Independent Control Over Size and Surface Density of Droplet Epitaxial Nanostructures Using Ultra-Low Arsenic Fluxes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, W.; Coleman, J.J. Semiconductor Quantum Dots. Curr. Opin. Solid State Mater. Sci. 2016, 20, 352–360. [Google Scholar] [CrossRef]
- Heitz, R.; Stier, O.; Mukhametzhanov, I.; Madhukar, A.; Bimberg, D. Quantum Size Effect in Self-Organized InAs/GaAs Quantum Dots. Phys. Rev. B 2000, 62, 11017–11028. [Google Scholar] [CrossRef]
- Gaan, S.; He, G.; Feenstra, R.M.; Walker, J.; Towe, E. Size, Shape, Composition, and Electronic Properties of InAs/GaAs Quantum Dots by Scanning Tunneling Microscopy and Spectroscopy. J. Appl. Phys. 2010, 108, 114315. [Google Scholar] [CrossRef]
- Mukhametzhanov, I.; Wei, Z.; Heitz, R.; Madhukar, A. Punctuated Island Growth: An Approach to Examination and Control of Quantum Dot Density, Size, and Shape Evolution. Appl. Phys. Lett. 1999, 75, 85–87. [Google Scholar] [CrossRef]
- Heitz, R.; Ramachandran, T.R.; Kalburge, A.; Xie, Q.; Mukhametzhanov, I.; Chen, P.; Madhukar, A. Observation of Reentrant 2D to 3D Morphology Transition in Highly Strained Epitaxy: InAs on GaAs. Phys. Rev. Lett. 1997, 78, 4071–4074. [Google Scholar] [CrossRef]
- Karrai, K.; Warburton, R.J.; Schulhauser, C.; Högele, A.; Urbaszek, B.; McGhee, E.J.; Govorov, A.O.; Garcia, J.M.; Gerardot, B.D.; Petroff, P.M. Hybridization of Electronic States in Quantum Dots through Photon Emission. Nature 2004, 427, 135–138. [Google Scholar] [CrossRef]
- Thompson, R.M.; Stevenson, R.M.; Shields, A.J.; Farrer, I.; Lobo, C.J.; Ritchie, D.A.; Leadbeater, M.L.; Pepper, M. Single-Photon Emission from Exciton Complexes in Individual Quantum Dots. Phys. Rev. B 2001, 64, 201302. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.M. Droplet Epitaxy for Advanced Optoelectronic Materials and Devices. J. Phys. D Appl. Phys. 2014, 47, 173001. [Google Scholar] [CrossRef]
- Gurioli, M.; Wang, Z.M.; Rastelli, A.; Kuroda, T.; Sanguinetti, S. Droplet Epitaxy of Semiconductor Nanostructures for Quantum Photonic Devices. Nat. Mater. 2019, 18, 799–810. [Google Scholar] [CrossRef]
- Huang, S.S.; Niu, Z.C.; Ni, H.Q.; Zhan, F.; Zhao, H.; Sun, Z.; Xia, J.B. Extremely Low Density InAs Quantum Dots with No Wetting Layer. Chin. Phys. Lett. 2007, 24, 1025–1028. [Google Scholar]
- Lee, J.H.; Wang, Z.M.; Ware, M.E.; Wijesundara, K.C.; Garrido, M.; Stinaff, E.A.; Salamo, G.J. Super Low Density InGaAs Semiconductor Ring-Shaped Nanostructures. Cryst. Growth Des. 2008, 8, 1945–1951. [Google Scholar] [CrossRef]
- Mantovani, V.; Sanguinetti, S.; Guzzi, M.; Grilli, E.; Gurioli, M.; Watanabe, K.; Koguchi, N. Low Density GaAs/AlGaAs Quantum Dots Grown by Molecular Beam Epitaxy. J. Appl. Phys. 2004, 96, 4416–4420. [Google Scholar] [CrossRef]
- Liang, B.L.; Wang, Z.M.; Lee, J.H.; Sablon, K.; Mazur, Y.I.; Salamo, G.J. Low Density InAs Quantum Dots Grown on GaAs nanoholes. Appl. Phys. Lett. 2006, 89, 043113. [Google Scholar] [CrossRef]
- Liang, B.L.; Wang, Z.M.; Wang, X.Y.; Lee, J.H.; Mazur, Y.I.; Shih, C.K.; Salamo, G.J. Energy Transfer within Ultralow Density Twin InAs Quantum Dots Grown by Droplet Epitaxy. ACS Nano 2008, 2, 2219–2224. [Google Scholar] [CrossRef]
- Sanguinetti, S.; Mano, T.; Oshima, M.; Tateno, T.; Wakaki, M.; Koguchi, N. Temperature Dependence of the Photoluminescence of InGaAs/GaAs Quantum Dot Structures without Wetting Layer. Appl. Phys. Lett. 2002, 81, 3067–3069. [Google Scholar] [CrossRef]
- Sanguinetti, S.; Watanabe, K.; Tateno, T.; Gurioli, M.; Werner, P.; Wakaki, M.; Koguchi, N. Modified Droplet Epitaxy GaAs/AlGaAs Quantum Dots Grown on a Variable Thickness Wetting Layer. J. Cryst. Growth 2003, 253, 7176. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Y.H.; Xu, B.; Tang, C.G.; Wang, Z.G.; Ding, F. Study of the Wetting Layer of InAs/GaAs Nanorings Grown by Droplet Epitaxy. Appl. Phys. Lett. 2008, 92, 063122. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, Z.M.; Kim, E.S.; Kim, N.Y.; Park, S.H.; Salamo, G.J. Various Quantum- and Nano-Structures by III-V Droplet Epitaxy on GaAs Substrates. Nanoscale Res. Lett. 2010, 5, 308–314. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, Z.M.; AbuWaar, Z.Y.; Salamo, G.J. Design of Nanostructure Complexes by Droplet Epitaxy. Cryst. Growth Des. 2009, 9, 715–721. [Google Scholar] [CrossRef]
- Chawner, J.M.A.; Chang, Y.; Hodgson, P.D.; Hayne, M.; Robson, A.J.; Sanchez, A.M.; Zhuang, Q. Control of Complex Quantum Structures in Droplet Epitaxy. Semicond. Sci. Technol. 2019, 34, 095011. [Google Scholar] [CrossRef]
- Noda, T.; Mano, T. Fabrication of a Complex InAs Ring-and-Dot Structure by Droplet Epitaxy. Appl. Surf. Sci. 2008, 254, 7777–7780. [Google Scholar] [CrossRef]
- Mano, T.; Abbarchi, M.; Kuroda, T.; Mastrandrea, C.A.; Vinattieri, A.; Sanguinetti, S.; Sakoda, K.; Gurioli, M. Ultra-Narrow Emission from Single GaAs Self-Assembled Quantum Dots Grown by Droplet Epitaxy. Nanotechnology 2009, 20, 395601. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Koguchi, N.; Gotoh, Y. Fabrication of GaAs Quantum Dots by Modified Droplet Epitaxy. Jpn. J. Appl. Phys. 2000, 39, L79–L81. [Google Scholar] [CrossRef]
- Balakirev, S.V.; Solodovnik, M.S.; Eremenko, M.M.; Chernenko, N.E.; Ageev, O.A. Anomalous Behavior of In Adatoms during Droplet Epitaxy on the AlGaAs Surfaces. Nanotechnology 2020, 31, 485604. [Google Scholar] [CrossRef]
- Lee, S.; Yeo, I.; Jo, M.K.; Jeong, Y.W.; Kim, T.G.; Kim, J.S.; Yi, K.S.; Han, I.K.; Song, J.D. Excition-Phonon Coupling Channels in a ‘Strain-Free’ GaAs Droplet Epitaxy Single Quantum Dot. Curr. Appl. Phys. 2018, 18, 829–833. [Google Scholar] [CrossRef]
- Kim, J.S.; Koguchi, N. Near Room Temperature Droplet Epitaxy for Fabrication of InAs Quantum Dots. Appl. Phys. Lett. 2004, 85, 5893–5895. [Google Scholar] [CrossRef]
- Balakirev, S.V.; Solodovnik, M.S.; Eremenko, M.M.; Konoplev, B.G.; Ageev, O.A. Mechanism of Nucleation and Critical Layer Formation during In/GaAs Droplet Epitaxy. Nanotechnology 2019, 30, 505601. [Google Scholar] [CrossRef]
- Heyn, C.; Stemmann, A.; Schramm, A.; Welsch, H.; Hansen, W.; Nemcsics, A. Regimes of GaAs Quantum Dot Self-Assembly by Droplet Epitaxy. Phys. Rev. B 2007, 76, 075317. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, Z.M.; Salamo, G.J. Observation of Change in Critical Thickness of In Droplet Formation on GaAs(100). J. Phys. Condens. Matter 2007, 19, 176223. [Google Scholar] [CrossRef]
- Kiravittaya, S.; Rastelli, A.; Schmidt, O.G. Self-Assembled InAs Quantum Dots on Patterned GaAs(001) Substrates: Formation and Shape Evolution. Appl. Phys. Lett. 2005, 87, 243112. [Google Scholar] [CrossRef]
- Kim, J.S.; Kawabe, M.; Koguchi, N. Ordering of High-Quality InAs Quantum Dots on Defect-Free Nanoholes. Appl. Phys. Lett. 2006, 88, 072107. [Google Scholar] [CrossRef]
- Song, H.Z.; Usuki, T.; Nakata, Y.; Yokoyama, N.; Sasakura, H.; Muto, S. Formation of InAs/GaAs Quantum Dots from a subcritical InAs Wetting Layer: A Reflection High-Energy Electron Diffraction and Theoretical Study. Phys. Rev. B 2006, 73, 115327. [Google Scholar] [CrossRef]
- Seravalli, L.; Trevisi, G.; Frigeri, P.; Rossi, F.; Buffagni, E.; Ferrari, C. Wetting Layer States in Low Density InAs/InGaAs Quantum Dots from Sub-Critical InAs Coverages. J. Phys. D Appl. Phys. 2013, 46, 315101. [Google Scholar] [CrossRef]
- Mano, T.; Watanabe, K.; Tsukamoto, S.; Fujioka, H.; Oshima, M.; Koguchi, N. Fabrication of InGaAs Quantum Dots on GaAs(001) by Droplet Epitaxy. J. Cryst. Growth 2000, 209, 504–508. [Google Scholar] [CrossRef]
- Benyoucef, M.; Zuerbig, V.; Reithmaier, J.P.; Kroh, T.; Schell, A.W.; Aichele, T.; Benson, O. Single-Photon Emission from Single InGaAs/GaAs Quantum Dots Grown by Droplet Epitaxy at High Substrate Temperature. Nanoscale Res. Lett. 2012, 7, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Mano, T.; Sakaki, H. Anisotropic Diffusion of In Atoms from an In Droplet and Formation of Elliptically Shaped InAs Quantum Dot Clusters on (100) GaAs. Cryst. Growth Des. 2011, 11, 726–728. [Google Scholar] [CrossRef]
- Stevens, M.A.; Tomasulo, S.; Maximenko, S.; Vandervelde, T.E.; Yakes, M.K. Surface Diffusion Measurements of In on InGaAs Enabled by Droplet Epitaxy. J. Appl. Phys. 2017, 121, 195302. [Google Scholar] [CrossRef]
- DeJarld, M.; Reyes, K.; Smereka, P.; Millunchick, J.M. Mechanisms of Ring and Island Formation in Lattice Mismatched Droplet Epitaxy. Appl. Phys. Lett. 2013, 102, 133107. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kim, D.H.; Hong, H.; Woo, J.-C.; Park, S.-J. Growth of InAs Nanocrystals on GaAs(100) by Droplet Epitaxy. J. Cryst. Growth 2000, 212, 67–73. [Google Scholar] [CrossRef]
- Mano, T.; Watanabe, K.; Tsukamoto, S.; Imanaka, Y.; Takamasu, T.; Fujioka, H.; Kido, G.; Oshima, M.; Koguchi, N. InAs Quantum Dots Growth by Modified Droplet Epitaxy Using Sulfur Termination. Jpn. J. Appl. Phys. 2000, 39, 4580–4583. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Y.H.; Xu, B.; Jin, P.; Wang, Z.G. Evolution of InAs Nanostructures Grown by Droplet Epitaxy. Appl. Phys. Lett. 2007, 91, 033112. [Google Scholar] [CrossRef]
- Gajjela, R.S.R.; Koenraad, P.M. Atomic-Scale Characterization of Droplet Epitaxy Quantum Dots. Nanomaterials 2021, 11, 85. [Google Scholar] [CrossRef]
- Park, S.I.; Trojak, O.J.; Lee, E.; Song, J.D.; Kyhm, J.; Han, I.; Kim, J.; Yi, G.-C.; Sapienza, L. GaAs Droplet Quantum Dots with Nanometer-Thin Capping Layer for Plasmonic Applications. Nanotechnology 2018, 29, 205602. [Google Scholar] [CrossRef]
- Ling, H.-S.; Lee, C.-P. Evolution of Self-Assembled InAs Quantum Ring Formation. J. Appl. Phys. 2007, 102, 024314. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zheng, C.X.; Tang, W.X.; Tersoff, J.; Jesson, D.E. Origin of Quantum Ring Formation During Droplet Epitaxy. Phys. Rev. Lett. 2013, 111, 036102. [Google Scholar] [CrossRef]
- Kim, J.S. Ga Migration on a Ga-rich and As-stabilized Surfaces: Ga-droplet and GaAs-nanostructure Formation. Mat. Sci. Semicon. Proc. 2017, 57, 70–76. [Google Scholar] [CrossRef]
- Li, X. Theory of Controllable Shape of Quantum Structures upon Droplet Epitaxy. J. Cryst. Growth 2013, 377, 59–63. [Google Scholar] [CrossRef]
- Balakirev, S.V.; Solodovnik, M.S.; Ageev, O.A. Hybrid Analytical-Monte Carlo Model of In/GaAs(001) Droplet Epitaxy: Theory and Experiment. Phys. Status Solidi B 2018, 255, 1700360. [Google Scholar] [CrossRef]
- Urbańczyk, A.; Hamhuis, G.J.; Nötzel, R. In Islands and Their Conversion to InAs Quantum Dots on GaAs(100): Structural and Optical Properties. J. Appl. Phys. 2010, 107, 014312. [Google Scholar] [CrossRef]
- Huang, S.; Kim, S.J.; Levy, R.; Pan, X.Q.; Goldman, R.S. Mechanisms of InAs/GaAs Quantum Dot Formation during Annealing of In Islands. Appl. Phys. Lett. 2013, 103, 132104. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Wang, Z.M.; Liang, B.; Lee, J.; Kim, E.-S.; Salamo, G.J. Origin of Nanohole Formation by Etching Based on Droplet Epitaxy. Nanoscale 2014, 6, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Stemmann, A.; Heyn, C.; Köppen, T.; Kipp, T.; Hansen, W. Local Droplet Etching of Nanoholes and Rings on GaAs and AlGaAs Surfaces. Appl. Phys. Lett. 2008, 93, 123108. [Google Scholar] [CrossRef]
- Vasilenko, M.A.; Neizvestny, I.G.; Shwartz, N.L. Formation of GaAs Nanostructures by Droplet Epitaxy–Monte Carlo Simulation. Comput. Mater. Sci. 2015, 102, 286–292. [Google Scholar] [CrossRef]
- Somaschini, C.; Bietti, S.; Koguchi, N.; Sanguinetti, S. Fabrication of Multiple Concentric Nanoring Structures. Nano Lett. 2009, 9, 3419–3424. [Google Scholar] [CrossRef]
- Hugues, M.; Teisseire, M.; Chauveau, J.-M.; Vinter, B.; Damilano, B.; Duboz, J.-Y.; Massies, J. Optical Determination of the Effective Wetting Layer Thickness and Composition in InAs/Ga(In)As Quantum Dots. Phys. Rev. B 2007, 76, 075335. [Google Scholar] [CrossRef]
- Fuster, D.; Gonzalez, Y.; Gonzalez, L. Fundamental Role of Arsenic Flux in Nanohole Formation by Ga Droplet Etching on GaAs(001). Nanoscale Res. Lett. 2014, 9, 309–314. [Google Scholar] [CrossRef]
- Reyes, K.; Smereka, P.; Nothern, D.; Millunchik, J.M.; Bietti, S.; Somaschini, C.; Sanguinetti, S.; Frigeri, C. Unified Model of Droplet Epitaxy for Compound Semiconductor Nanostructures: Experiments and Theory. Phys. Rev. B 2013, 87, 165406. [Google Scholar] [CrossRef]


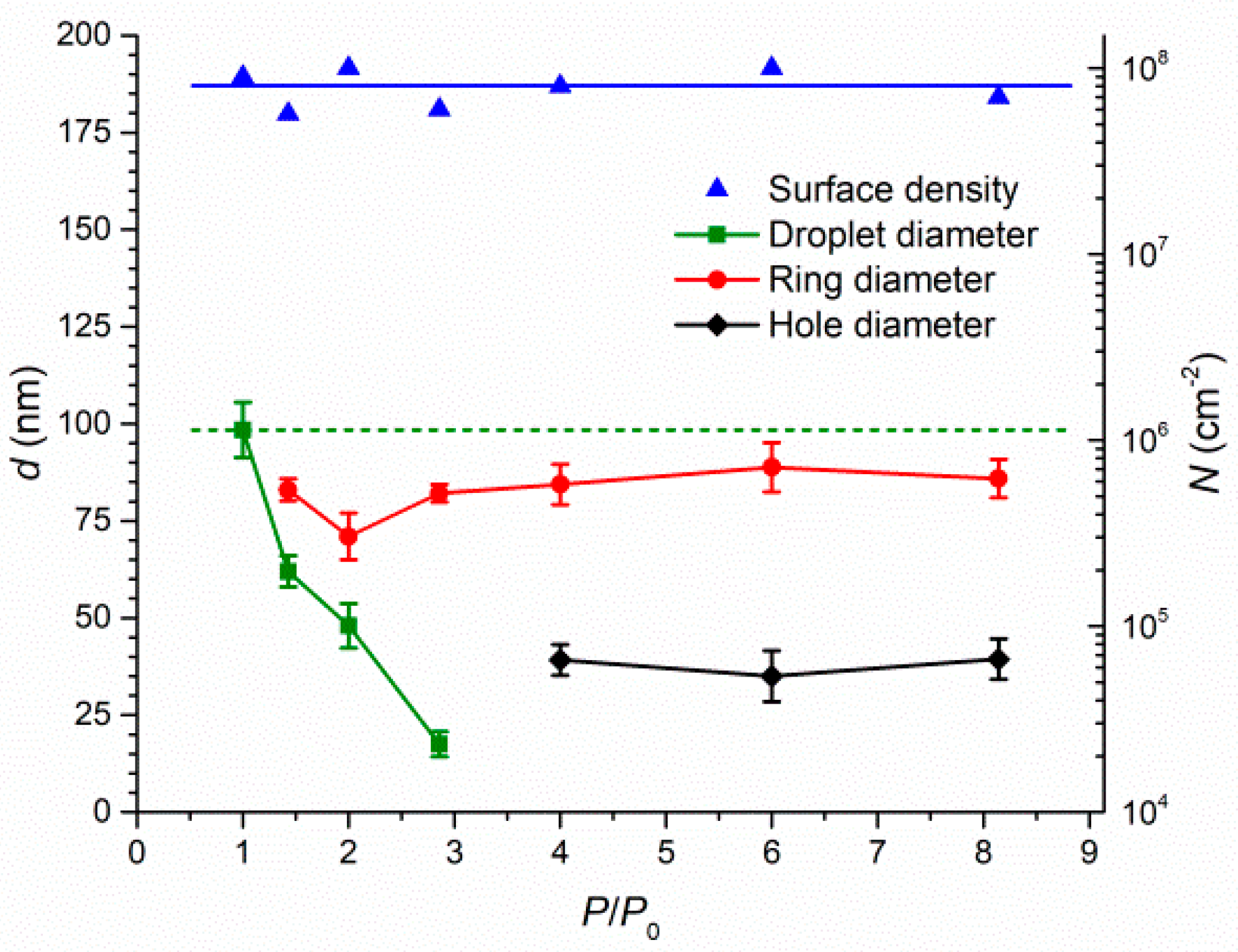

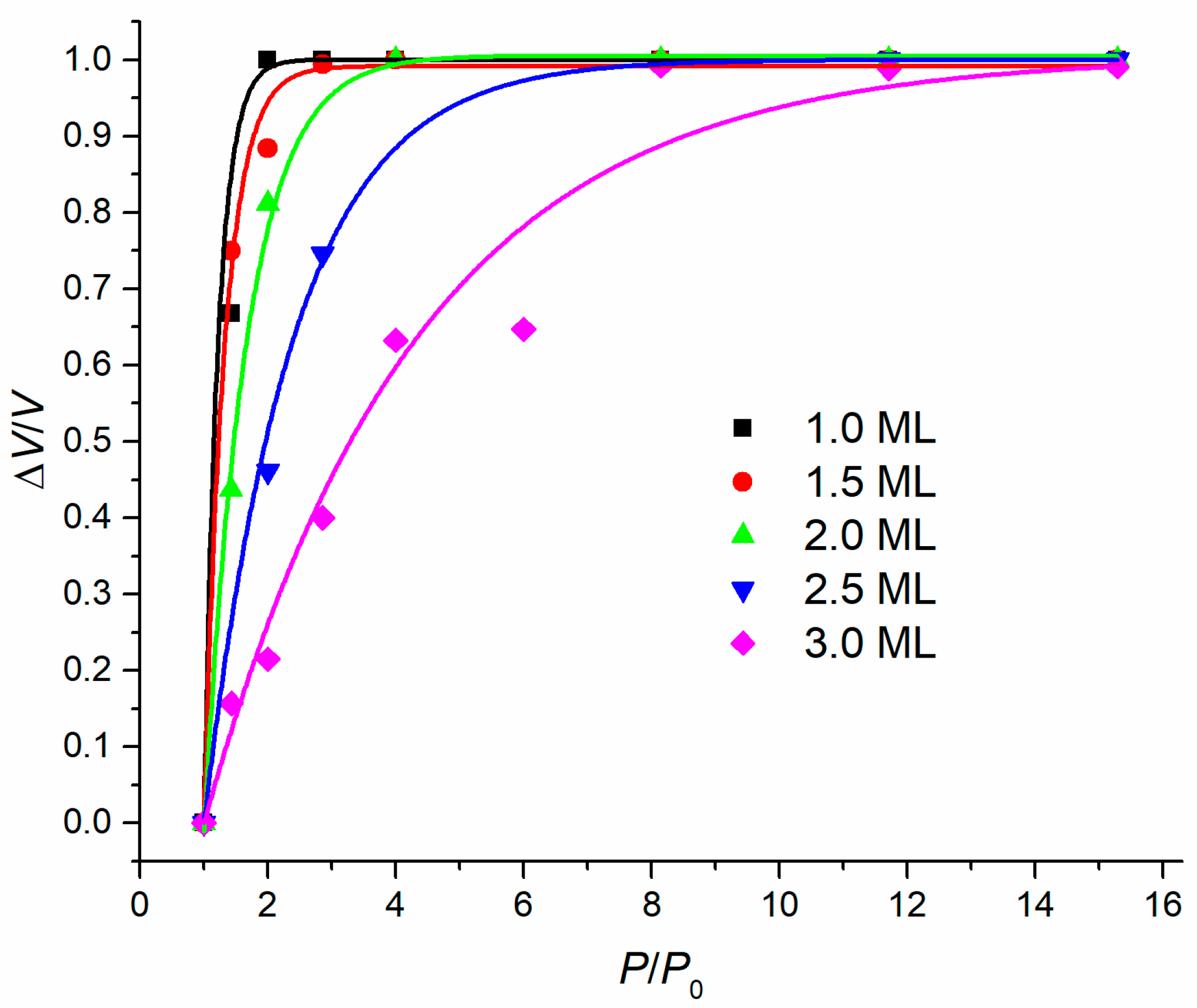

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakirev, S.V.; Chernenko, N.E.; Eremenko, M.M.; Ageev, O.A.; Solodovnik, M.S. Independent Control Over Size and Surface Density of Droplet Epitaxial Nanostructures Using Ultra-Low Arsenic Fluxes. Nanomaterials 2021, 11, 1184. https://doi.org/10.3390/nano11051184
Balakirev SV, Chernenko NE, Eremenko MM, Ageev OA, Solodovnik MS. Independent Control Over Size and Surface Density of Droplet Epitaxial Nanostructures Using Ultra-Low Arsenic Fluxes. Nanomaterials. 2021; 11(5):1184. https://doi.org/10.3390/nano11051184
Chicago/Turabian StyleBalakirev, Sergey V., Natalia E. Chernenko, Mikhail M. Eremenko, Oleg A. Ageev, and Maxim S. Solodovnik. 2021. "Independent Control Over Size and Surface Density of Droplet Epitaxial Nanostructures Using Ultra-Low Arsenic Fluxes" Nanomaterials 11, no. 5: 1184. https://doi.org/10.3390/nano11051184
APA StyleBalakirev, S. V., Chernenko, N. E., Eremenko, M. M., Ageev, O. A., & Solodovnik, M. S. (2021). Independent Control Over Size and Surface Density of Droplet Epitaxial Nanostructures Using Ultra-Low Arsenic Fluxes. Nanomaterials, 11(5), 1184. https://doi.org/10.3390/nano11051184








