Nanostructured ZnFe2O4: An Exotic Energy Material
Abstract
:1. Introduction
2. Material Properties of ZnFe2O4
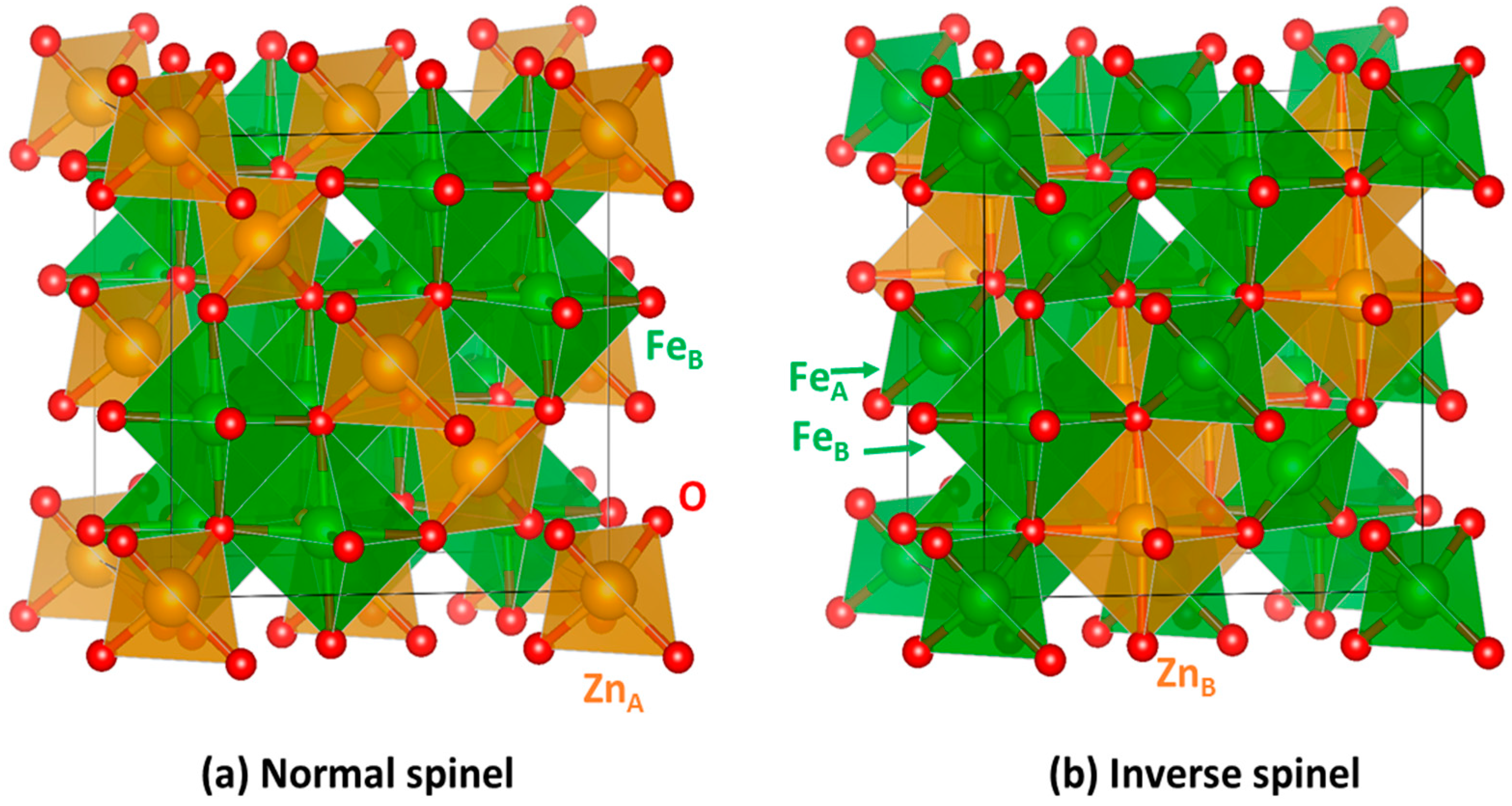
2.1. Bulk Crystalline and Spin Structure
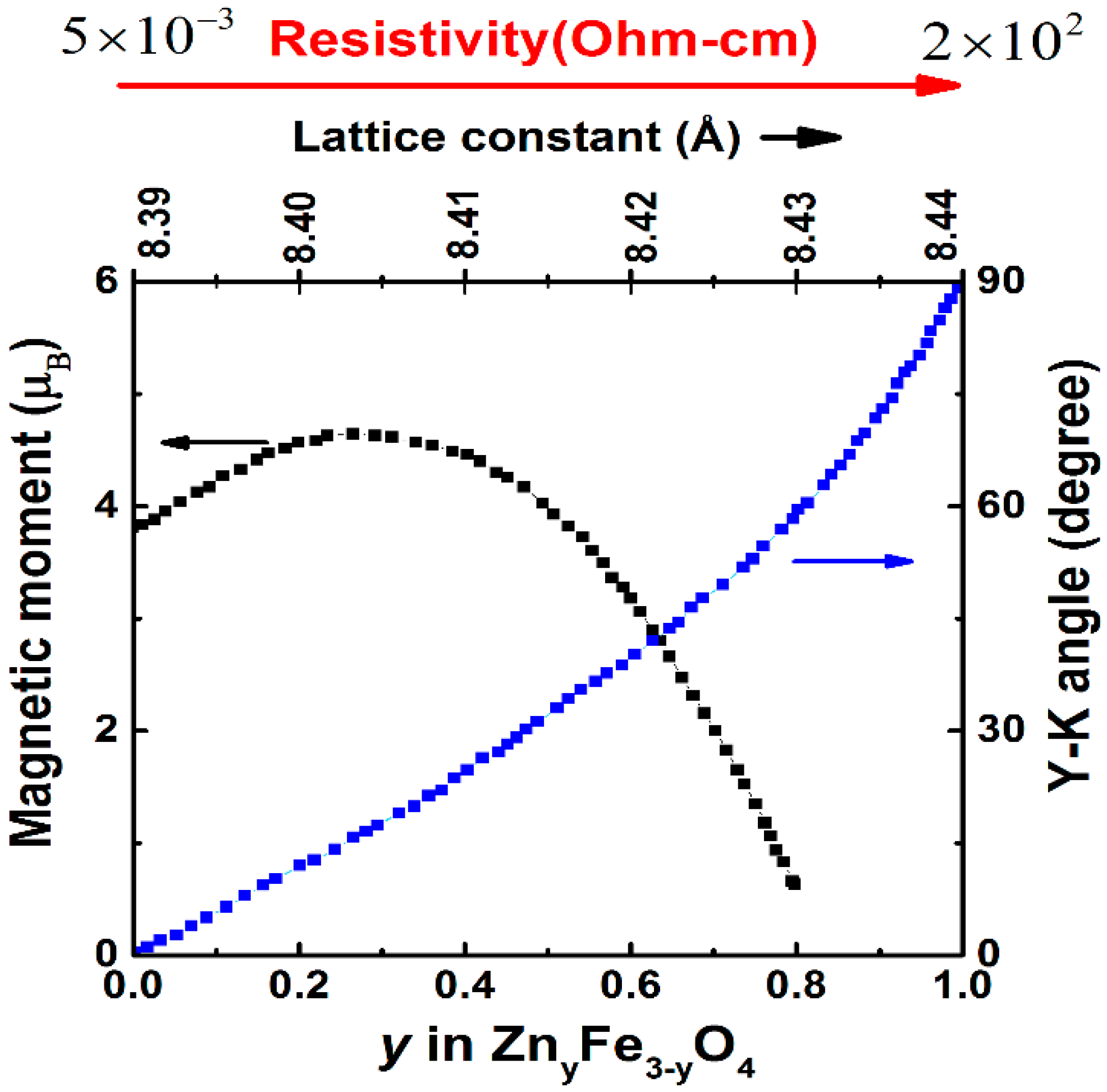
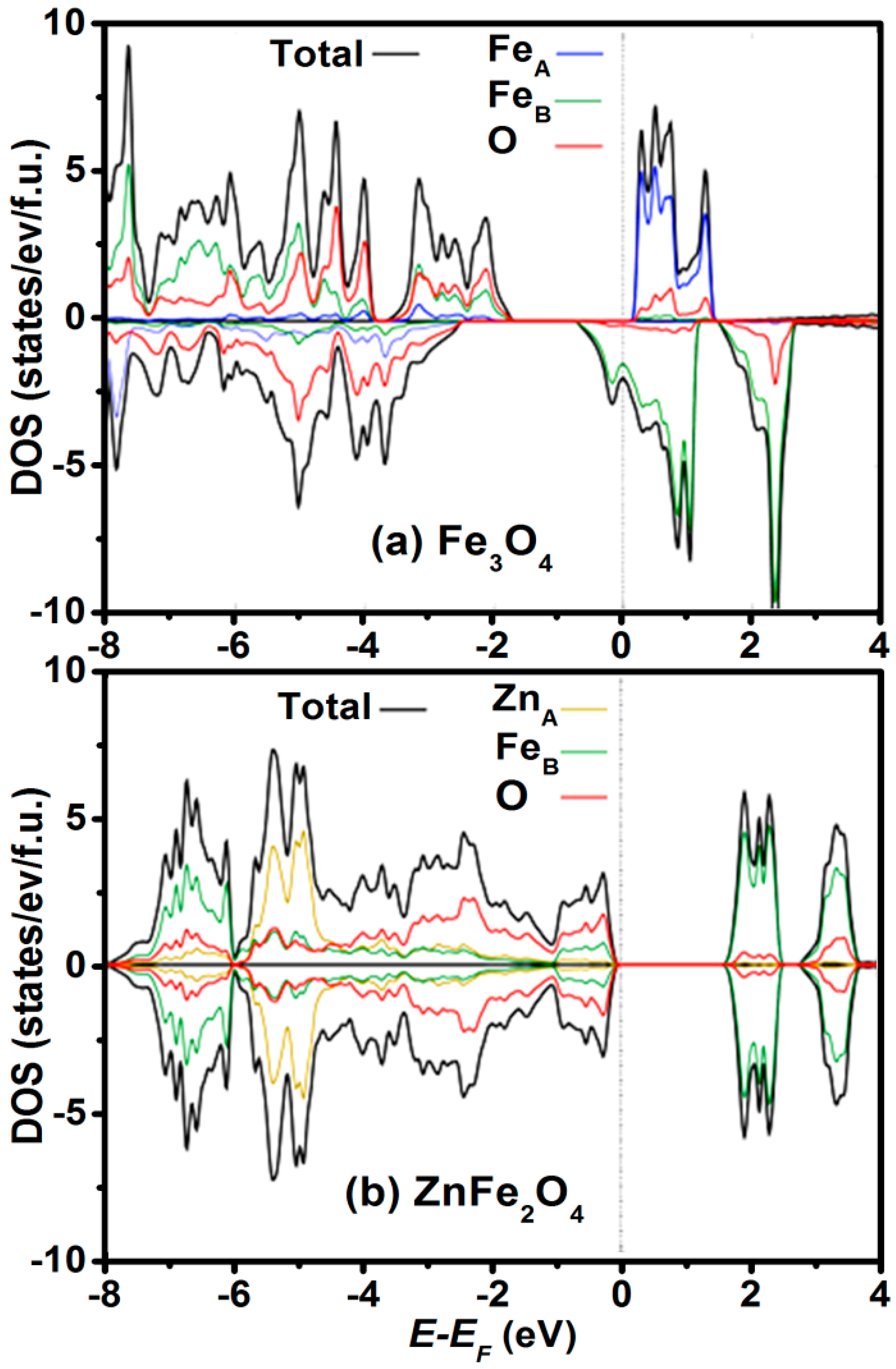
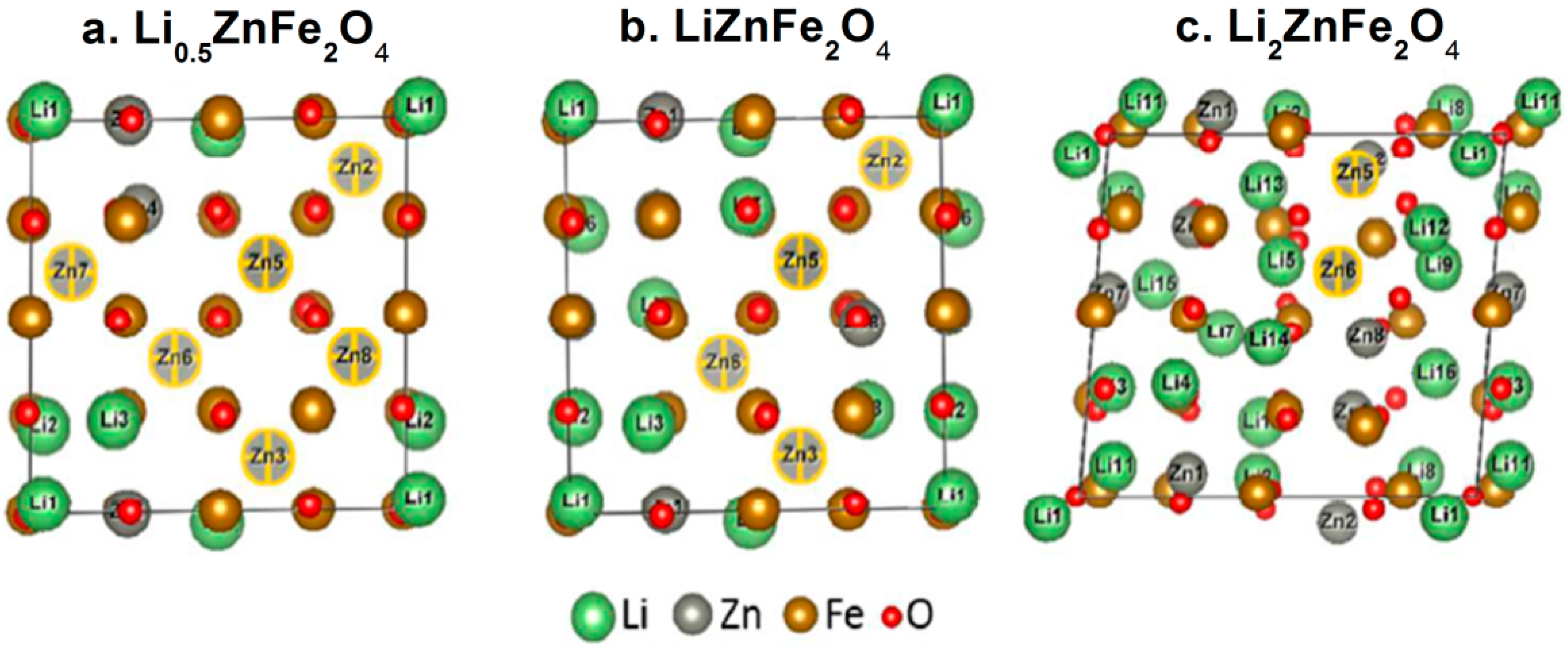
2.2. Cation Engineering in Nano Regime
3. Various ZnFe2O4 Nanostructure Morphologies
3.1. Nanoparticles (1 nm < Particle Size < 100 nm)
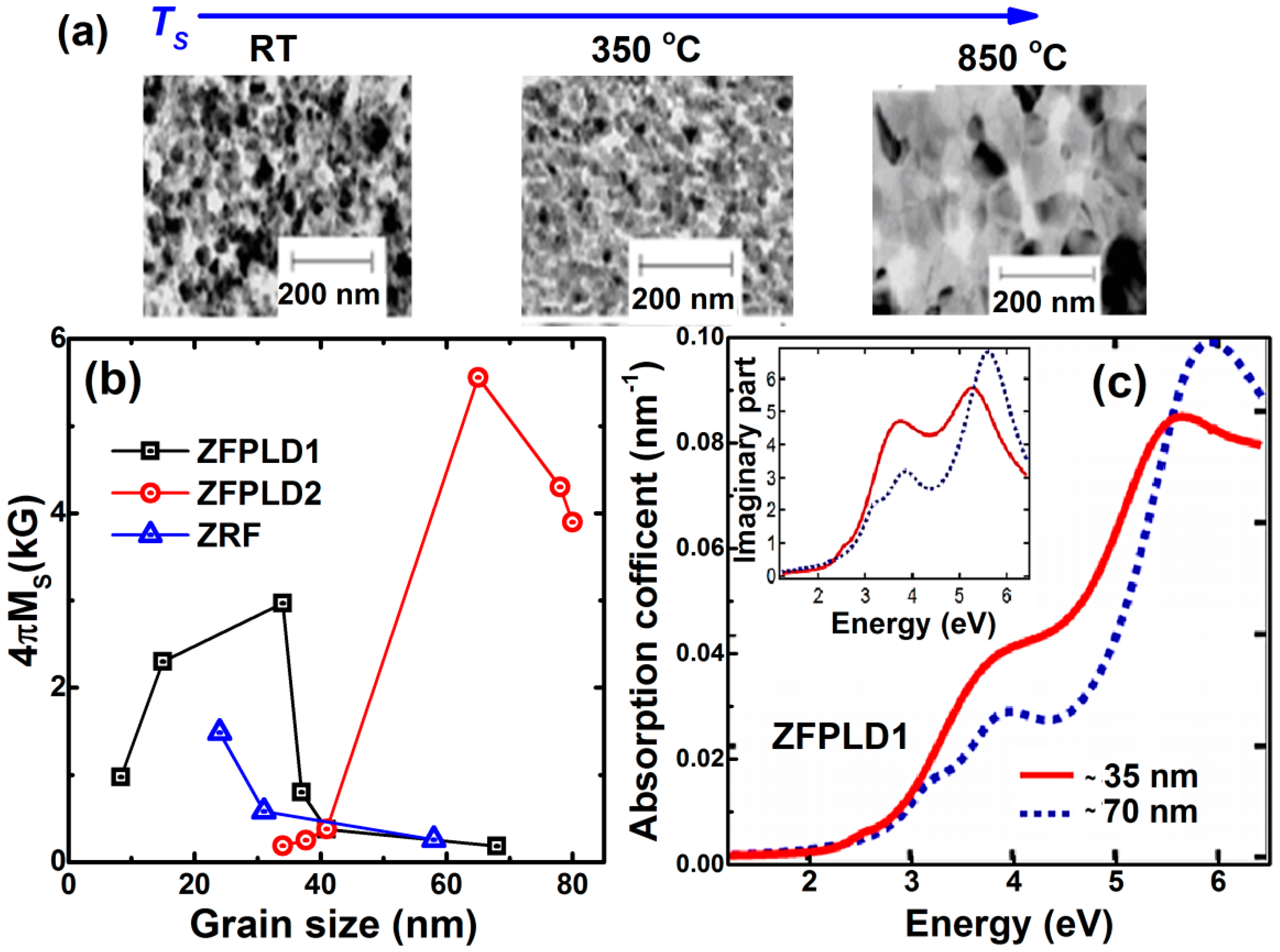
3.2. Nanocrystalline Thin Films (1 nm < Grain Size < 100 nm)
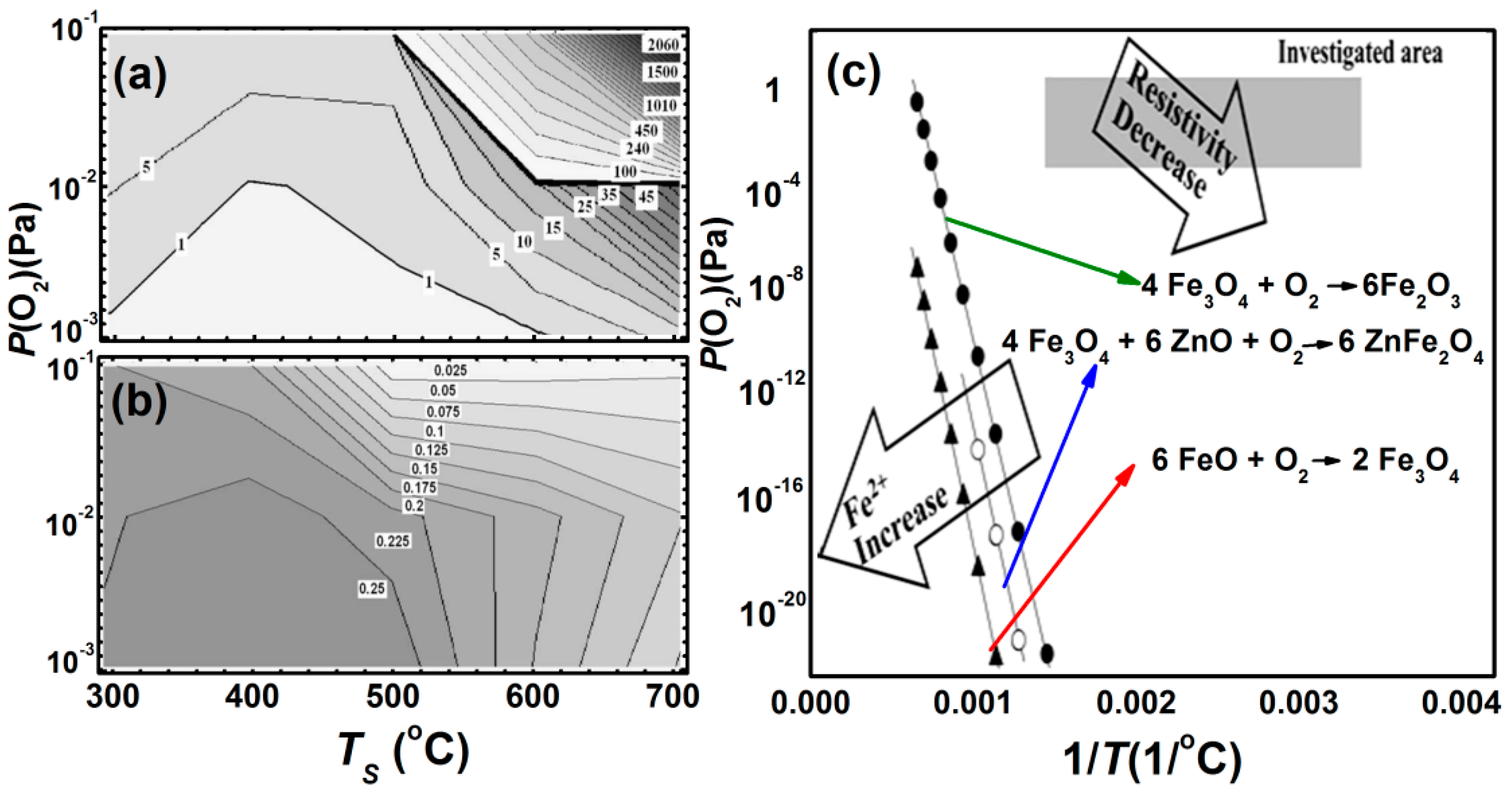
3.3. Epitaxial Films (1 nm < Nano-Thick < 200 nm)
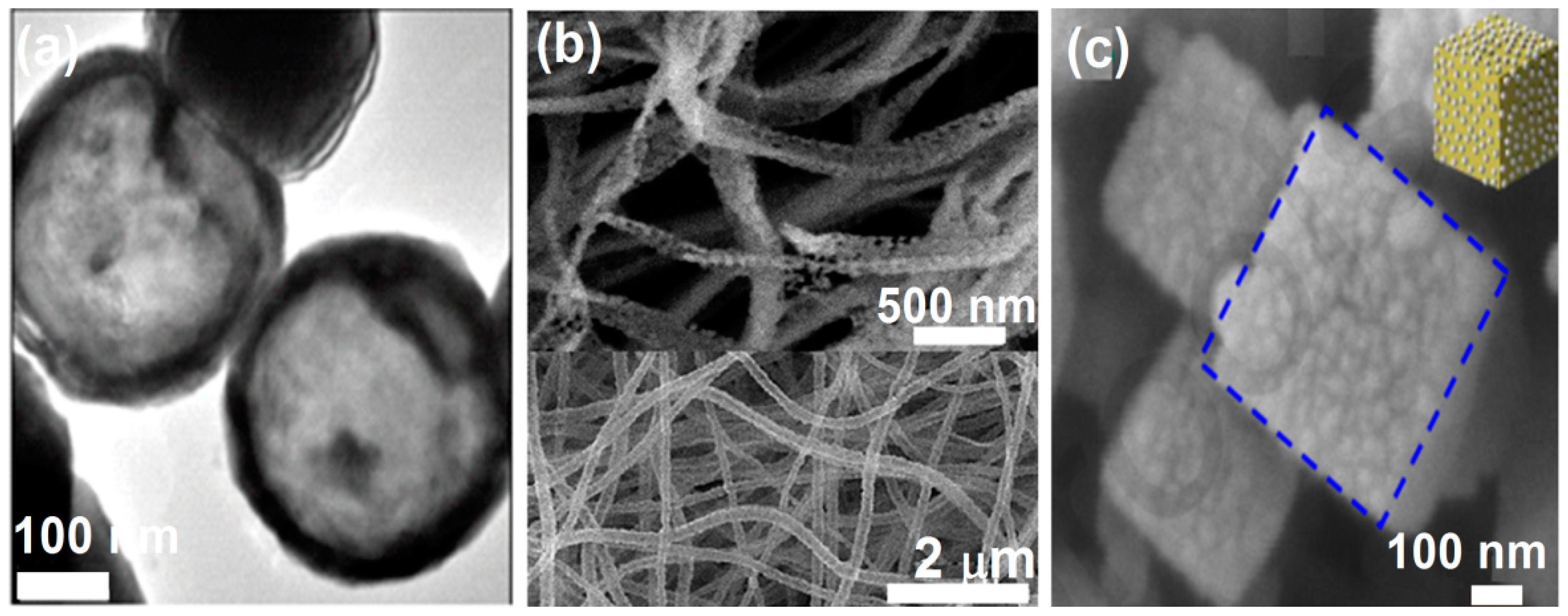
3.4. Other Nanostructured ZnFe2O4 Geometries
4. Applications
4.1. Exchange Coupling
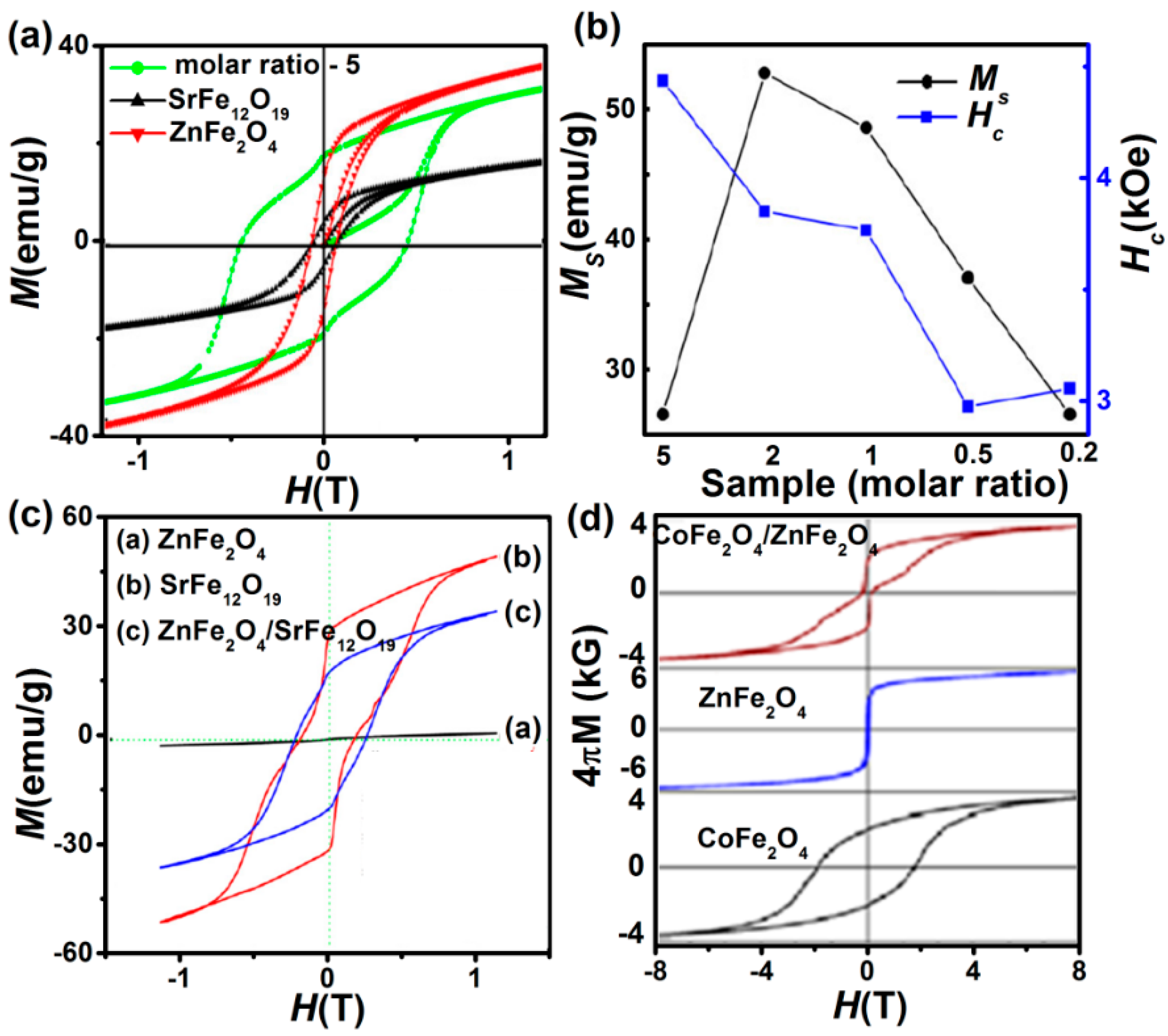
4.1.1. Exchange Spring System (Soft + Hard Ferrite)
4.1.2. Exchange Bias (AFM/FM Interfaces)
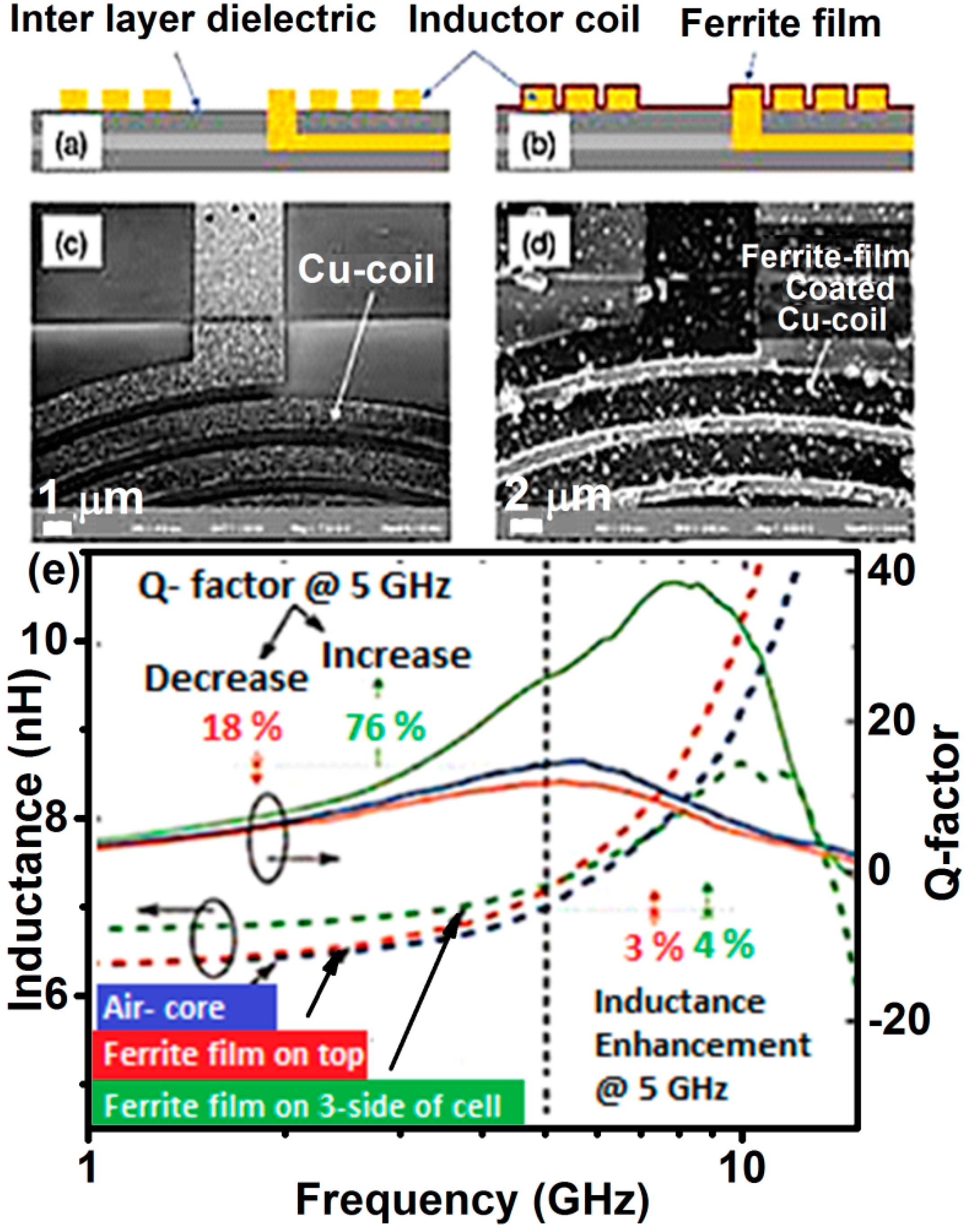
4.2. High-Frequency Applications
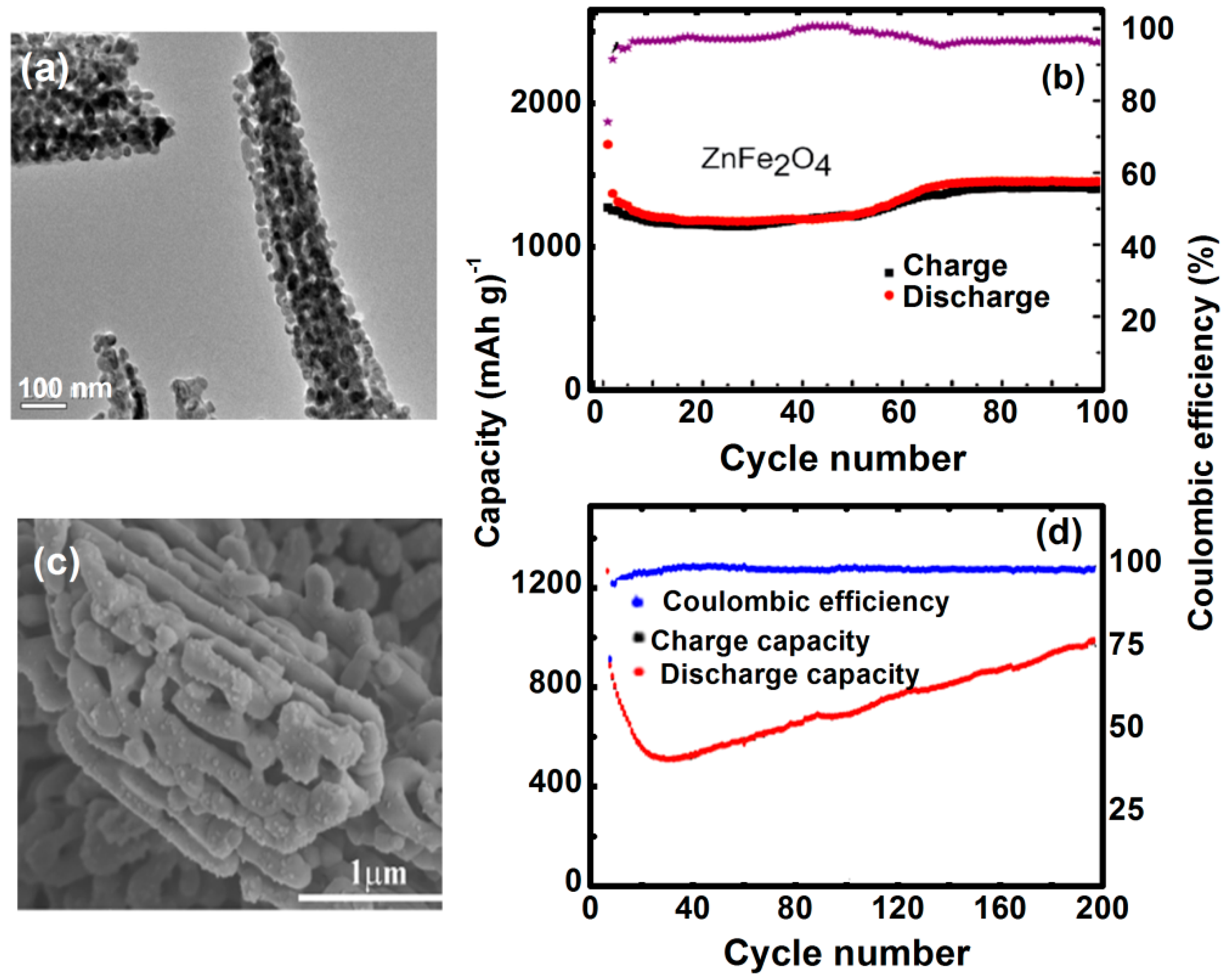
4.3. Lithium-Ion Batteries
| Morphology | Reversible Capacity mAh g−1 | Cycle | Current Rate mA g−1 | Ref. |
|---|---|---|---|---|
| Thin film | 434 | 100 | 10 | [78] |
| Nanoparticles | 841 | 50 | 60 | [79] |
| Nanofibers | 733 | 30 | 60 | [80] |
| Nano-octahedrons | 910 | 80 | 60 | [81] |
| Nanorod | 900 | 50 | 100 | [82] |
| Cubic nanoparticles | 367 | 50 | 60 | [83] |
| Hollow spheres | 900 | 50 | 65 | [70] |
| Hollow microspheres | 1200 | 120 | 100 | [84] |
| Hollow nanospheres | 1101 | 120 | 200 | [85] |
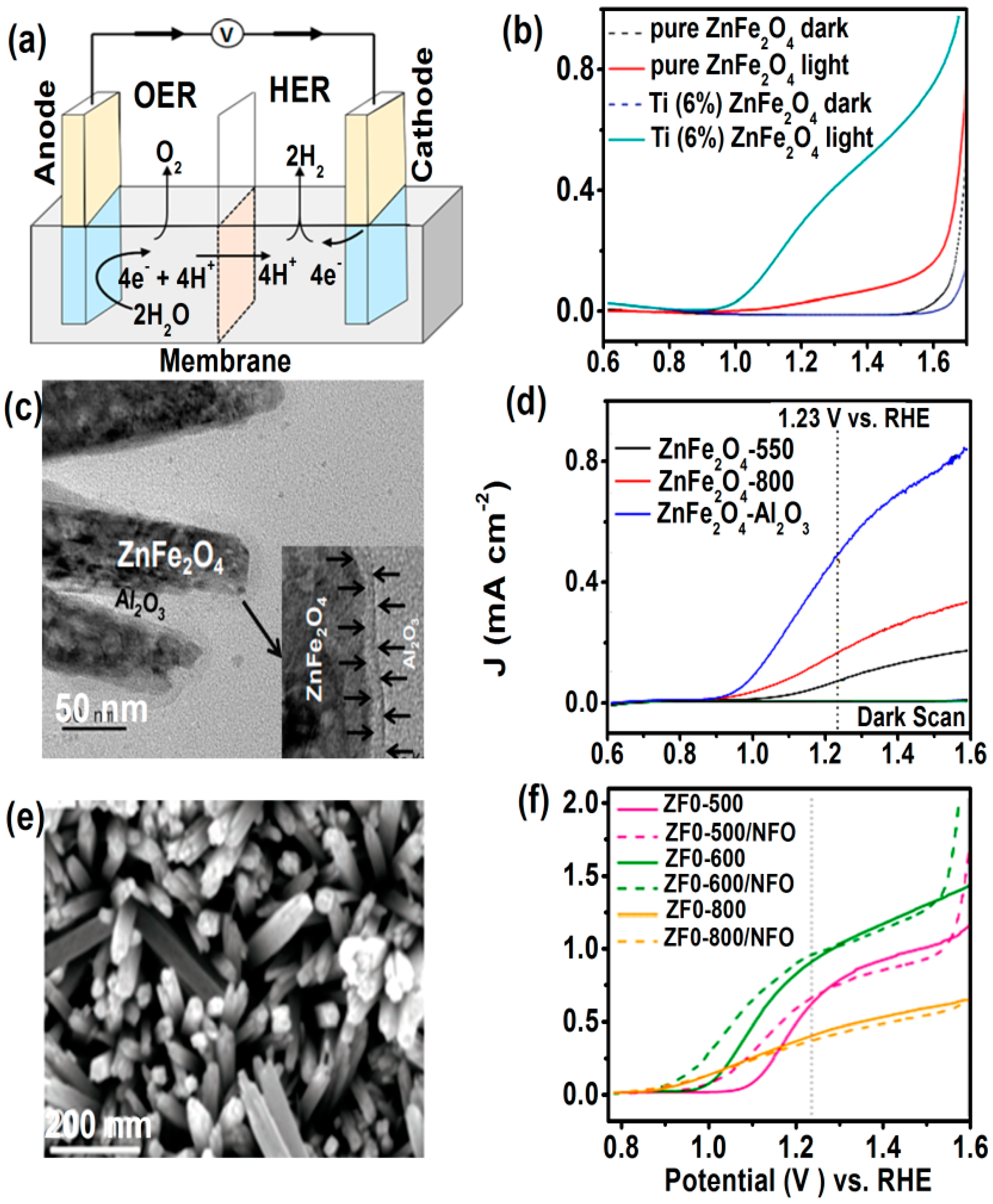
4.4. Photoelectrochemical (PEC) Water Splitting
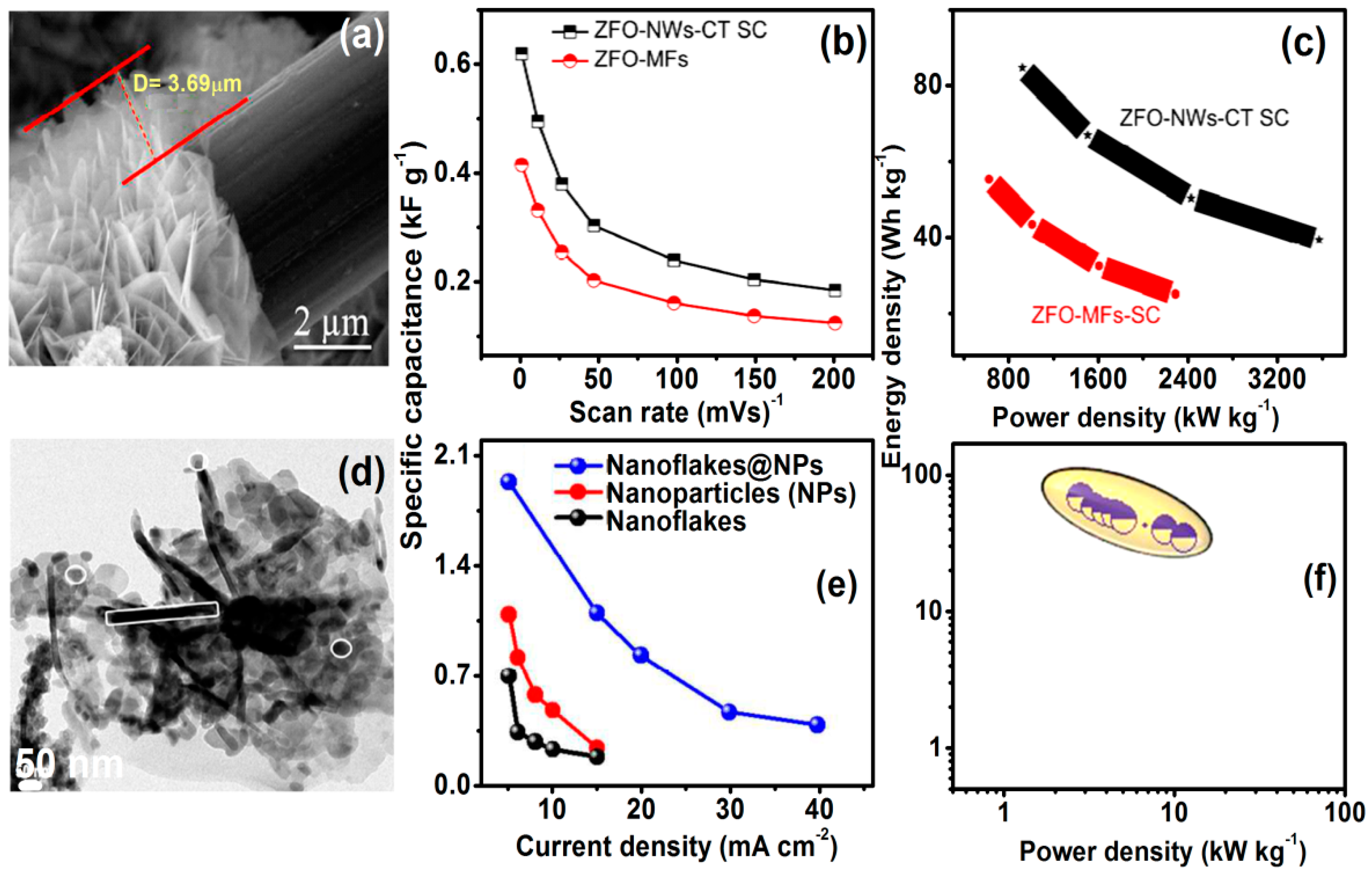
4.5. Electrochemical Supercapacitors
5. Conclusions
- Different spintronics devices, possibly with low-energy operation cost, can be constructed by using an inverted stoichiometric ZnFe2O4 thin film as ferrimagnetic layer in magnetic tunnel junctions, as a barrier layer in spin filtering devices, oxygen-deficient ZnyFe3−yO4−δ thin film as a conducting layer could be used in homo-epitaxial devices, provided with a fine control of the stoichiometry during the growth.
- Inverted ZnFe2O4 thin layer with low microwave loss can be a potential material for high-frequency applications, such as 5G mobile communication.
- Inverted ZnFe2O4 nanostructures are emerging photoanode material for photoelectrochemical solar fuel productions. Cation disorder in ZnFe2O4 facilitates photogenerated charge separation and increased charge carrier transport.
- ZnFe2O4 used as an electrode in a Li-ion battery demonstrated large charge/discharge capacity and cycle stability. Highly porous surface and wide voids in ZnFe2O4 nanostructures play a critical role in enhancing electrochemical reactions. The suitable cathode and stable electrolyte materials are the prerequisite to form ZnFe2O4-based Li-ion battery considering high working voltage of electrode.
- Various ZnFe2O4-based heterostructures and nanocomposites with high conducting property can boost cycle stability and energy density for high-performance supercapacitors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dusastre, V.; Martiradonna, L. Materials for sustainable energy. Nat. Mater. 2017, 16, 15. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Chua, C.S.; Kushwaha, A.; Liew, S.L.; Suresh, V.; Chi, D. All earth abundant materials for low cost solar-driven hydrogen production. Mater. Lett. 2016, 183, 183–186. [Google Scholar] [CrossRef]
- Jang, J.-W.; Du, C.; Ye, Y.; Lin, Y.; Yao, X.; Thorne, J.; Liu, E.; McMahon, G.; Zhu, J.; Javey, A.; et al. Enabling unassisted solar water splitting by iron oxide and silicon. Nat. Commun. 2015, 6, 7447–7452. [Google Scholar]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Abd Elkodous, M.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced materials and technologies for supercapacitors used in energy conversion and storage: A review. Environ. Chem. Lett. 2021, 19, 375–439. [Google Scholar]
- Wong, H.; Iwai, H. The road to miniaturization. Phys. World 2005, 18, 40–44. [Google Scholar] [CrossRef]
- Lorenz, M.; Rao, M.S.R.; Venkatesan, T.; Fortunato, E.; Barquinha, P.; Branquinho, R.; Salgueiro, D.; Martins, R.; Carlos, E.; Liu, A.; et al. The 2016 oxide electronic materials and oxide interfaces roadmap. J. Phys. D Appl. Phys. 2016, 49, 433001. [Google Scholar] [CrossRef]
- Coll, M.; Fontcuberta, J.; Althammer, M.; Bibes, M.; Boschker, H.; Calleja, A.; Cheng, G.; Cuoco, M.; Dittmann, R.; Dkhil, B.; et al. Towards oxide electronics: A roadmap. Appl. Surf. Sci. 2019, 482, 1–93. [Google Scholar]
- Giustino, F.; Lee, J.H.; Trier, F.; Bibes, M.; Winter, S.M.; Valentí, R.; Son, Y.-W.; Taillefer, L.; Heil, C.; Figueroa, A.I.; et al. The 2021 quantum materials roadmap. J. Phys. Mater. 2020, 3, 042006. [Google Scholar] [CrossRef]
- Fritsch, D. Electronic and optical properties of spinel zinc ferrite: Ab initio hybrid functional calculations. J. Phys. Condens. Matter 2018, 30, 095502. [Google Scholar] [CrossRef] [Green Version]
- Heda, N.L.; Panwar, K.; Kumar, K.; Ahuja, B.L. Performance of hybrid functional in linear combination of atomic orbitals scheme in predicting electronic response in spinel ferrites ZnFe2O4 and CdFe2O4. J. Mater. Sci. 2020, 55, 3912–3925. [Google Scholar] [CrossRef]
- Ulpe, A.C.; Bauerfeind, K.C.; Bredow, T. Influence of spin state and cation distribution on stability and electronic properties of ternary transition-metal oxides. ACS Omega 2019, 4, 4138–4146. [Google Scholar] [CrossRef] [PubMed]
- Ulpe, A.C.; Bredow, T. GW-BSE calculations of electronic band gap and optical spectrum of ZnFe2O4: Effect of cation distribution and spin configuration. Chem. Phys. Chem. 2020, 21, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.G. Modern Microwave Ferrites. IEEE Trans. Magn. 2012, 48, 1075–1104. [Google Scholar] [CrossRef]
- Lišková-Jakubisová, E.; Višňovský, Š.; Široký, P.; Hrabovský, D.; Pištora, J.; Sahoo, S.C.; Prasad, S.; Venkataramani, N.; Bohra, M.; Krishnan, R. Nanocrystalline zinc ferrite films studied by magneto-optical spectroscopy. J. Appl. Phys. 2015, 117, 17B726. [Google Scholar] [CrossRef]
- Zviagin, V.; Kumar, Y.; Lorite, I.; Esquinazi, P.; Grundmann, M.; Schmidt-Grund, R. Ellipsometric investigation of ZnFe2O4 thin films in relation to magnetic properties. Appl. Phys. Lett. 2016, 108, 131901. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.E.; Kim, J.H.; Lee, J.S. Ferrites: Emerging light absorbers for solar water splitting. J. Mater. Chem. A 2020, 8, 9447–9482. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Shringi, S.N.; Srivastava, R.G.; Nanadikar, N.G. Magnetic ordering and domain-wall relaxation in zinc-ferrous ferrites. Phys. Rev. B 1976, 14, 2032–2040. [Google Scholar] [CrossRef]
- Bohra, M.; Prasad, S.; Venkataramani, N.; Sahoo, S.C.; Kumar, N.; Krishnan, R. Low temperature magnetization studies of nanocrystalline Zn-ferrite thin films. IEEE Trans. Magn. 2013, 49, 4249–4252. [Google Scholar] [CrossRef]
- Nakashima, S.; Fujita, K.; Tanaka, K.; Hirao, K. High magnetization and the high-temperature superparamagnetic transition with intercluster interaction in disordered zinc ferrite thin film. J. Phys. Condens. Matter. 2005, 17, 137–149. [Google Scholar] [CrossRef]
- Jedrecy, N.; Hebert, C.; Perriere, J.; Nistor, M.; Millon, E. Magnetic and magnetotransport properties of ZnxFe3−xO4−y thin films. J. Appl. Phys. 2014, 116, 213903. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Chattopadhyay, K.; Guérault, H.; Greneche, J.-M. Magnetic properties of nanostructured ferrimagnetic zinc ferrite. J. Phys. Condens. Matter. 2000, 12, 7795–7805. [Google Scholar] [CrossRef]
- Hufnagel, A.G.; Peters, K.; Müller, A.; Scheu, C.; Fattakhova-Rohlfing, D.; Bein, T. Zinc ferrite photoanode nanomorphologies with favorable kinetics for water-splitting. Adv. Funct. Mater. 2016, 26, 4435–4443. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Bhise, S.C.; Patil, S.K.; Patil, S.A.; Pawar, D.K.; Ghule, A.V.; Patil, P.S.; Kolekar, S.S. Mechanochemical growth of a porous ZnFe2O4 nano-flake thin film as an electrode for supercapacitor application. RSC Adv. 2015, 5, 45935–45942. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Shao, H. High capacity ZnFe2O4 anode material for lithium ion batteries. Electrochim. Acta 2011, 56, 9433–9438. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Gupta, A.K.; Kumar, P.; Rajak, R.; Mobin, S.M. Electrochemical energy storage properties of solvothermally driven ZnFe2O4 microspheres. Mater. Res. Express 2019, 6, 095534. [Google Scholar] [CrossRef]
- Kamazawa, K.; Tsunoda, Y.; Kadowaki, H.; Kohn, K. Magnetic neutron scattering measurements on a single crystal of frustrated ZnFe2O4. Phys. Rev. B 2003, 68, 024412. [Google Scholar] [CrossRef]
- Bohra, M.; Agarwal, N.; Singh, V. A short review on verwey transition in nanostructured Fe3O4 materials. J. Nanomater. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Kumar, R.S.; Grinblat, F.; Aphesteguy, J.C.; Saccone, F.D.; Errandonea, D. In-situ high-pressure x-ray diffraction study of zinc ferrite nanoparticles. Solid State Sci. 2016, 56, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B. Magnetism and the Chemical Bond; Interscience: New York, NY, USA, 1963. [Google Scholar]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Srivastava, M.; Alla, S.K.; Meena, S.S.; Gupta, N.; Mandal, R.K.; Prasad, N.K. ZnxFe3−xO4 (0.01 ≤ x ≤ 0.8) nanoparticles for controlled magnetic hyperthermia application. New J. Chem. 2018, 42, 7144–7153. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, Q.; Xie, Q.; Jiangong, L. Aqueous solution preparation, structure, and magnetic properties of nano-granular ZnxFe3−xO4 ferrite films. Nanoscale Res. Lett. 2010, 5, 1518–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Csonka, G.I.; Perdew, J.P.; Ruzsinszky, A.; Philipsen, P.H.T.; Lebègue, S.; Paier, J.; Vydrov, O.A.; Ángyán, J.G. Assessing the performance of recent density functionals for bulk solids. Phys. Rev. B 2009, 79, 155107. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Bohra, M.; Arras, R.; Bobo, J.-F.; Singh, V.; Kumar, N.; Chou, H. Multiple spintronic functionalities into single zinc-ferrous ferrite thin films. J. Alloys Compd. 2021. submitted for publication. [Google Scholar]
- Desai, M.; Prasad, S.; Venkataramani, N.; Samajdar, I.; Nigam, A.K.; Krishnan, R. Annealing induced structural change in sputter deposited copper ferrite thin films and its impact on magnetic properties. J. Appl. Phys. 2002, 91, 2220–2227. [Google Scholar] [CrossRef] [Green Version]
- Bohra, M.; Prasad, S.; Kumar, N.; Misra, D.S.; Sahoo, S.C.; Venkataramani, N. Large room temperature magnetization in nanocrystalline zinc ferrite thin films. Appl. Phys. Lett. 2006, 88, 262506. [Google Scholar] [CrossRef]
- Cobos, M.A.; Presa, P.D.L.; Llorente, I.; Alonso, J.M.; García-Escorial, A.; Marína, P.; Hernando, A.; Jiménez, J.A. Magnetic phase diagram of nanostructured zinc ferrite as a function of inversion degree δ. J. Phys. Chem. C 2019, 123, 17472–17482. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, Q.; Goya, G.F.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 nanocrystals: Synthesis and magnetic properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef] [Green Version]
- Granone, L.I.; Ulpe, A.C.; Robben, L.; Klimke, S.; Jahns, M.; Renz, F.; Gesing, T.M.; Bredow, T.; Dillert, R.; Bahnemann, D.W. Effect of the degree of inversion on optical properties of spinel ZnFe2O4. Phys. Chem. Chem. Phys. 2018, 20, 28267–28278. [Google Scholar] [CrossRef] [Green Version]
- Zviagin, V.; Sturm, C.; Esquinazi, P.D.; Grundmann, M.; Schmidt-Grund, R. Control of magnetic properties in spinel ZnFe2O4 thin films through intrinsic defect manipulation. J. Appl. Phys. 2020, 128, 165702. [Google Scholar] [CrossRef]
- Marcu, A.; Yanagida, T.; Nagashima, K.; Tanaka, H.; Kawai, T. Transport properties of ZnFe2O4−δ thin films. J. Appl. Phys. 2007, 102, 023713. [Google Scholar] [CrossRef]
- Rivero, M.; Campo, A.D.; Mayoral, Á.; Mazario, E.; Sánchez-Marcos, J.; Muñoz-Bonilla, A. Synthesis and structural characterization of ZnxFe3−xO4 ferrite nanoparticles obtained by an electrochemical method. RSC Adv. 2016, 6, 40067–40076. [Google Scholar] [CrossRef]
- Venkateshvaran, D.; Althammer, M.; Nielsen, A.; Geprägs, S.; Ramachandra Rao, M.S.; Sebastian, T.; Goennenwein, B.; Opel, M.; Gross, R. Epitaxial ZnxFe3−xO4 thin films: A spintronic material with tunable electrical and magnetic properties. Phys. Rev. B 2009, 79, 134405. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.N.; Venkataramani, N.; Prasad, S.; Krishnan, R. Effect of thickness on magnetic and microwave properties of RF-sputtered Zn-ferrite thin films. AIP Adv. 2017, 7, 056102. [Google Scholar]
- Hwang, H.; Shin, H.; Lee, W.-J. Effects of calcination temperature for rate capability of triple-shelled ZnFe2O4 hollow microspheres for lithium ion battery anodes. Sci. Rep. 2017, 7, 46378. [Google Scholar] [CrossRef] [Green Version]
- Takaobushi, J.; Tanaka, H.; Kawai, T.; Ueda, S.; Kim, J.-J.; Kobata, M.; Ikenaga, E.; Yabashi, M.; Kobayashi, K.; Nishino, Y.; et al. Fe3−xZnxO4 thin film as tunable high Curie temperature ferromagnetic semiconductor. Appl. Phys. Lett. 2006, 89, 242507. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tanaka, H.; Kawai, T. The control of cluster-glass transition temperature in spinel-type ZnFe2O4−δ thin film. Jpn. J. Appl. Phys. 2001, 40, L545–L547. [Google Scholar] [CrossRef]
- Lorenz, M.; Brandt, M.; Mexner, K.; Brachwitz, K.; Ziese, M.; Esquinazi, P.; Hochmuth, H.; Grundmann, M. Ferrimagnetic ZnFe2O4 thin films on SrTiO3 single crystals with highly tunable electrical conductivity. Phys. Status Solidi RRL 2011, 5, 438–440. [Google Scholar] [CrossRef]
- Saha, P.; Rakshit, R.; Alam, M.; Mandal, K. Magnetic and electronic properties of Zn doped Fe3O4 hollow nanospheres. Phys. Rev. Appl. 2019, 11, 024059–024069. [Google Scholar] [CrossRef]
- Bohra, M.; Prasad, S.; Venkataramani, N.; Kumar, N.; Sahoo, S.C.; Krishnan, R. Narrow ferromagnetic resonance line width polycrystalline Zn-ferrite thin films. IEEE Trans. Magn. 2011, 47, 345–348. [Google Scholar] [CrossRef]
- Sai, R.; Endo, Y.; Shimada, Y.; Yamaguchi, M.; Shivashankar, S.A. Oriented nanometric aggregates of partially inverted zinc ferrite: One-step processing and tunable high-frequency magnetic properties. J. Appl. Phys. 2015, 117, 17E511. [Google Scholar]
- Sai, R.; Shivashankar, S.A.; Yamaguchi, M.; Bhat, N. Magnetic nanoferrites for RF CMOS: Enabling 5G and beyond. Electrochem. Soc. Interface 2017, 26, 71–76. [Google Scholar]
- Sai, R.; Vinoy, K.J.; Bhat, N.; Shivashankar, S.A. CMOS-compatible and scalable deposition of nanocrystalline zinc ferrite thin film to improve inductance density of integrated RF inductor. IEEE Trans. Magn. 2013, 49, 4323–4326. [Google Scholar]
- Ameer, S.; Gul, I.H.; Mahmood, N.; Mujahid, M. Semiconductor-to-metallic flipping in a ZnFe2O4–graphene based smart nano-system: Temperature/microwave magneto-dielectric spectroscopy. Mater. Charact. 2015, 99, 254–265. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Y.; Wang, X.; Ma, Q.; Zhang, A.; Yang, P. Electrospun ZnFe2O4 nanotubes and nanobelts: Morphology evolution, formation mechanism and Fenton-like photocatalytic activities. Mater. Chem. Phys. 2018, 207, 114–122. [Google Scholar]
- Wang, K.; Zhan, S.; Sun, H.; Zhang, D.; Wang, J. Hollow porous core–shell ZnFe2O4/AgCl nanocubes coated with EDTA and Ag nanoparticles for enhanced photocatalytic performances of visible-light-driven. Chem. Eng. J. 2020, 400, 125908. [Google Scholar]
- Sahoo, S.C.; Venkataramani, N.; Prasad, S.; Bohra, M.; Krishnan, R. Magnetic Properties of Nanocrystalline CoFe2O4/ZnFe2O4 Bilayers. J. Supercond. Nov. Magn. 2012, 25, 2653–2657. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, Z.; Wu, Y.; Gao, Z.; Xie, H. The magnetic and photocatalytic properties of nanocomposites SrFe12O19 /ZnFe2O4. J. Magn. Magn. Mater. 2018, 465, 1–8. [Google Scholar]
- Taiping, X.; Longjun, X.; Chenglun, L.; Yuan, W. Magnetic composite ZnFe2O4/SrFe12O19: Preparation, characterization, and photocatalytic activity under visible light. Appl. Surf. Sci. 2013, 273, 684–691. [Google Scholar]
- Bohra, M.; Singh, V.; Sowwan, M.; Bobo, J.-F.; Chung, C.-J.; Clemens, B. Influence of packaging on the surface oxidation and magnetic properties of cobalt nanocrystals. J. Phys. D Appl. Phys. 2014, 47, 305002. [Google Scholar] [CrossRef]
- Lin, W.-J.; Chang, W.-C.; Qi, X. Exchange bias and magneto-resistance in an all-oxide spin valve with multi-ferroic BiFeO3 as the pinning layer. Acta Mater. 2013, 61, 7444–7453. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Zhang, D.; Adair, K.; Feng, K.; Sun, N.; Zheng, H.; Shao, H.; Zhong, J.; Ma, Y. Carbon coated bimetallic sulfide nanodots/carbon nanorod heterostructure enabling long-life lithium-ion batteries. J. Mater. Chem. 2017, 5, 25625–25631. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierarchical mesoporous Bi-component-active ZnO/ZnFe2O4 sub-microcubes as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Yu, S.-H.; Lee, S.H.; Lee, D.J.; Sung, Y.-E.; Hyeon, T. Conversion reaction-based oxide nanomaterials for lithium ion battery anodes. Small 2016, 12, 2146–2172. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 1488–1504. [Google Scholar]
- Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Bai, Y.J. Enhanced electrochemical performance of Zn-doped Fe3O4 with carbon coating. Electrochim. Acta 2014, 117, 230–238. [Google Scholar] [CrossRef]
- Guo, X.; Lu, X.; Fang, X.; Mao, Y.; Wang, Z.; Chen, L.; Xu, X.; Yang, H.; Liu, Y. Lithium storage in hollow spherical ZnFe2O4 as anode materials for lithium ion batteries. Electrochem. Commun. 2010, 12, 847–850. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Kloepsch, R.; Krueger, S.; Fiedler, M.; Schmitz, R.; Baither, D.; Winter, M.; Passerini, S. Carbon coated ZnFe2O4 nanoparticles for advanced lithium-ion anodes. Adv. Energy Mater. 2013, 3, 513–523. [Google Scholar] [CrossRef]
- Teh, P.F.; Pramana, S.S.; Kim, C.; Chen, C.M.; Chuang, C.H.; Sharma, Y.; Cabana, J.; Madhavi, S. Electrochemical reactivity with lithium of spinel-type ZnFe2–yCryO4 (0≤ y ≤ 2). J. Phys. Chem. C 2013, 117, 24213–24223. [Google Scholar]
- Bourrioux, S. Laser-Pyrolysed ZnFe2O4 Anode for Lithium-Ion Batteries: Understanding of the Lithium Storage Mechanisms. Ph.D. Thesis, Université Grenoble Alpes and NTU Singapore, Singapore, 2018. [Google Scholar]
- Zhang, Y.; Pelliccione, C.J.; Brady, A.B.; Guo, H.; Smith, P.F.; Liu, P.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Probing the Li insertion mechanism of ZnFe2O4 in Li-ion batteries: A combined X-ray diffraction, extended X-ray absorption fine structure, and density functional theory study. Chem. Mater. 2017, 29, 4282–4292. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Liu, P. A first principles study of spinel ZnFe2O4 for electrode materials in lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 26322–26329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, Z.; Xiong, W.L. Metal oxide hollow nanostructures for lithium ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar]
- Hou, X.; Wang, X.; Yao, L.; Hu, S.; Wu, Y.; Liu, X. Facile synthesis of ZnFe2O4 with inflorescence spicate architecture as anode materials for lithium-ion batteries with outstanding performance. New J. Chem. 2015, 39, 1943–1952. [Google Scholar] [CrossRef]
- Nuli, Y.N.; Chu, Y.Q.; Qin, Q.Z. Nanocrystalline ZnFe2O4 and Ag-doped ZnFe2O4 films used as new anode materials for Li-ion batteries. J. Electrochem. Soc. 2003, 151, A1077–A1083. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Li-storage and cyclability of urea combustion derived ZnFe2O4 as anode for li-ion batteries. Electrochim. Acta 2008, 53, 2380–2385. [Google Scholar] [CrossRef]
- Pei, F.T.; Sharma, Y.; Pramana, S.S.; Srinivasan, M. Nanoweb anodes composed of one-dimensional, high aspect ratio, size tunable electrospun ZnFe2O4 nanofibers for lithium ion batteries. J. Mater. Chem. 2011, 21, 14999–15008. [Google Scholar]
- Xing, Z.; Ju, Z.; Yang, J. One-step hydrothermal synthesis of ZnFe2O4 nano-octahedrons as a high capacity anode material for Li-ion batteries. Nano Res. 2012, 5, 477–485. [Google Scholar] [CrossRef]
- Zhong, X.B.; Yang, Z.Z.; Wang, H.Y.; Lu, L.; Jin, B.; Zha, M.; Jiang, Q.C. A novel approach to facilely synthesize mesoporous ZnFe2O4 nanorods for lithium ion batteries. J. Power Source 2016, 306, 718–723. [Google Scholar] [CrossRef]
- Zhong, X.B.; Jin, B.; Yang, Z.Z.; Wang, C.; Wang, H.Y. Facile shape design and fabrication of ZnFe2O4 as an anode material for Li-ion batteries. RSC Adv. 2014, 4, 55173–55178. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, L.; Qi, H.; Yue, H.; Zhang, T.; Zhao, X.; Chen, G.; Wei, Y.; Wang, C.; Zhang, D. Nanosheet assembled hollow ZnFe2O4 microsphere as anode for lithium-ion batteries. J. Alloy. Compd. 2018, 762, 480–487. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Wang, K.; Han, X.; Wang, M.; Zhu, Y.; Liu, L. Complete hollow ZnFe2O4 nanospheres with huge internal space synthesized by a simple solvothermal method as anode for lithium ion batteries. Appl. Surf. Sci. 2018, 462, 955–962. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Zhu, H.; Wang, X. Hierarchical bead chain ZnFe2O4-PEDOT composites with enhanced Li-ion storage properties as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2020, 529, 147078. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 1–13. [Google Scholar]
- Polo, A.; Lhermitte, C.R.; Dozzi, M.V.; Selli, E.; Sivula, K. Hydrogenation of ZnFe2O4 flat films: Effects of the pre-annealing temperature on the photoanodes efficiency for water oxidation. Surfaces 2020, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Jang, Y.J.; Choi, S.H.; Lee, B.J.; Kim, J.H.; Park, Y.B.; Nam, C.M.; Kim, H.G.; Lee, J.S. A multitude of modifications strategy of ZnFe2O4 nanorod photoanodes for enhanced photoelectrochemical water splitting activity. J. Mater. Chem. A 2018, 6, 12693–12700. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jang, J.W.; Kim, J.Y.; Choi, S.H.; Magesh, G.; Lee, J.; Lee, J.S. Awakening solar water-splitting activity of ZnFe2O4 nanorods by hybrid microwave annealing. Adv. Energy Mater. 2014, 5, 1401933. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, Z.; Guo, Z.; Ruan, M.; Li, X. A promising p-type Co-ZnFe2O4 nanorod film as a photocathode for photoelectrochemical water splitting. Chem. Commun. 2020, 56, 5279–5282. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Wang, X.; Qian, Q.; Zhang, S.; Li, Z.; Zou, Z. A facile spray pyrolysis method to prepare Ti-doped ZnFe2O4 for boosting photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 7571–7577. [Google Scholar] [CrossRef]
- Sahu, T.K.; Shah, A.K.; Gogoi, G.; Patra, A.S.; Ansari, M.S.; Qureshi, M. Effect of surface overlayer in enhancing the photoelectrochemical water oxidation of in-situ grown one dimensional spinel zinc ferrite nanorods directly onto the substrate. Chem. Commun. 2018, 54, 10483–10486. [Google Scholar] [CrossRef]
- Guijarro, N.; Bornoz, P.; Prévot, M.; Yu, X.; Zhu, X.; Johnson, M.; Jeanbourquin, X.; Formal, F.L.; Sivula, K. Evaluating spinel ferrites MFe2O4 (M = Cu, Mg, Zn) as photoanodes for solar water oxidation: Prospects and limitations. Sustain. Energy Fuels 2018, 2, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Guijarro, N.; Liu, Y.; Schouwink, P.; Wells, R.A.; Formal, F.L.; Sun, S.; Gao, C.; Sivula, K. Spinel structural disorder influences solar-water-splitting performance of ZnFe2O4 nanorod photoanodes. Adv. Mater. 2018, 30, 1801612. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Ye, R.; Guo, X.; Wang, S.; Wang, X.; Xiang, Q. A facile strategy for ZnFe2O4 coating preparing by electrophoretic deposition and its supercapacitor performances. J. Mater. Sci. Mater. Electron. 2018, 29, 5454–5458. [Google Scholar] [CrossRef]
- Israr, M.; Iqbal, J.; Arshad, A.; Aisida, S.O.; Ahmad, I. A unique ZnFe2O4/graphene nanoplatelets nanocomposite for electrochemical energy storage and efficient visible light driven catalysis for the degradation of organic noxious in wastewater. J. Phys. Chem. Solids 2020, 140, 109333. [Google Scholar] [CrossRef]
- Javed, M.S.; Jiang, Z.; Yang, Q.; Wang, X.; Han, X.; Zhang, C.; Gu, X.; Hu, C. Exploring Li-ion hopping behaviour in zinc ferrite and promoting performance for flexible solid-state supercapacitor. Electrochem. Acta 2019, 295, 558–568. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Kolekar, S.S.; Chang, J.Y.; Ye, Z.; Ghule, A.V. Anchoring ultrafine ZnFe2O4/C nanoparticles on 3D ZnFe2O4 nanoflakes for boosting cycle stability and energy density of flexible asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26016–26028. [Google Scholar] [CrossRef] [PubMed]

 x = 0.104;
x = 0.104;  x = 0.134;
x = 0.134;  x = 0.159;
x = 0.159;  x = 0.203).
x = 0.203).
 x = 0.104;
x = 0.104;  x = 0.134;
x = 0.134;  x = 0.159;
x = 0.159;  x = 0.203).
x = 0.203).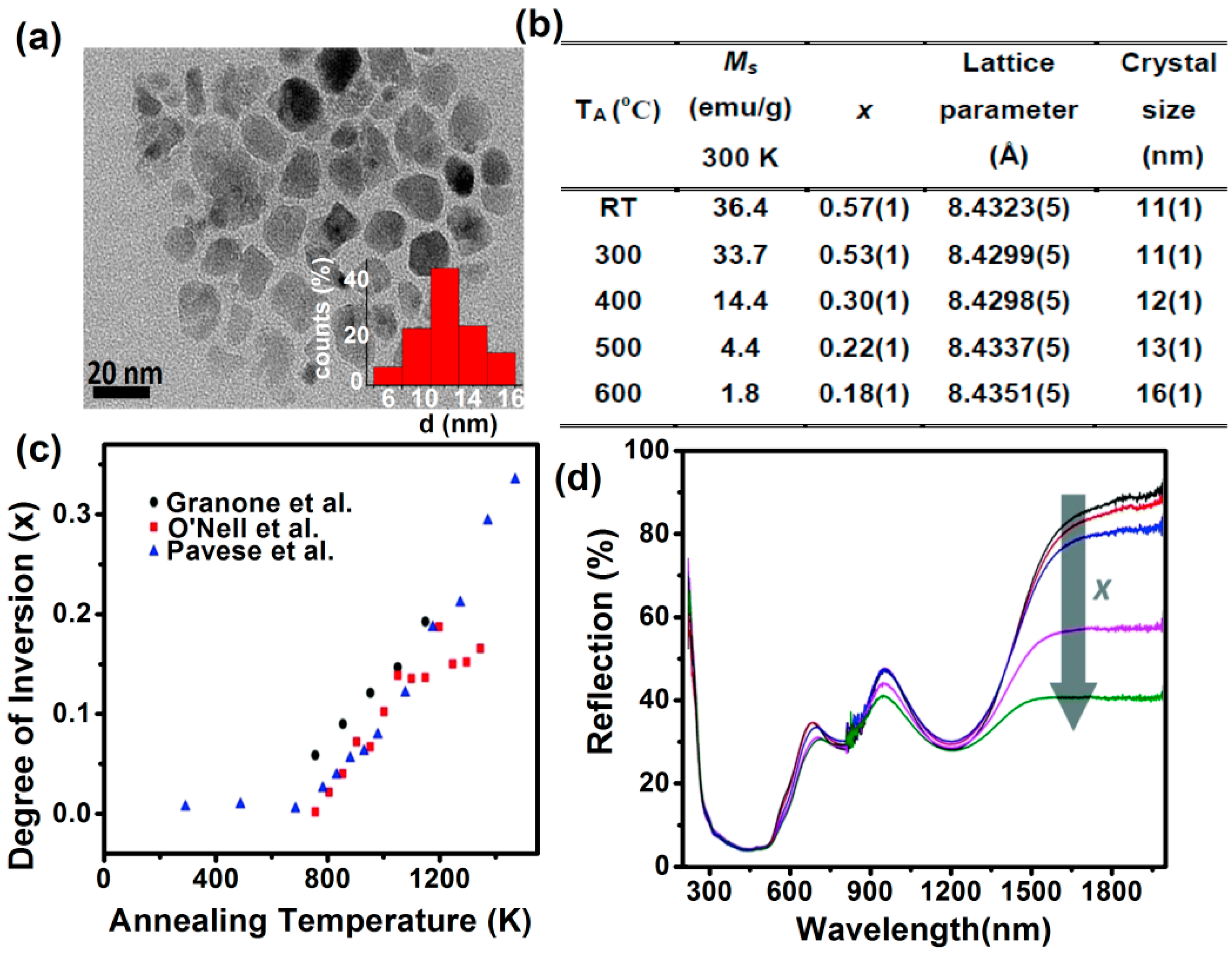


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohra, M.; Alman, V.; Arras, R. Nanostructured ZnFe2O4: An Exotic Energy Material. Nanomaterials 2021, 11, 1286. https://doi.org/10.3390/nano11051286
Bohra M, Alman V, Arras R. Nanostructured ZnFe2O4: An Exotic Energy Material. Nanomaterials. 2021; 11(5):1286. https://doi.org/10.3390/nano11051286
Chicago/Turabian StyleBohra, Murtaza, Vidya Alman, and Rémi Arras. 2021. "Nanostructured ZnFe2O4: An Exotic Energy Material" Nanomaterials 11, no. 5: 1286. https://doi.org/10.3390/nano11051286
APA StyleBohra, M., Alman, V., & Arras, R. (2021). Nanostructured ZnFe2O4: An Exotic Energy Material. Nanomaterials, 11(5), 1286. https://doi.org/10.3390/nano11051286






