Influence of Physicochemical Characteristics and Stability of Gold and Silver Nanoparticles on Biological Effects and Translocation across an Intestinal Barrier—A Case Study from In Vitro to In Silico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanomaterials
2.2. Determination of Size Distribution and Ionic Concentration via Single Particle Inductively Coupled Plasma Mass Spectroscopy (spICP-MS)
2.3. Determination of Size Distribution, Particle Shape and Thickness of Protein Corona via Transmission Electron Microscope (TEM)
2.4. Determination of Surface Charge via Electrical Asymmetrical Flow Field-Flow Fractionation Coupled to an UV–VIS-Detector (EAF4-UV-VIS)
2.5. Cell Lines and Cultivation
2.6. Translocation Studies
2.7. Characterization of the In Vitro Model via Electron Microscopy
2.8. Cell Viability Assay
2.9. Determination of the Generation of Reactive Oxygen Species (ROS) via Quantitative Polymerase Chain Reaction (PCR)
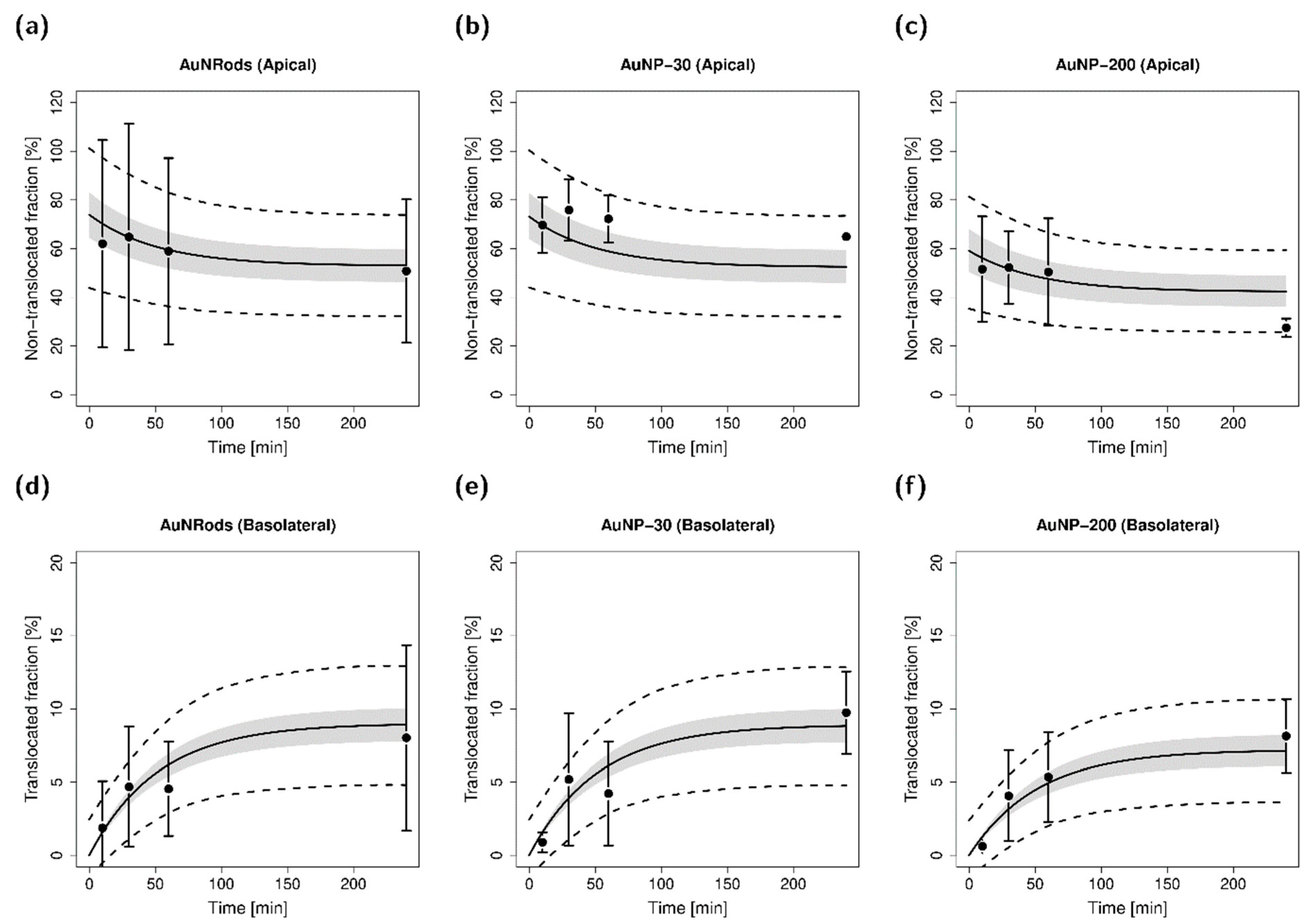
2.10. Mathematical Characterization of Particle Translocation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Nanomaterial Characterization
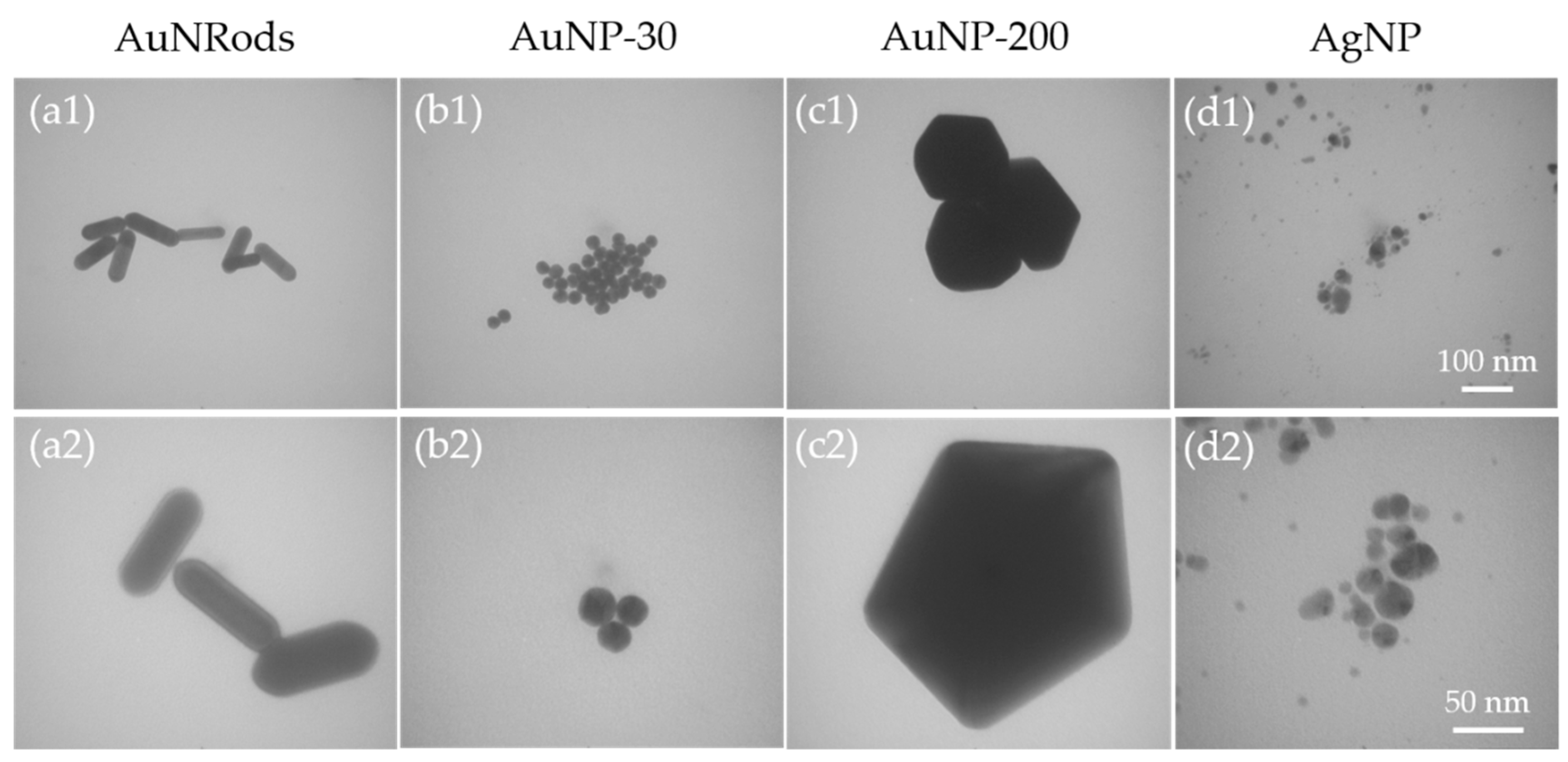
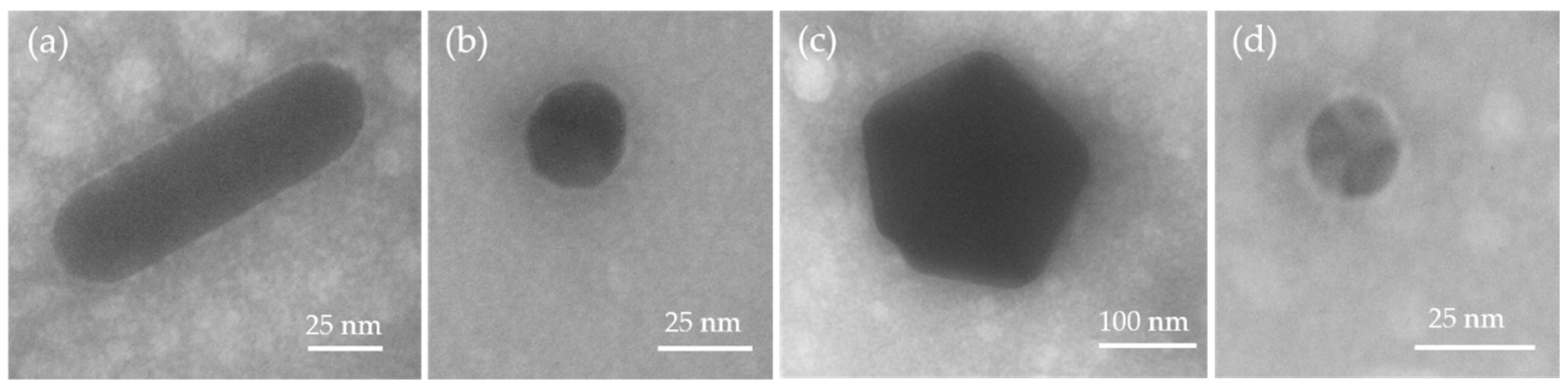
3.1.1. Size and Shape Analysis of the Nanoparticles via Transmission Electron Microscopy (TEM)
3.1.2. Stability Analysis in Cell Culture Medium (CCM)
3.1.3. Protein Corona Formation in Cell Culture Medium (CCM)
3.1.4. Determination of the Surface Charge by EAF4-UV–VIS
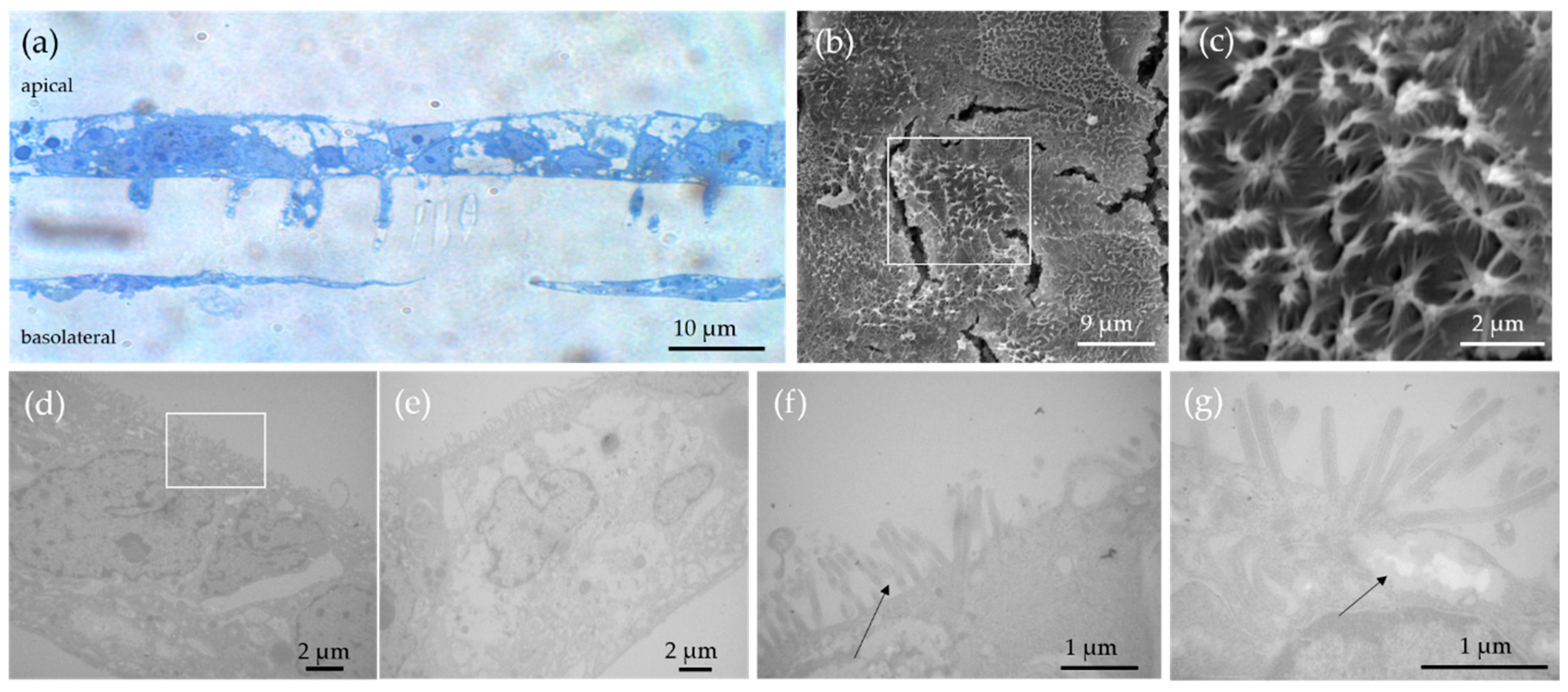
3.2. Characterization of the In Vitro Model by Light and Electron Microscopy
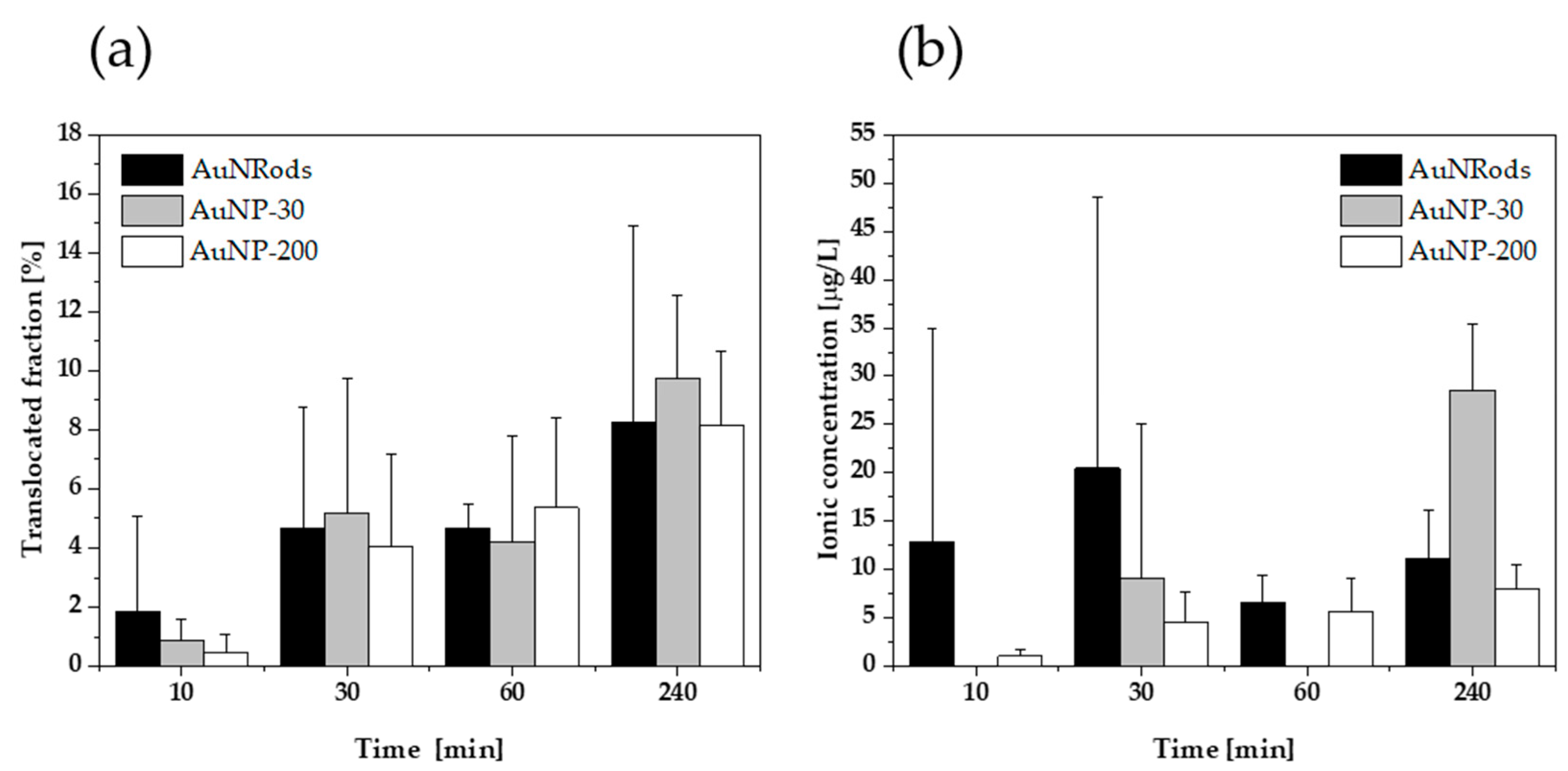
3.3. Gastrointestinal Translocation of mNPs
3.4. Mathematical Characterization of Particle Translocation
3.5. Cellular Uptake and Cell Viability
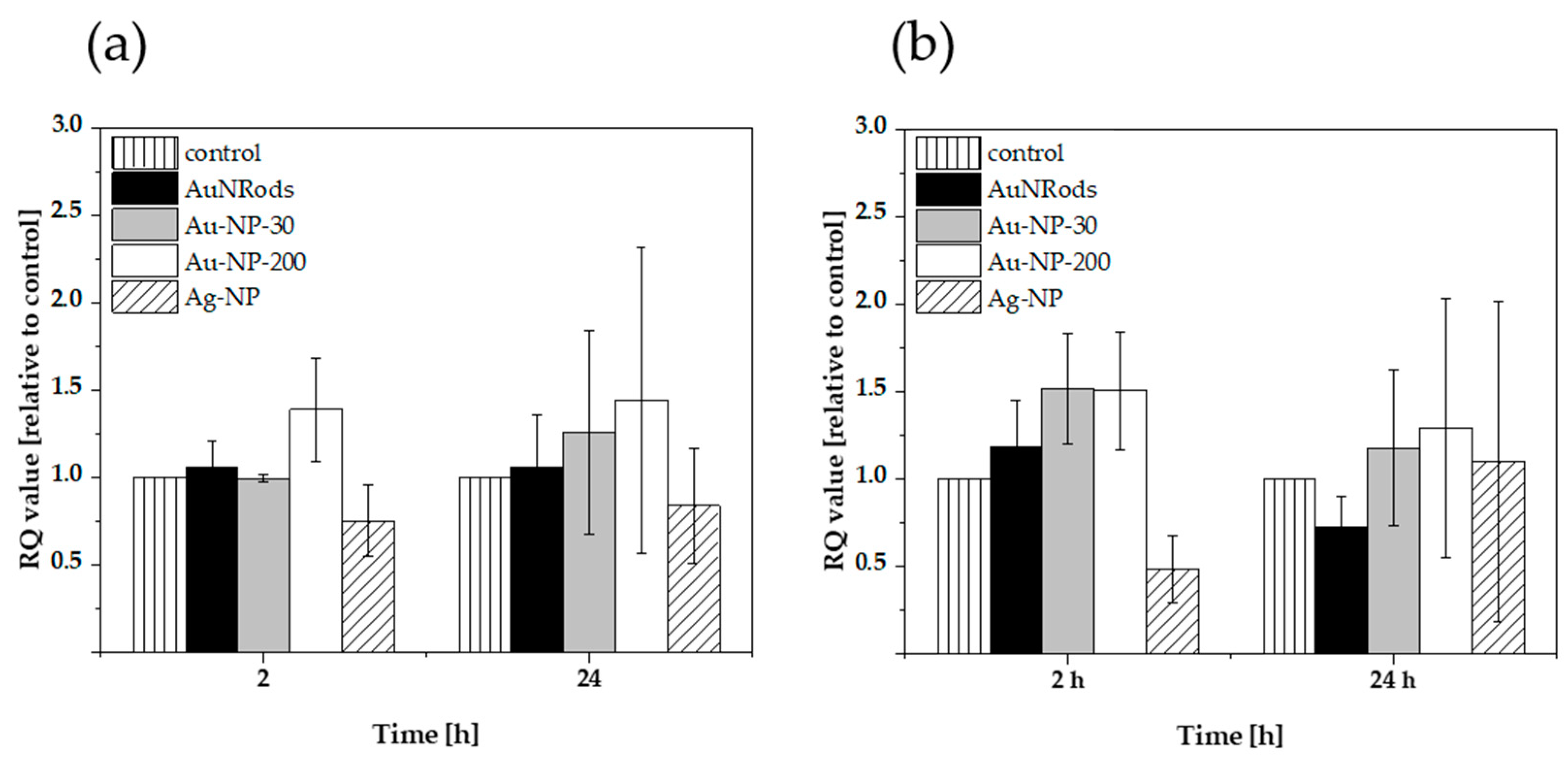
3.6. Expression of Oxidative Stress Genes after mNP Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adawi, H.I.; Newbold, M.A.; Reed, J.M.; Vance, M.E.; Feitshans, I.L.; Bickford, L.R.; Lewinski, N.A. Nano-enabled personal care products: Current developments in consumer safety. NanoImpact 2018, 11, 170–179. [Google Scholar] [CrossRef]
- Soeteman-Hernandez, L.G.; Apostolova, M.D.; Bekker, C.; Dekkers, S.; Grafström, R.C.; Groenewold, M.; Handzhiyski, Y.; Herbeck-Engel, P.; Hoehener, K.; Karagkiozaki, V.; et al. Safe innovation approach: Towards an agile system for dealing with innovations. Mater. Today Commun. 2019, 20, 100548. [Google Scholar] [CrossRef]
- Lynch, I. Compendium of Projects in the European NanoSafety Cluster; University of Birmingham: Birmingham, UK, 2017. [Google Scholar]
- Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of safe-by-design for nanomaterial development and safe innovation: Why we need a comprehensive approach. Nanomaterials 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Barton, L.E.; Auffan, M.; Bertrand, M.; Barakat, M.; Santaella, C.; Masion, A.; Borschneck, D.; Olivi, L.; Roche, N.; Wiesner, M.R.; et al. Transformation of pristine and citrate-functionalized CeO2 nanoparticles in a laboratory-scale activated sludge reactor. Environ. Sci. Technol. 2014, 48, 7289–7296. [Google Scholar] [CrossRef] [Green Version]
- Loiodice, S.; da Costa, A.; Atienzar, F. Current trends in in silico, in vitro toxicology, and safety biomarkers in early drug development. Drug Chem. Toxicol. 2019, 42, 113–121. [Google Scholar] [CrossRef]
- Kramer, J.A.; Sagartz, J.E.; Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 2007, 6, 636–649. [Google Scholar] [CrossRef]
- Hjorth, R.; van Hove, L.; Wickson, F. What can nanosafety learn from drug development? The feasibility of “safety by design.”. Nanotoxicology 2017, 11, 305–312. [Google Scholar] [PubMed] [Green Version]
- Goesmann, H.; Feldmann, C. Nanoparticulate functional materials. Angew. Chemie Int. Ed. 2010, 49, 1362–1395. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, D.; Gao, Y.; Mu, L.; Zhou, Q. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ. Int. 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Crater, J.S.; Carrier, R.L. Barrier Properties of Gastrointestinal Mucus to Nanoparticle Transport. Macromol. Biosci. 2010, 10, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Szentkuti, L.; Lorenz, K. The thickness of the mucus layer in different segments of the rat intestine. Histochem. J. 1995, 27, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Loretz, B.; Bernkop-Schnürch, A. In vitro cytotoxicity testing of non-thiolated and thiolated chitosan nanoparticles for oral gene delivery. Nanotoxicology 2007, 1, 139–148. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; La Barbera, G.; Amici, A.; Laganà, A. The liposome-protein corona in mice and humans and its implications for in vivo delivery. J. Mater. Chem. B 2014, 2, 7419–7428. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Blume, J.E.; Manning, W.C.; Troiano, G.; Hornburg, D.; Figa, M.; Hesterberg, L.; Platt, T.L.; Zhao, X.; Cuaresma, R.A.; Everley, P.A.; et al. Rapid, deep and precise profiling of the plasma proteome with multi-nanoparticle protein corona. Nat. Commun. 2020, 11, 3662. [Google Scholar] [CrossRef] [PubMed]
- Faunce, T.A.; White, J.; Matthaei, K.I. Integrated research into the nanoparticle-protein corona: A new focus for safe, sustainable and equitable development of nanomedicines. Nanomedicine 2008, 3, 859–866. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.C.; Maynard, A.D. Exposure Assessment Approaches for Engineered Nanomaterials. Risk Anal. 2010, 30, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Castranova, V. Overview of current toxicological knowledge of engineered nanoparticles. J. Occup. Environ. Med. 2011, 53. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; Silva, D.D.; Costa, J.; Soares, M.E.; De Lourdes Bastos, M.; Carmo, H. Study of the intestinal uptake and permeability of gold nanoparticles using both in vitro and in vivo approaches. Nanotechnology 2020, 31, 195102. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; de Almeida, M.P.; Araújo, A.M.; Bastos, M. de L.; Carmo, H. Gold nanoparticles induce oxidative stress and apoptosis in human kidney cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, X.; Gray, P.; Zheng, J.; Croley, T.R.; Fu, P.P.; Yin, J.J. Influences of simulated gastrointestinal environment on physicochemical properties of gold nanoparticles and their implications on intestinal epithelial permeability. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2019, 37, 116–131. [Google Scholar] [CrossRef]
- Lefebvre, D.E.; Venema, K.; Gombau, L.; Valerio, L.G.; Raju, J.; Bondy, G.S.; Bouwmeester, H.; Singh, R.P.; Clippinger, A.J.; Collnot, E.-M.; et al. Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices. Nanotoxicology 2015, 9, 523–542. [Google Scholar] [CrossRef]
- Mwilu, S.K.; El Badawy, A.M.; Bradham, K.; Nelson, C.; Thomas, D.; Scheckel, K.G.; Tolaymat, T.; Ma, L.; Rogers, K.R. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Sci. Total Environ. 2013, 447, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Wiele, T.R.; Oomen, A.G.; Wragg, J.; Cave, M.; Minekus, M.; Hack, A.; Cornelis, C.; Rompelberg, C.J.M.; De Zwart, L.L.; Klinck, B.; et al. Comparison of five in vitro digestion models to in vivo experimental results: Lead bioaccessibility in the human gastrointestinal tract. J. Environ. Sci. Health Part A Toxic Subst. Environ. Eng. 2007, 42, 1203–1211. [Google Scholar] [CrossRef]
- Peters, R.; Kramer, E.; Oomen, A.G.; Herrera Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.P.; Weigel, S.; et al. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Miret, S.; Abrahamse, L.; De Groene, E.M. Comparison of in vitro models for the prediction of compound absorption across the human intestinal mucosa. J. Biomol. Screen. 2004, 9, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Rietjens, I.M.C.M.; Singh, M.P.; Atkins, T.M.; Purkait, T.K.; Xu, Z.; Regli, S.; Shukaliak, A.; Clark, R.J.; Mitchell, B.S.; et al. Cytotoxicity of surface-functionalized silicon and germanium nanoparticles: The dominant role of surface charges. Nanoscale 2013, 5, 4870–4883. [Google Scholar] [CrossRef] [Green Version]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Tromp, P.; Helsper, J.P.F.G.; Van Der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Kloet, S.K.; Kezic, S.; Kuper, F.; Park, M.V.D.Z.; Bellmann, S.; van der Zande, M.; Le Gac, S.; Krystek, P.; Peters, R.J.B.; et al. Progress and future of in vitro models to study translocation of nanoparticles. Arch. Toxicol. 2015, 89, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Behrens, I.; Vila Pena, A.I.; Alonso, M.J.; Kissel, T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharm. Res. 2002, 19, 1185–1193. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The gut barrier: New acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 2012, 46. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Poortman, J.; Peters, R.J.; Wijma, E.; Kramer, E.; Makama, S.; Puspitaninganindita, K.; Marvin, H.J.P.; Peijnenburg, A.A.C.M.; Hendriksen, P.J.M. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano 2011, 5, 4091–4103. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef]
- Des Rieux, A.; Ragnarsson, E.G.E.; Gullberg, E.; Préat, V.; Schneider, Y.J.; Artursson, P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 2005, 25, 455–465. [Google Scholar] [CrossRef]
- Antunes, F.; Andrade, F.; Araújo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef]
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef]
- Abbott Chalew, T.E.; Schwab, K.J. Toxicity of commercially available engineered nanoparticles to Caco-2 and SW480 human intestinal epithelial cells. Cell Biol. Toxicol. 2013, 29, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.E.; Fröhlich, E. Cytotoxicity of nanoparticles contained in food on intestinal cells and the gut microbiota. Int. J. Mol. Sci. 2016, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnology 2008, 19, 75104. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; He, L.; McClements, D.J.; Xiao, H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J. Agric. Food Chem. 2015, 63, 8044–8049. [Google Scholar] [CrossRef]
- Susewind, J.; De Souza Carvalho-Wodarz, C.; Repnik, U.; Collnot, E.M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2016, 10, 53–62. [Google Scholar] [CrossRef]
- Williams, K.M.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J. Nanobiotechnol. 2016, 14, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Zhang, J.; Li, M.; Yang, D.; Liu, D.; Song, S.; Fu, J.; Zhang, H.; Dai, W.; Wang, X.; et al. Proteomic analysis of intracellular protein corona of nanoparticles elucidates nano-trafficking network and nano-bio interactions. Theranostics 2020, 10, 1213–1229. [Google Scholar] [CrossRef]
- Kämpfer, A.A.M.; Urbán, P.; La Spina, R.; Jiménez, I.O.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Ongoing inflammation enhances the toxicity of engineered nanomaterials: Application of an in vitro co-culture model of the healthy and inflamed intestine. Toxicol. Vitr. 2020, 63. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Carpinone, P.; Alshahrani, S.; Alsulays, B.; Ansari, M.; Anwer, M.; Alshehri, S.; Alshetaili, A. Influence of chitosan coating on the oral bioavailability of gold nanoparticles in rats. Saudi Pharm. J. 2019, 27, 171–175. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001, 90, 1927–1936. [Google Scholar] [CrossRef]
- Orr, S.E.; Gokulan, K.; Boudreau, M.; Cerniglia, C.E.; Khare, S. Alteration in the mRNA expression of genes associated with gastrointestinal permeability and ileal TNF-α secretion due to the exposure of silver nanoparticles in Sprague-Dawley rats. J. Nanobiotechnol. 2019, 17, 63. [Google Scholar] [CrossRef]
- Heikkinen, A.T.; Korjamo, T.; Mönkkönen, J. Modelling of drug disposition kinetics in in vitro intestinal absorption cell models. In Proceedings of the Basic and Clinical Pharmacology and Toxicology. Basic Clin. Pharmacol. Toxicol. 2010, 106, 180–188. [Google Scholar] [CrossRef]
- Bachler, G.; Losert, S.; Umehara, Y.; von Goetz, N.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; Rothen-Rutishauser, B.; Hungerbuehler, K. Translocation of gold nanoparticles across the lung epithelial tissue barrier: Combining in vitro and in silico methods to substitute in vivo experiments. Part. Fibre Toxicol. 2015, 12. [Google Scholar] [CrossRef] [Green Version]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Royce, S.G.; Sarkar, S.; Thorley, A.; Schwander, S.; Ryan, M.P.; Porter, A.E.; Chung, K.F.; Tetley, T.D.; Zhang, J.; et al. Modeling in vitro cellular responses to silver nanoparticles. J. Toxicol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [Green Version]
- Cirtiu, C.-M.; Fleury, N.; Stephan, C. Assessing the Fate of Nanoparticles in Biological Fluids Using SP-ICP-MS, Application Note Perkin Elmer. 2015. Available online: https://resources.perkinelmer.com/lab-solutions/resources/docs/APP_012008_01-NexION-350S-Fate-of-NPs-in-Bio-Fluids.pdf (accessed on 7 May 2021).
- Vidmar, J.; Loeschner, K.; Correia, M.; Larsen, E.H.; Manser, P.; Wichser, A.; Boodhia, K.; Al-Ahmady, Z.S.; Ruiz, J.; Astruc, D.; et al. Translocation of silver nanoparticles in the: Ex vivo human placenta perfusion model characterized by single particle ICP-MS. Nanoscale 2018, 10, 11980–11991. [Google Scholar] [CrossRef] [Green Version]
- Tuoriniemi, J.; Cornelis, G.; Hassellöv, M. Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles. Anal. Chem. 2012, 84, 3965–3972. [Google Scholar] [CrossRef]
- Laborda, F.; Jiménez-Lamana, J.; Bolea, E.; Castillo, J.R. Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J. Anal. At. Spectrom. 2013, 28, 1220–1232. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Drexel, R.; Siupa, A.; Carnell-Morris, P.; Carboni, M.; Sullivan, J.; Meier, F. Fast and Purification-Free Characterization of Bio-Nanoparticles in Biological Media by Electrical Asymmetrical Flow Field-Flow Fractionation Hyphenated with Multi-Angle Light Scattering and Nanoparticle Tracking Analysis Detection. Molecules 2020, 25, 4703. [Google Scholar] [CrossRef] [PubMed]
- Von Smoluchowski, M. Zur kinetischen Theorie der Brownschen Molekularbewegung und der Suspensionen. Ann. Phys. 1906, 326, 756–780. [Google Scholar] [CrossRef] [Green Version]
- Hesler, M.; Schwarz, D.H.; Dähnhardt-Pfeiffer, S.; Wagner, S.; von Briesen, H.; Wenz, G.; Kohl, Y. Synthesis and in vitro evaluation of cyclodextrin hyaluronic acid conjugates as a new candidate for intestinal drug carrier for steroid hormones. Eur. J. Pharm. Sci. 2020, 143, 1–12. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. Vitr. 2019, 61, 104610. [Google Scholar] [CrossRef]
- Elmokadem, A.; Riggs, M.M.; Baron, K.T. Quantitative Systems Pharmacology and Physiologically-Based Pharmacokinetic Modeling With mrgsolve: A Hands-On Tutorial. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Menzel, M.; Bienert, R.; Bremser, W.; Girod, M.; Rolf, S.; Thünemann, A.F.; Emmerling, F. Certified Reference Material BAM-N001 Particle Size Parameters of Nano Silver. Federal Institute for Materials Research and Testing (BAM), 2013; pp. 1–23. Available online: https://rrr.bam.de/RRR/Content/DE/Downloads/RM-Zertifikate/RM-Partikelgroessen/bam_n001repe.pdf?__blob=publicationFile (accessed on 31 March 2021).
- Peters, R.; Herrera-Rivera, Z.; Undas, A.; Van Der Lee, M.; Marvin, H.; Bouwmeester, H.; Weigel, S. Single particle ICP-MS combined with a data evaluation tool as a routine technique for the analysis of nanoparticles in complex matrices. J. Anal. At. Spectrom. 2015, 30, 1274–1285. [Google Scholar] [CrossRef]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Levak, M.; Burić, P.; Dutour Sikirić, M.; Domazet Jurašin, D.; Mikac, N.; Bačić, N.; Drexel, R.; Meier, F.; Jakšić, Ž.; Lyons, D.M. Effect of Protein Corona on Silver Nanoparticle Stabilization and Ion Release Kinetics in Artificial Seawater. Environ. Sci. Technol. 2017, 51, 1259–1266. [Google Scholar] [CrossRef]
- Martin, M.N.; Allen, A.J.; Maccuspie, R.I.; Hackley, V.A. Dissolution, agglomerate morphology, and stability limits of protein-coated silver nanoparticles. Langmuir 2014, 30, 11442–11452. [Google Scholar] [CrossRef]
- Aueviriyavit, S.; Phummiratch, D.; Maniratanachote, R. Mechanistic study on the biological effects of silver and gold nanoparticles in Caco-2 cells-Induction of the Nrf2/HO-1 pathway by high concentrations of silver nanoparticles. Toxicol. Lett. 2014, 224, 73–83. [Google Scholar] [CrossRef]
- GPX1 Glutathione Peroxidase 1 [Homo Sapiens (Human)] GeneNCBI. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=2876 (accessed on 25 February 2021).
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.; Mandal, D.; Dash, S.K.; Das, S.; Dey, A.; Dey, S.K.; Das, D.; et al. Surface modification minimizes the toxicity of silver nanoparticles: An in vitro and in vivo study. J. Biol. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and gold nanoparticles exposure to in Vitro cultured retina—Studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Bajak, E.; Fabbri, M.; Ponti, J.; Gioria, S.; Ojea-Jiménez, I.; Collotta, A.; Mariani, V.; Gilliland, D.; Rossi, F.; Gribaldo, L. Changes in Caco-2 cells transcriptome profiles upon exposure to gold nanoparticles. Toxicol. Lett. 2015, 233, 187–199. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]


| spICP-MS Median Size (nm) | TEM Diameter (nm) | spICP-MS Median Size (nm) | TEM Diameter (nm) | |
|---|---|---|---|---|
| Stock Suspension | Cell Culture Medium | |||
| AuNRods | 57.4 ± 0.5 | 36.0 ± 5.7 | 54.5 ± 0.9 | 31.2 ± 7.5 |
| AuNP-30 | 28.0 ± 0.1 | 24.0 ± 2.2 | 26.0 ± 1.0 | 25 ± 5.5 |
| AuNP-200 | 191.6 ± 1.4 | 214 ± 20.1 | 176.2 ± 1.2 | 222 ± 18.7 |
| AgNP | 20.8 ± 0.2 | 15.2 ± 7.1 | 21.3 ± 0.5 | 15.5 ± 7.4 |
| mNP | Solvent | Electrophoretic Mobility (1 × 10−8 m2/(V s)) | ζ-Potential (mV) |
|---|---|---|---|
| AuNRods | UPW | −2.74 ± 0.22 | −35.0 ± 2.8 |
| AuNP-30 | −4.61 ± 0.39 | −59.0 ± 5.0 | |
| AuNP-200 | −2.36 ± 0.10 | −30.2 ± 1.2 | |
| AgNP | −2.99 ± 0.15 | −38.2 ± 1.9 | |
| AuNRods | CCM | −2.68 ± 0.14 | −34.2 ± 1.8 |
| AuNP-30 | −4.41 ± 0.32 | −56.4 ± 4.1 | |
| AuNP-200 | −1.81 ± 0.11 | −23.1 ± 1.4 | |
| AgNP | −5.35 ± 0.35 | −68.5 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohl, Y.; Hesler, M.; Drexel, R.; Kovar, L.; Dähnhardt-Pfeiffer, S.; Selzer, D.; Wagner, S.; Lehr, T.; von Briesen, H.; Meier, F. Influence of Physicochemical Characteristics and Stability of Gold and Silver Nanoparticles on Biological Effects and Translocation across an Intestinal Barrier—A Case Study from In Vitro to In Silico. Nanomaterials 2021, 11, 1358. https://doi.org/10.3390/nano11061358
Kohl Y, Hesler M, Drexel R, Kovar L, Dähnhardt-Pfeiffer S, Selzer D, Wagner S, Lehr T, von Briesen H, Meier F. Influence of Physicochemical Characteristics and Stability of Gold and Silver Nanoparticles on Biological Effects and Translocation across an Intestinal Barrier—A Case Study from In Vitro to In Silico. Nanomaterials. 2021; 11(6):1358. https://doi.org/10.3390/nano11061358
Chicago/Turabian StyleKohl, Yvonne, Michelle Hesler, Roland Drexel, Lukas Kovar, Stephan Dähnhardt-Pfeiffer, Dominik Selzer, Sylvia Wagner, Thorsten Lehr, Hagen von Briesen, and Florian Meier. 2021. "Influence of Physicochemical Characteristics and Stability of Gold and Silver Nanoparticles on Biological Effects and Translocation across an Intestinal Barrier—A Case Study from In Vitro to In Silico" Nanomaterials 11, no. 6: 1358. https://doi.org/10.3390/nano11061358
APA StyleKohl, Y., Hesler, M., Drexel, R., Kovar, L., Dähnhardt-Pfeiffer, S., Selzer, D., Wagner, S., Lehr, T., von Briesen, H., & Meier, F. (2021). Influence of Physicochemical Characteristics and Stability of Gold and Silver Nanoparticles on Biological Effects and Translocation across an Intestinal Barrier—A Case Study from In Vitro to In Silico. Nanomaterials, 11(6), 1358. https://doi.org/10.3390/nano11061358