Biocompatibility of Bacterial Magnetosomes as MRI Contrast Agent: A Long-Term In Vivo Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Magnetosomes (BMs)
2.2. Preparation of the Polyclonal Antibody of BMs
2.3. Transmission Electron Microscopy (TEM) Analysis
2.4. Size and Zeta Potential Measurement
2.5. Cell Culture
2.6. BMs Internalization into Magcrophages
2.7. Immunocytofluorescence (ICF) Assay
2.8. Prussian Blue Staining
2.9. Animal Experiment
2.10. Mice Magnetic Resonance Imaging (MRI)
2.11. Immunohistochemistry (IHC) and HE Staining of Mice Tissue
2.12. The Distribution of BMs in Mice
2.13. ICP-MS
2.14. Cardiac Perfusion
2.15. Statistical Analysis
3. Results
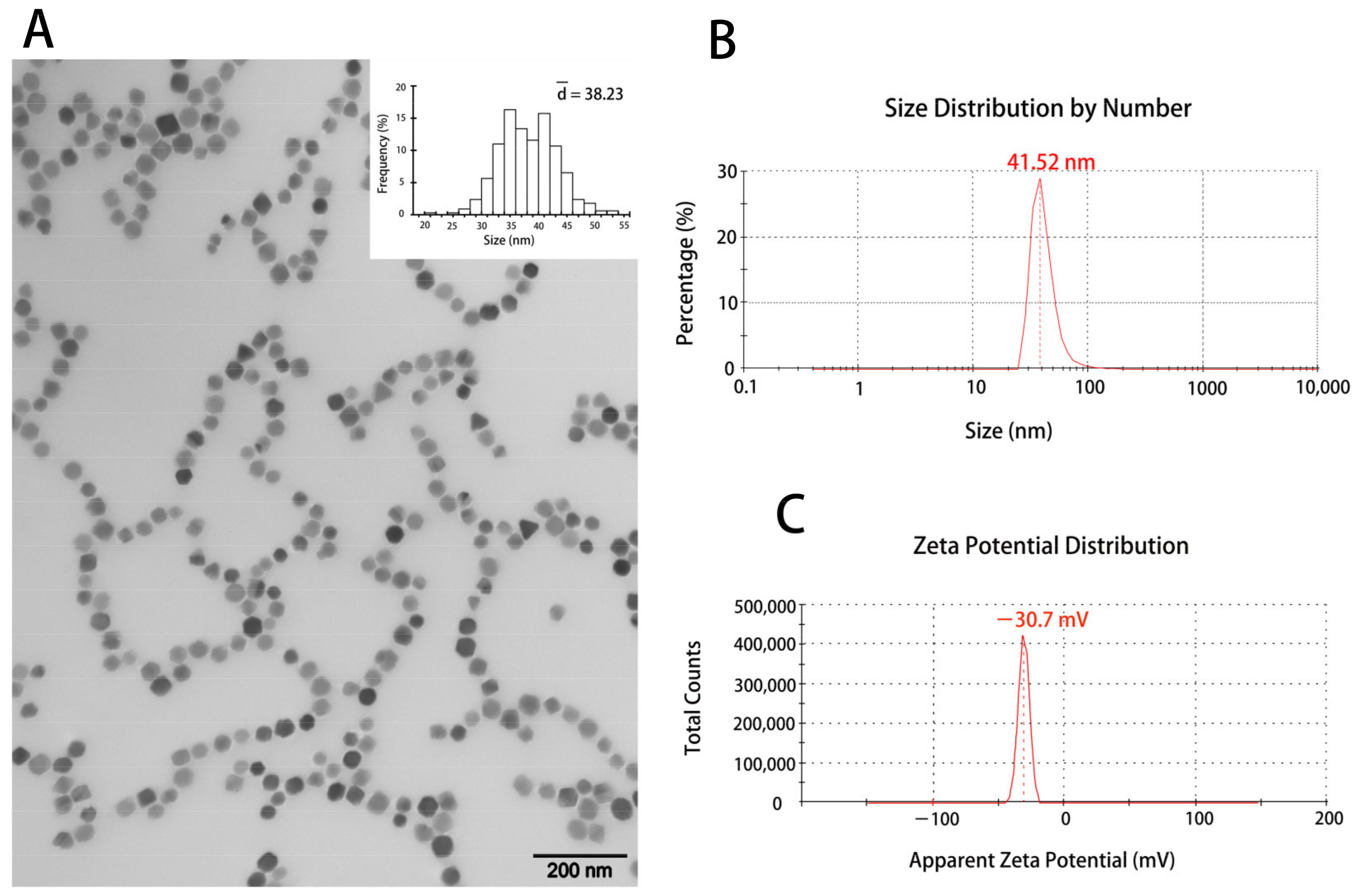
3.1. The Characterization of the Magnetosomes (BMs)
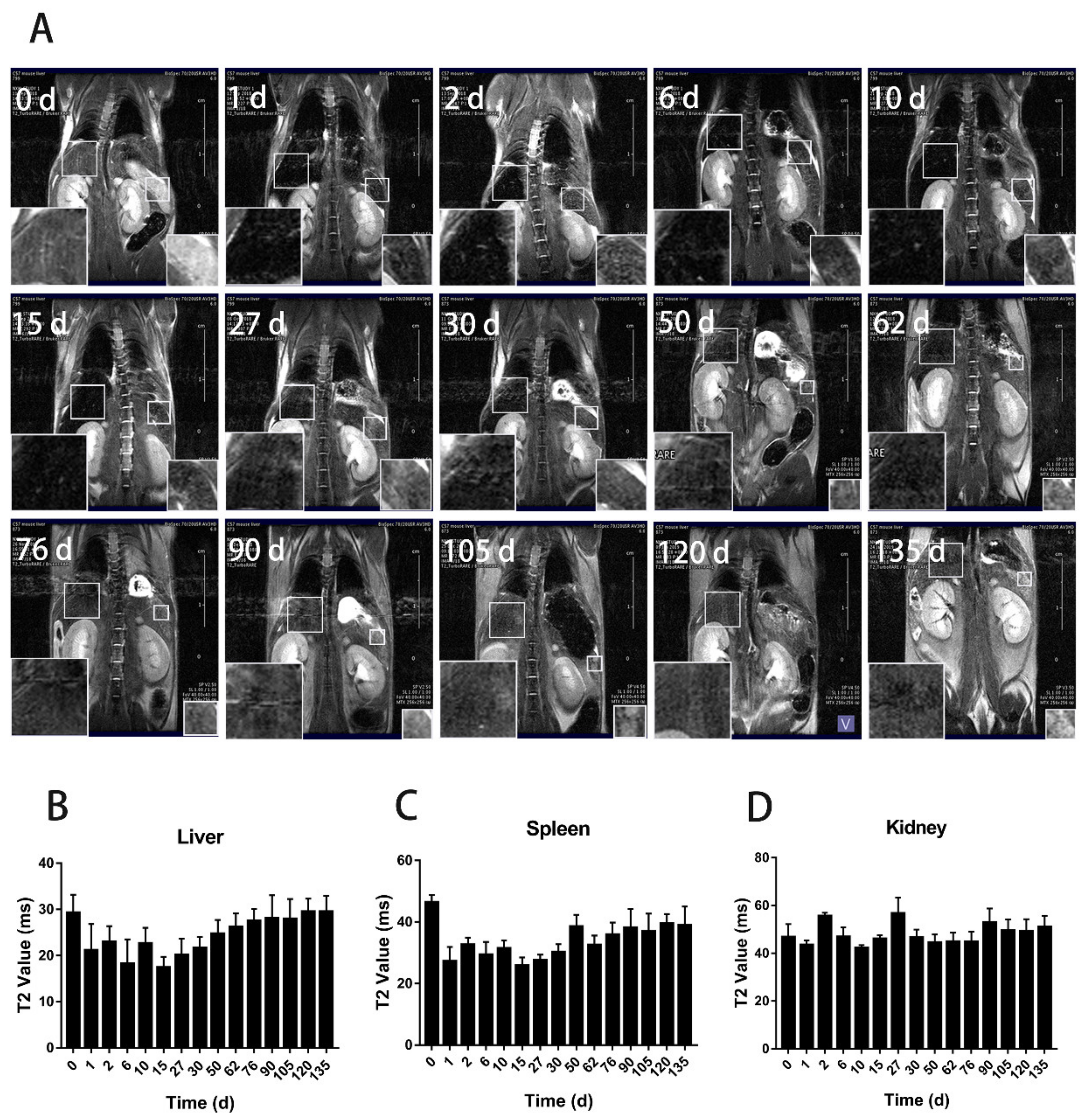
3.2. The Distribution of BMs in Mice by MRI
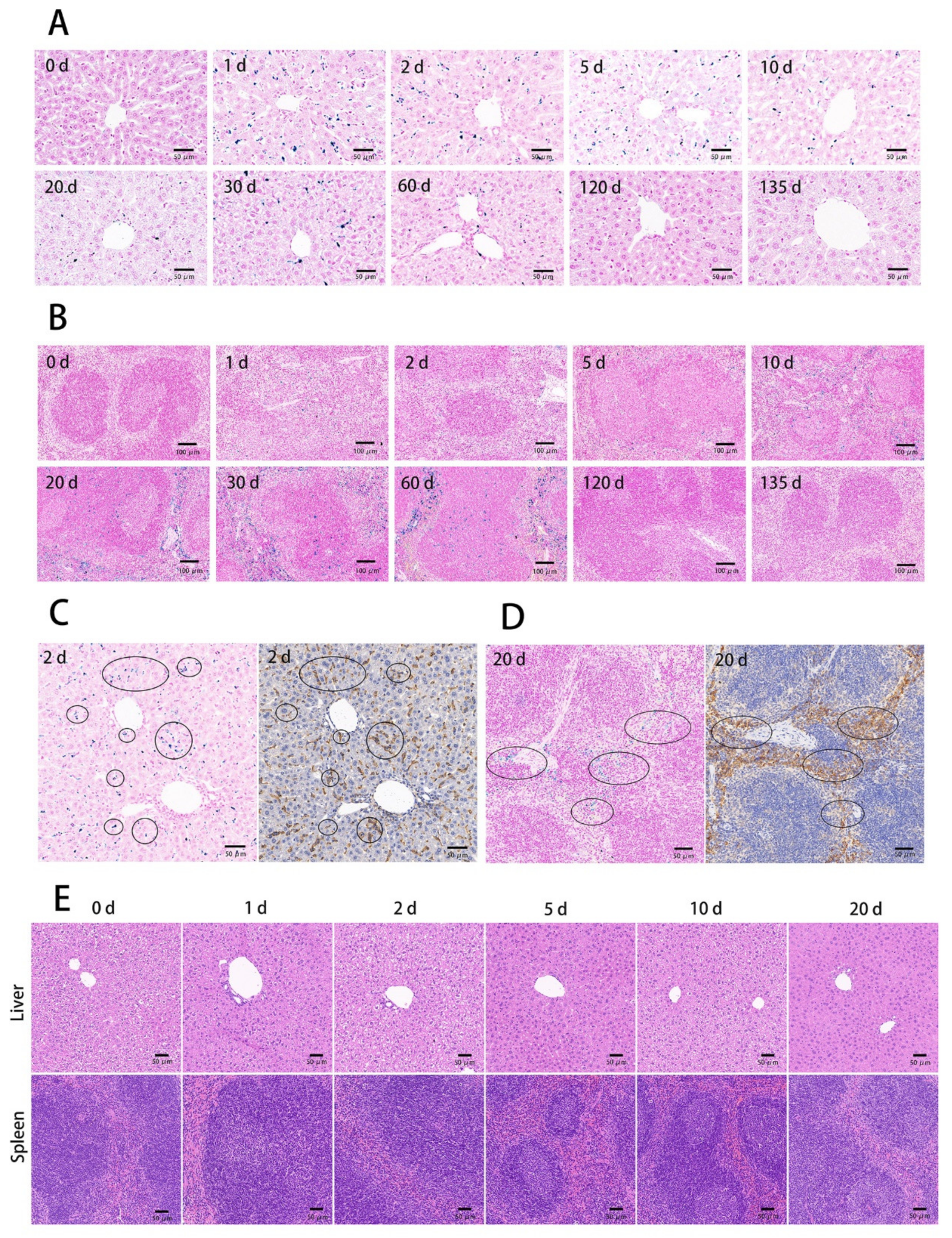
3.3. The Tissue Ultrathin Section Staining
3.4. ICP-MS Analysis
3.5. The Internalization and Elimination of BMs in Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blakemore, R. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Balkwill, D.L.; Maratea, D.; Blakemore, R.P. Ultrastructure of a Magnetotactic Spirillum. J. Bacteriol. 1980, 141, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Gorby, Y.A.; Beveridge, T.J.; Blakemore, R.P. Characterization of the bacterial magnetosome membrane. J. Bacteriol. 1988, 170, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Farina, M.; Esquivel, D.M.S.; de Barros, H.G.L. Magnetic iron-sulphur crystals from a magnetotactic microorganism. Nature 1990, 343, 256–258. [Google Scholar] [CrossRef]
- Frankel, R.B.; Blakemore, R.P.; Wolfe, R.S. Magnetite in Freshwater Magnetotactic Bacteria. Science 1979, 203, 1355–1356. [Google Scholar] [CrossRef] [Green Version]
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Genet. 2004, 2, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Abreu, F.P.; Silva, K.T.; Farina, M.; Keim, C.N.; Lins, U. Greigite magnetosome membrane ultrastructure in ’Candidatus Magnetoglobus multicellularis’. Int. Microbiol. 2008, 11, 75–80. [Google Scholar] [PubMed]
- Grünberg, K.; Müller, E.C.; Otto, A.; Reszka, R.; Linder, D.; Kube, M.; Reinhardt, R.; Schüler, D. Biochemical and Proteomic Analysis of the Magnetosome Membrane in Magnetospirillum gryphiswaldense. Appl. Environ. Microbiol. 2004, 70, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, D.; Schüler, D. Magnetotactic Bacteria and Magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef]
- Schüler, D.; Frankel, R.B. Bacterial magnetosomes: Microbiology, biomineralization and biotechnological applications. Appl. Microbiol. Biotechnol. 1999, 52, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Bazylinski, D.A.; Garratt-Reed, A.J.; Frankel, R.B. Electron microscopic studies of magnetosomes in magnetotactic bacteria. Microsc. Res. Tech. 1994, 27, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Dieudonné, A.; Pignol, D.; Prévéral, S. Magnetosomes: Biogenic iron nanoparticles produced by environmental bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 3637–3649. [Google Scholar] [CrossRef]
- Scheffel, A.; Gruska, M.; Faivre, D.; Linaroudis, A.; Plitzko, J.M.; Schüler, D. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nat. Cell Biol. 2006, 440, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Applications of magnetotactic bacteria and magnetosome for cancer treatment: A review emphasizing on practical and mechanistic aspects. Drug Discov. Today 2020, 25, 1444–1452. [Google Scholar] [CrossRef]
- Hu, L.L.; Zhang, F.; Wang, Z.; You, X.F.; Nie, L.; Wang, H.X.; Song, T.; Yang, W.H. Comparison of the 1H1H NMR Relaxation Enhancement Produced by Bacterial Magnetosomes and Synthetic Iron Oxide Nanoparticles for Potential Use as MR Molecular Probes. IEEE Trans. Appl. Supercond. 2010, 20, 822–825. [Google Scholar] [CrossRef]
- Tartaj, P.; del Puerto Morales, M.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Boucher, M.; Geffroy, F.; Prévéral, S.; Bellanger, L.; Selingue, E.; Adryanczyk-Perrier, G.; Pean, M.; Lefèvre, C.T.; Pignol, D.; Ginet, N.; et al. Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 2016, 121, 167–178. [Google Scholar] [CrossRef]
- Benoit, M.R.; Mayer, D.; Barak, Y.; Chen, I.Y.; Hu, W.; Cheng, Z.; Wang, S.X.; Spielman, D.M.; Gambhir, S.S.; Matin, A. Visualizing Implanted Tumors in Mice with Magnetic Resonance Imaging Using Magnetotactic Bacteria. Clin. Cancer Res. 2009, 15, 5170–5177. [Google Scholar] [CrossRef] [Green Version]
- Mériaux, S.; Boucher, M.; Marty, B.; Lalatonne, Y.; Prévéral, S.; Motte, L.; Lefèvre, C.T.; Geffroy, F.; Lethimonnier, F.; Péan, M.; et al. Magnetosomes, Biogenic Magnetic Nanomaterials for Brain Molecular Imaging with 17.2 T MRI Scanner. Adv. Healthc. Mater. 2015, 4, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Erdal, E.; Demirbilek, M.; Yeh, Y.; Akbal, Ö.; Ruff, L.; Bozkurt, D.; Cabuk, A.; Senel, Y.; Gumuskaya, B.; Algın, O.; et al. A Comparative Study of Receptor-Targeted Magnetosome and HSA-Coated Iron Oxide Nanoparticles as MRI Contrast-Enhancing Agent in Animal Cancer Model. Appl. Biochem. Biotechnol. 2018, 185, 91–113. [Google Scholar] [CrossRef]
- Kraupner, A.; Eberbeck, D.; Heinke, D.; Uebe, R.; Schüler, D.; Briel, A. Bacterial magnetosomes-nature’s powerful contribution to MPI tracer research. Nanoscale 2017, 9, 5788–5793. [Google Scholar] [CrossRef]
- Sun, J.; Tang, T.; Duan, J.; Xu, P.-X.; Wang, Z.; Zhang, Y.; Wu, L.; Li, Y. Biocompatibility of bacterial magnetosomes: Acute toxicity, immunotoxicity and cytotoxicity. Nanotoxicology 2010, 4, 271–283. [Google Scholar] [CrossRef]
- Yan, L.; Yue, X.; Zhang, S.; Chen, P.; Xu, Z.; Li, Y.; Li, H. Biocompatibility evaluation of magnetosomes formed by Acidithiobacillus ferrooxidans. Mater. Sci. Eng. C 2012, 32, 1802–1807. [Google Scholar] [CrossRef]
- Xiang, Z.; Yang, X.; Xu, J.; Lai, W.; Wang, Z.; Hu, Z.; Tian, J.; Geng, L.; Fang, Q. Tumor detection using magnetosome nanoparticles functionalized with a newly screened EGFR/HER2 targeting peptide. Biomaterials 2017, 115, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-García, J.; Das, R.; Garaio, E.; García, J.Á.; Phan, M.H.; Srikanth, H.; Orue, I.; et al. Unlocking the potential of magnetotactic bacteria as magnetic hyperthermia agents. Small 2019, 15, 1902626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Fèvre, R.; Durand-Dubief, M.; Chebbi, I.; Mandawala, C.; Lagroix, F.; Valet, J.-P.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; et al. Enhanced antitumor efficacy of biocompatible magnetosomes for the magnetic hyperthermia treatment of glioblastoma. Theranostics 2017, 7, 4618–4631. [Google Scholar] [CrossRef]
- Xiang, Z.; Jiang, G.; Yang, X.; Fan, D.; Nan, X.; Li, D.; Hu, Z.; Fang, Q. Peptosome Coadministration Improves Nanoparticle Delivery to Tumors through NRP1-Mediated Co-Endocytosis. Biomolecules 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Hu, J.; Liu, L.; Li, L.; Wang, X.; Zhang, H.; Jiang, W.; Tian, J.; Li, Y.; Li, J. Surface expression of protein A on magnetosomes and capture of pathogenic bacteria by magnetosome/antibody complexes. Front. Microbiol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Matsunaga, T.; Hashimoto, K.; Nakamura, N.; Nakamura, K.; Hashimoto, S. Phagocytosis of bacterial magnetite by leucocytes. Appl. Microbiol. Biotechnol. 1989, 31, 401–405. [Google Scholar] [CrossRef]
- Honda, T.; Tanaka, T.; Yoshino, T. Stoichiometrically Controlled Immobilization of Multiple Enzymes on Magnetic Nanoparticles by the Magnetosome Display System for Efficient Cellulose Hydrolysis. Biomacromolecules 2015, 16, 3863–3868. [Google Scholar] [CrossRef]
- Xiang, L.; Wei, J.; Jianbo, S.; Guili, W.; Feng, G.; Ying, L. Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett. Appl. Microbiol. 2007, 45, 75–81. [Google Scholar] [CrossRef]
- Hamdous, Y.; Chebbi, I.; Mandawala, C.; Le Fèvre, R.; Guyot, F.; Seksek, O.; Alphandéry, E. Biocompatible coated magnetosome minerals with various organization and cellular interaction properties induce cytotoxicity towards RG-2 and GL-261 glioma cells in the presence of an alternating magnetic field. J. Nanobiotechnol. 2017, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Rodríguez, R.E.; Díaz, R.C. Copper and Zinc Levels in Urine: Relationship to Various Individual Factors. J. Trace Elem. Med. Biol. 1995, 9, 200–209. [Google Scholar] [CrossRef]
- Du, B.; Yu, M.; Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 2018, 3, 358–374. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1559S–1566S. [Google Scholar] [CrossRef] [Green Version]
- Dobrinska, M.R. Enterohepatic circulation of drugs. J. Clin. Pharmacol. 1989, 29, 577–580. [Google Scholar] [CrossRef]
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic Circulation. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, X.; Lai, W.; Li, D.; Tian, J.; Hu, Z.; Fang, Q. Biocompatibility of Bacterial Magnetosomes as MRI Contrast Agent: A Long-Term In Vivo Follow-Up Study. Nanomaterials 2021, 11, 1235. https://doi.org/10.3390/nano11051235
Nan X, Lai W, Li D, Tian J, Hu Z, Fang Q. Biocompatibility of Bacterial Magnetosomes as MRI Contrast Agent: A Long-Term In Vivo Follow-Up Study. Nanomaterials. 2021; 11(5):1235. https://doi.org/10.3390/nano11051235
Chicago/Turabian StyleNan, Xiaohui, Wenjia Lai, Dan Li, Jiesheng Tian, Zhiyuan Hu, and Qiaojun Fang. 2021. "Biocompatibility of Bacterial Magnetosomes as MRI Contrast Agent: A Long-Term In Vivo Follow-Up Study" Nanomaterials 11, no. 5: 1235. https://doi.org/10.3390/nano11051235






