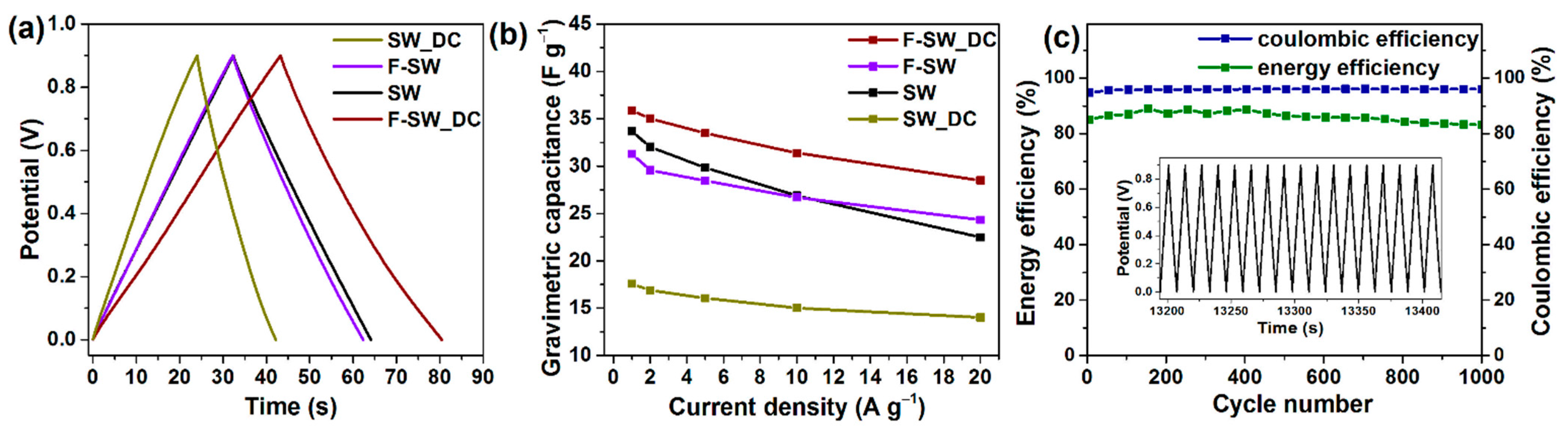
3.2. Electrochemical Behavior of SWCNT-Based Electrodes
3.3. F-SWCNTs after Electrochemical Tests
3.4. Electrochemical Performance of Aged Electrodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.; Wang, H.; Hu, X.; Cai, W.; Zhang, C.; Wang, M.; Zang, Z. Sodium Benzenesulfonate Modified Poly (3,4-Ethylenedioxythiophene):Polystyrene Sulfonate with Improved Wettability and Work Function for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2021, 5, 2000573. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Wang, H.; Yang, K.; Hu, X.; Sun, K.; Lu, S.; Zang, Z. Small Molecule Modulator at the Interface for Efficient Perovskite Solar Cells with High Short-Circuit Current Density and Hysteresis Free. Adv. Electron. Mater. 2020, 6, 2000604. [Google Scholar] [CrossRef]
- Massé, R.C.; Liu, C.; Li, Y.; Mai, L.; Cao, G. Energy Storage through Intercalation Reactions: Electrodes for Rechargeable Batteries. National Sci. Rev. 2017, 4, 26–53. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-Based Supercapacitors for Efficient Energy Storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Pacheco-Catalán, D.; Miranda-Hernández, M. Environmentally Friendly Supercapacitors. In Materials for Sustainable Energy Applications: Conversion, Storage, Transmission, and Consumption; Pan Stanford Publishing Pte. Ltd.: New York, NY, USA, 2016; pp. 351–445. [Google Scholar]
- Najib, S.; Erdem, E. Current Progress Achieved in Novel Materials for Supercapacitor Electrodes: Mini Review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Zang, X.; Wang, X.; Gu, X.; Shao, Q.; Cao, N. Recent Advances in Fluorine-Doped/Fluorinated Carbon-Based Materials for Supercapacitors. Energy Storage Mater. 2020, 30, 367–384. [Google Scholar] [CrossRef]
- Shlaykhova, E.V.; Okotrub, A.V.; Fedoseeva, Y.V.; Fedorovskaya, E.O.; Mel’gunova, E.A.; Mel’gunov, M.S.; Koroteev, V.O.; Makarova, A.A.; Zhou, J.; Song, H.; et al. Iron Induced Porosity of the Templated Carbon for Enhancement of Electrochemical Capacitance. Appl. Surf. Sci. 2021, 543, 148565. [Google Scholar] [CrossRef]
- Deng, L.; Gu, Y.; Gao, Y.; Ma, Z.; Fan, G. Carbon Nanotubes/Holey Graphene Hybrid Film as Binder-Free Electrode for Flexible Supercapacitors. J. Colloid Interface Sci. 2017, 494, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Yan, L.; Zhao, S.; Zuo, Z.; Wang, X.; Wang, C.; Hou, M. Fluorine-Doped Graphene/Nanosized Carbide-Derived Carbon Composites for High-Performance Supercapacitor. Nano 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Asgari, M.; Lohrasbi, E. Comparison of Single-Walled and Multiwalled Carbon Nanotubes Durability as Pt Support in Gas Diffusion Electrodes. ISRN Electrochem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable Materials for Electrochemical Capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Yuksel, R.; Sarioba, Z.; Cirpan, A.; Hiralal, P.; Unalan, H.E. Transparent and Flexible Supercapacitors with Single Walled Carbon Nanotube Thin Film Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 15434–15439. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chemie Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, L.L.; Lee, S.; Oh, J.; Lee, K.-S.; Potts, J.R.; Ji, J.; Zhao, X.; Ruoff, R.S.; Park, S. Generation of B-Doped Graphene Nanoplatelets Using a Solution Process and Their Supercapacitor Applications. ACS Nano 2013, 7, 19–26. [Google Scholar] [CrossRef]
- Bahuguna, G.; Chaudhary, S.; Sharma, R.K.; Gupta, R. Electrophilic Fluorination of Graphitic Carbon for Enhancement in Electric Double-Layer Capacitance. Energy Technol. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Long, P.; Gao, Y.; Qin, C.; Cao, C.; Feng, Y.; Feng, W. Hydrothermal Preparation of Fluorinated Graphene Hydrogel for High-Performance Supercapacitors. J. Power Sources 2016, 312, 146–155. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, K.H.; Heo, J.K.; Lee, Y.H. Fabrication of Supercapacitor Electrodes using Fluorinated Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 8812–8815. [Google Scholar] [CrossRef]
- Lee, Y.-S. Surface-Fluorinated Carbon Materials for Supercapacitor. In Advanced Fluoride-Based Materials for Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2015; pp. 375–386. [Google Scholar]
- Fedoseeva, Y.V.; Arkhipov, V.E.; Maksimovskiy, E.A.; Gusel’nikov, A.V.; Mikhlin, Y.L.; Zhuravlev, K.S.; Senkovskiy, B.V.; Larionov, S.V.; Bulusheva, L.G.; Okotrub, A.V. Fluorinated Surface of Carbon Nanotube Buckypaper for Uniform Growth of CdS Nanoparticles. J. Phys. Chem. C 2017, 121, 19182–19190. [Google Scholar] [CrossRef]
- Marcoux, P.R.; Schreiber, J.; Batail, P.; Lefrant, S.; Renouard, J.; Jacob, G.; Albertini, D.; Mevellec, J.-Y. A Spectroscopic Study of the Fluorination and Defluorination Reactions on Single-Walled Carbon Nanotubes. Phys. Chem. Chem. Phys. 2002, 4, 2278–2285. [Google Scholar] [CrossRef] [Green Version]
- Krestinin, A.V.; Kharitonov, A.P.; Shul’ga, Y.M.; Zhigalina, O.M.; Knerel’man, E.I.; Dubois, M.; Brzhezinskaya, M.M.; Vinogradov, A.S.; Preobrazhenskii, A.B.; Zvereva, G.I.; et al. Fabrication and characterization of fluorinated single-walled carbon nanotubes. Nanotechnologies Russ. 2009, 4, 60–78. [Google Scholar] [CrossRef]
- Gurova, O.A.; Arhipov, V.E.; Koroteev, V.O.; Guselnikova, T.Y.; Asanov, I.P.; Sedelnikova, O.V.; Okotrub, A.V. Purification of Single-Walled Carbon Nanotubes Using Acid Treatment and Magnetic Separation. Phys. Status Solidi B 2019, 256, 1800742. [Google Scholar] [CrossRef]
- Lobiak, E.V.; Bulusheva, L.G.; Galitsky, A.A.; Smirnov, D.A.; Flahaut, E.; Okotrub, A.V. Structure and Electrochemical Properties of Carbon Nanotubes Synthesized with Catalysts Obtained by Decomposition of Co, Ni, and Fe Polyoxomolybdates Supported by MgO. J. Struct. Chem. 2018, 59, 786–792. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Filho, A.G.S.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing Carbon Nanotube Samples with Resonance Raman Scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Miyata, Y.; Mizuno, K.; Kataura, H. Purity and Defect Characterization of Single-Wall Carbon Nanotubes Using Raman Spectroscopy. J. Nanomater. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Heller, D.A.; Barone, P.W.; Swanson, J.P.; Mayrhofer, R.M.; Strano, M.S. Using Raman Spectroscopy to Elucidate the Aggregation State of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2004, 108, 6905–6909. [Google Scholar] [CrossRef]
- Levshov, D.I.; Slabodyan, Y.S.; Tonkikh, A.A.; Michel, T.; Roshal’, S.B.; Yuzyuk, Y.I. Specific Features of Tangential Modes in Raman Scattering Spectra of Semiconducting Single-Walled Carbon Nanotubes with a Large Diameter. Phys. Solid State 2017, 59, 594–600. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Lobiak, E.V.; Fedoseeva, Y.V.; Mevellec, J.-Y.; Makarova, A.A.; Flahaut, E.; Okotrub, A.V. Effect of Ultrasound Pretreatment on Bromination of Double-Walled Carbon Nanotubes. Synth. Met. 2020, 259, 116233. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Bulusheva, L.G.; Asanov, I.P.; Yushina, I.V.; Okotrub, A.V. Energy Shift of Collective Electron Excitations in Highly Corrugated Graphitic Nanostructures: Experimental and Theoretical Investigation. Appl. Phys. Lett. 2014, 104, 161905. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedoseeva, Y.V.; Flahaut, E.; Rio, J.; Ewels, C.P.; Koroteev, V.O.; Van Lier, G.; Vyalikh, D.V.; Okotrub, A. V Effect of the Fluorination Technique on the Surface-Fluorination Patterning of Double-Walled Carbon Nanotubes. Beilstein J. Nanotechnol. 2017, 8, 1688–1698. [Google Scholar] [CrossRef] [Green Version]
- Fedoseeva, Y.V.; Bulusheva, L.G.; Koroteev, V.O.; Mevellec, J.-Y.; Senkovskiy, B.V.; Flahaut, E.; Okotrub, A.V. Preferred Attachment of Fluorine near Oxygen-Containing Groups on the Surface of Double-Walled Carbon Nanotubes. Appl. Surf. Sci. 2020, 504, 144357. [Google Scholar] [CrossRef] [Green Version]
- Dementjev, A.P.; Eletskii, A.V.; Maslakov, K.I.; Rakov, E.G.; Sukhoverhov, V.F.; Naumkin, A.V. Fluorination of Carbon Nanostructures and Their Comparative Investigation by XPS and XAES Spectroscopy. Fuller. Nanotub. Carbon Nanostructures 2006, 14, 287–296. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Yudanov, N.F.; Asanov, I.P.; Vyalikh, D.V.; Bulusheva, L.G. Anisotropy of Chemical Bonding in Semifluorinated Graphite C 2 F Revealed with Angle-Resolved X-ray Absorption Spectroscopy. ACS Nano 2013, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Asanov, I.P.; Bulusheva, L.G.; Dubois, M.; Yudanov, N.F.; Alexeev, A.V.; Makarova, T.L.; Okotrub, A.V. Graphene Nanochains and Nanoislands in the Layers of Room-Temperature Fluorinated Graphite. Carbon 2013, 59, 518–529. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Tur, V.A.; Fedorovskaya, E.O.; Asanov, I.P.; Pontiroli, D.; Ricco, M.; Okotrub, A.V. Structure and Supercapacitor Performance of Graphene Materials Obtained from Brominated and Fluorinated Graphites. Carbon 2014, 78, 137–146. [Google Scholar] [CrossRef]
- Ruiz, V.; Santamaria, R.; Granda, M.; Blanco, C. Long-term cycling of carbon-based supercapacitors in aqueous media. Electrochim. Acta 2009, 54, 4481–4486. [Google Scholar] [CrossRef] [Green Version]
- Fic, K.; Meller, M.; Frackowiak, E. Strategies for enhancing the performance of carbon/carbon supercapacitors in aqueous electrolytes. Electrochim. Acta 2014, 128, 210–217. [Google Scholar] [CrossRef]
- Bichat, M.P.; Raymundo-Pinero, E.; Beguin, F. High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte. Carbon 2010, 48, 4351–4361. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Grebel, H. Asymmetric Supercapacitors: Optical and Thermal Effects When Active Carbon Electrodes Are Embedded with Nano-Scale Semiconductor Dots. C 2021, 7, 7. [Google Scholar] [CrossRef]
- Nishchakova, A.D.; Grebenkina, M.A.; Shlyakhova, E.V.; Shubin, Y.V.; Kovalenko, K.A.; Asanov, I.P.; Fedoseeva, Y.V.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Porosity and Composition of Nitrogen-Doped Carbon Materials Templated by the Thermolysis Products of Calcium Tartrate and Their Performance in Electrochemical Capacitors. J. Alloys Compd. 2021, 858, 158259. [Google Scholar] [CrossRef]
- Hu, C.-C.; Su, J.-H.; Wen, T.-C. Modification of Multi-Walled Carbon Nanotubes for Electric Double-Layer Capacitors: Tube Opening and Surface Functionalization. J. Phys. Chem. Solids 2007, 68, 2353–2362. [Google Scholar] [CrossRef]
- Fedorovskaya, E.O.; Bulusheva, L.G.; Kurenya, A.G.; Asanov, I.P.; Okotrub, A.V. Effect of Oxidative Treatment on the Electrochemical Properties of Aligned Multi-Walled Carbon Nanotubes. Russ. J. Electrochem. 2016, 52, 441–448. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Asanov, I.P.; Fonseca, A.; Nagy, J.B. Comparative Study on the Electronic Structure of Arc-Discharge and Catalytic Carbon Nanotubes. J. Phys. Chem. B 2001, 105, 4853–4859. [Google Scholar] [CrossRef]
- Blume, R.; Rosenthal, D.; Tessonnier, J.-P.; Li, H.; Knop-Gericke, A.; Schlögl, R. Characterizing Graphitic Carbon with X-ray Photoelectron Spectroscopy: A Step-by-Step Approach. ChemCatChem 2015, 7, 2871–2881. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Wang, Y.M.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR STUDY. J. Phys. Chem. C 2008, 43, 16869–16878. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béquin, F. Appropriate Methods for Evaluating the Efficiency and Capacitive Behaviour of Different Types of Supercapacitors. Electrochim. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J. Recent Advances in Flexible Supercapacitors Based on Carbon Nanotubes and Graphene. Sci. China Mater. 2018, 61, 210–232. [Google Scholar] [CrossRef] [Green Version]
- Bulusheva, L.G.; Arkhipov, V.E.; Fedorovskaya, E.O.; Zhang, S.; Kurenya, A.G.; Kanygin, M.A.; Asanov, I.P.; Tsygankova, A.R.; Chen, X.; Song, H.; et al. Fabrication of Free-Standing Aligned Multiwalled Carbon Nanotube Array for Li-ion Batteries. J. Power Sources 2016, 311, 42–48. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Shandakov, S.D.; Timmermans, M.Y.; Kauppinen, E.I. Aerosol Synthesis and Applications of Single-Walled Carbon Nanotubes. Russ. Chem. Rev. 2011, 80, 771–786. [Google Scholar] [CrossRef]
- Popov, K.M.; Arkhipov, V.E.; Kurenya, A.G.; Fedorovskaya, E.O.; Kovalenko, K.A.; Okotrub, A.V.; Bulusheva, L.G. Supercapacitor Performance of Binder-Free Buckypapers from Multiwall Carbon Nanotubes Synthesized at Different Temperatures. Phys. Status Solidi B 2016, 253, 2406–2412. [Google Scholar] [CrossRef]
- Lv, S.; Ma, L.; Shen, X.; Tong, H. Recent Design and Control of Carbon Materials for Supercapacitors. J. Mater. Sci. 2021, 56, 1919–1942. [Google Scholar] [CrossRef]
- Bahr, J.L.; Mickelson, E.T.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Dissolution of Small Diameter Single-Wall Carbon Nanotubes in Organic Solvents? Chem. Commun. 2001, 193–194. [Google Scholar] [CrossRef]
- Jung, M.J.; Jeong, E.; Lee, Y.S. The Surface Chemical Properties of Multi-Walled Carbon Nanotubes Modified by Thermal Fluorination for Electric Double-Layer Capacitor. Appl. Surf. Sci. 2015, 347, 250–257. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh Volumetric Capacitance and Cyclic Stability of Fluorine and Nitrogen Co-doped Carbon Microspheres. Nat. Commun. 2015, 6, 8503. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Kim, K.H.; Jung, M.J.; Park, J.H.; Lee, Y.S. Fluorination Effect of Activated Carbons on Performance of Asymmetric Capacitive Deionization. Appl. Surf. Sci. 2017, 409, 117–123. [Google Scholar] [CrossRef]
- Pinakov, D.V.; Chekhova, G.N.; Okotrub, A.V.; Asanov, I.P.; Shubin, Y.V.; Fedorovskaya, E.O.; Plyusnin, P.E.; Bulusheva, L.G. Structure and Supercapacitor Properties of Few-Layer Low-Fluorinated Graphene Materials. J. Mater. Sci. 2018, 53, 13053–13066. [Google Scholar] [CrossRef]
- Lavskaya, Y.V.; Bulusheva, L.G.; Okotrub, A.V.; Yudanov, N.F.; Vyalikh, D.V.; Fonseca, A. Comparative Study of Fluorinated Single- and Few-Wall Carbon Nanotubes by X-ray Photoelectron and X-ray Absorption Spectroscopy. Carbon 2009, 47, 1629–1636. [Google Scholar] [CrossRef]
- Ye, J.-S.; Liu, X.; Cui, H.F.; Zhang, W.-D.; Sheu, F.-S.; Lim, T.M. Electrochemical Oxidation of Multi-Walled Carbon Nanotubes and its Application to Electrochemical Double Layer Capacitors. Electrochem. Commun. 2005, 7, 249–255. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Bulusheva, L.G.; Okotrub, A.V.; Vyalikh, D.V.; Fonseca, A. A Comparative Study of Argon Ion Irradiated Pristine and Fluorinated Single-Wall Carbon Nanotubes. J. Chem. Phys. 2010, 133, 224706. [Google Scholar] [CrossRef] [PubMed]
- Emmenegger, C.; Mauron, P.; Sudan, P.; Wenger, P.; Hermann, V.; Gallay, R.; Züttel, A. Investigation of Electrochemical Double-Layer (ECDL) Capacitors Electrodes Based on Carbon Nanotubes and Activated Carbon Materials. J. Pow. Sourc. 2003, 124, 321–329. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, K.-W.; Ma, S.B.; Kim, K.B. Fabrication and Electrochemical Properties of Carbon Nanotube Film Electrodes. Carbon 2006, 44, 1963–1968. [Google Scholar] [CrossRef]
- Huang, C.; Grobert, N.; Watt, A.A.R.; Johnston, C.; Crossley, A.; Young, N.P.; Grant, P.S. Layer-by-Layer Spray Depostion and Unzipping of Single-Walled Carbon Nanotube-Based Thin Film Electrodes for Electrochemical Capacitors. Carbon 2013, 61, 525–536. [Google Scholar] [CrossRef]
- Coromina, H.M.; Adeniran, B.; Mokaya, R.; Walsh, D.A. Bridging the Performance Gap between Electric Double-Layer Capacitors and Batteries with High-Energy/High-Power Carbon Nanotube-Based Electrodes. J. Mater. Chem. A 2016, 4, 14586–14594. [Google Scholar] [CrossRef] [Green Version]



| Film | SSA, m2 g−1 | F, at% | CG, F g−1 | CV, F cm−3 | CG Drop, % |
|---|---|---|---|---|---|
| SW | 1135 | – | 29 | 18 | 41 |
| SW_DC | 840 | – | 21 | 34 | 43 |
| F-SW | 753 | 14 | 32 | 19 | 32 |
| F-SW_DC | 494 | 23 | 33 | 44 | 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurova, O.A.; Sysoev, V.I.; Lobiak, E.V.; Makarova, A.A.; Asanov, I.P.; Okotrub, A.V.; Kulik, L.V.; Bulusheva, L.G. Enhancement of Volumetric Capacitance of Binder-Free Single-Walled Carbon Nanotube Film via Fluorination. Nanomaterials 2021, 11, 1135. https://doi.org/10.3390/nano11051135
Gurova OA, Sysoev VI, Lobiak EV, Makarova AA, Asanov IP, Okotrub AV, Kulik LV, Bulusheva LG. Enhancement of Volumetric Capacitance of Binder-Free Single-Walled Carbon Nanotube Film via Fluorination. Nanomaterials. 2021; 11(5):1135. https://doi.org/10.3390/nano11051135
Chicago/Turabian StyleGurova, Olga A., Vitalii I. Sysoev, Egor V. Lobiak, Anna A. Makarova, Igor P. Asanov, Alexander V. Okotrub, Leonid V. Kulik, and Lyubov G. Bulusheva. 2021. "Enhancement of Volumetric Capacitance of Binder-Free Single-Walled Carbon Nanotube Film via Fluorination" Nanomaterials 11, no. 5: 1135. https://doi.org/10.3390/nano11051135
APA StyleGurova, O. A., Sysoev, V. I., Lobiak, E. V., Makarova, A. A., Asanov, I. P., Okotrub, A. V., Kulik, L. V., & Bulusheva, L. G. (2021). Enhancement of Volumetric Capacitance of Binder-Free Single-Walled Carbon Nanotube Film via Fluorination. Nanomaterials, 11(5), 1135. https://doi.org/10.3390/nano11051135







