Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extract
2.3. Biosynthesis of Gold Nanoparticles (AuNPs)
2.4. Synthesis of Hydroxyapatite (HA) Nanopowder
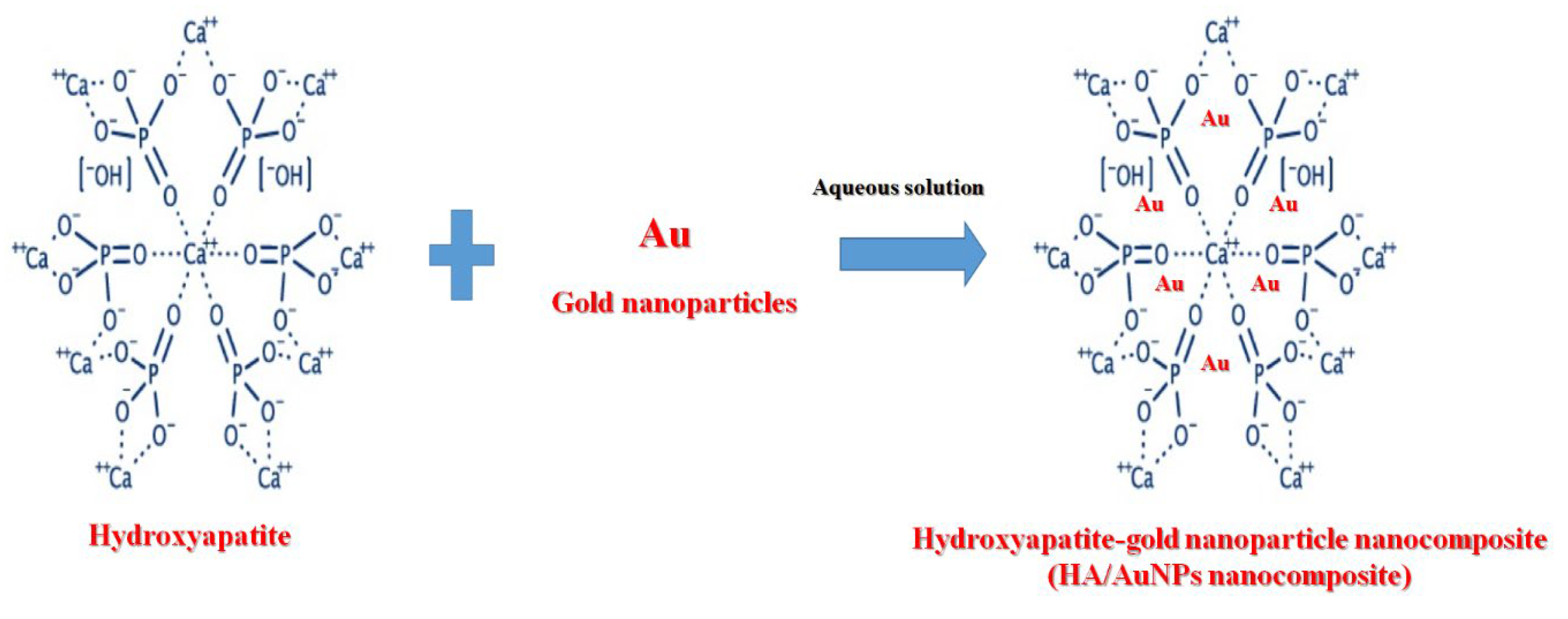
2.5. Preparation of Hydroxyapatite (HA)/Gold (Au) Nanocomposite
2.6. Physico-Chemical Characterization
2.7. Adsorption Studies
2.8. Antibacterial Studies
3. Result and Discussion
3.1. Synthesis Mechanism
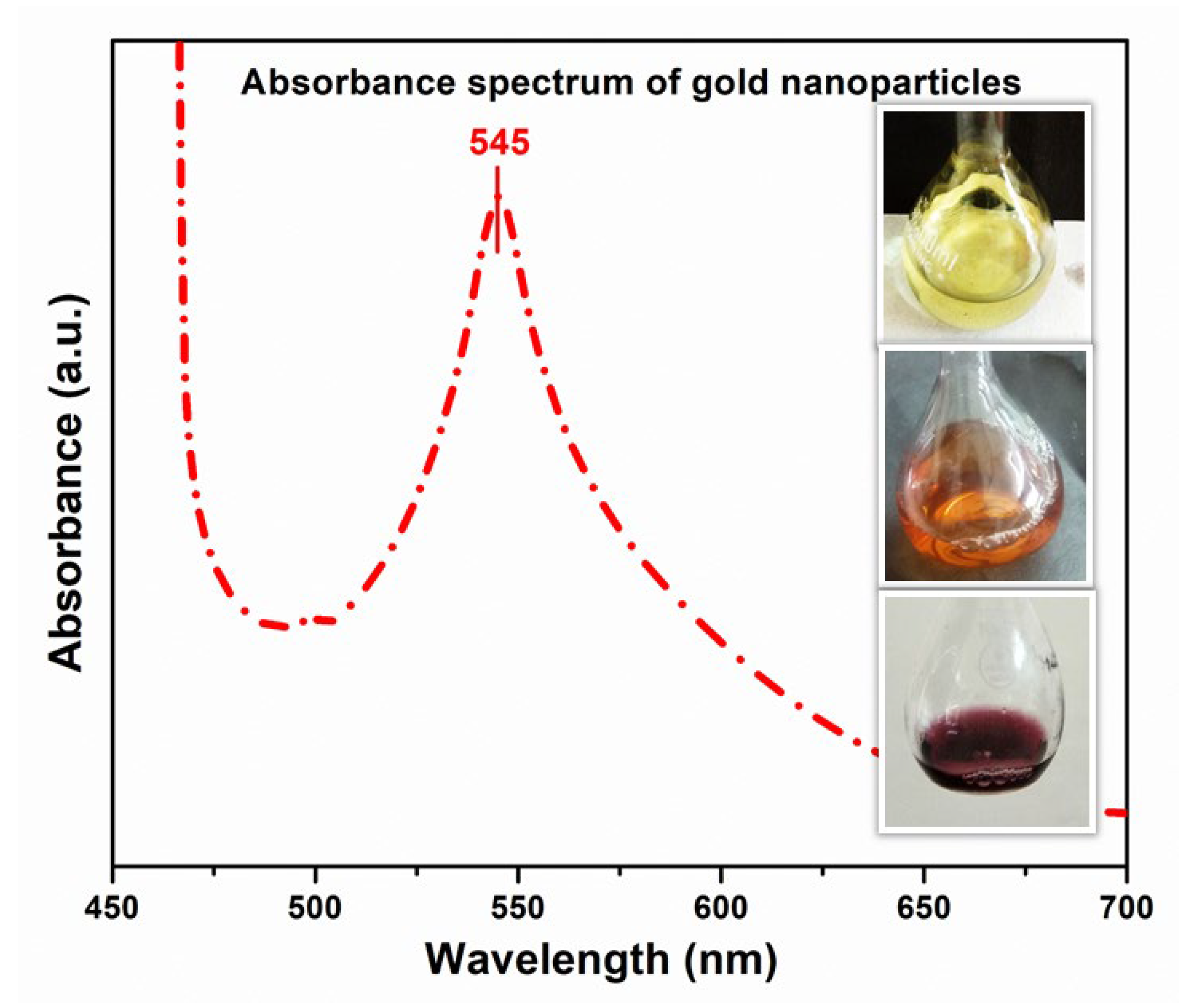
3.2. Confirmation of Gold Nanoparticles (AuNPs) By Using UV-Vis Spectroscopy
3.3. XRD Analysis
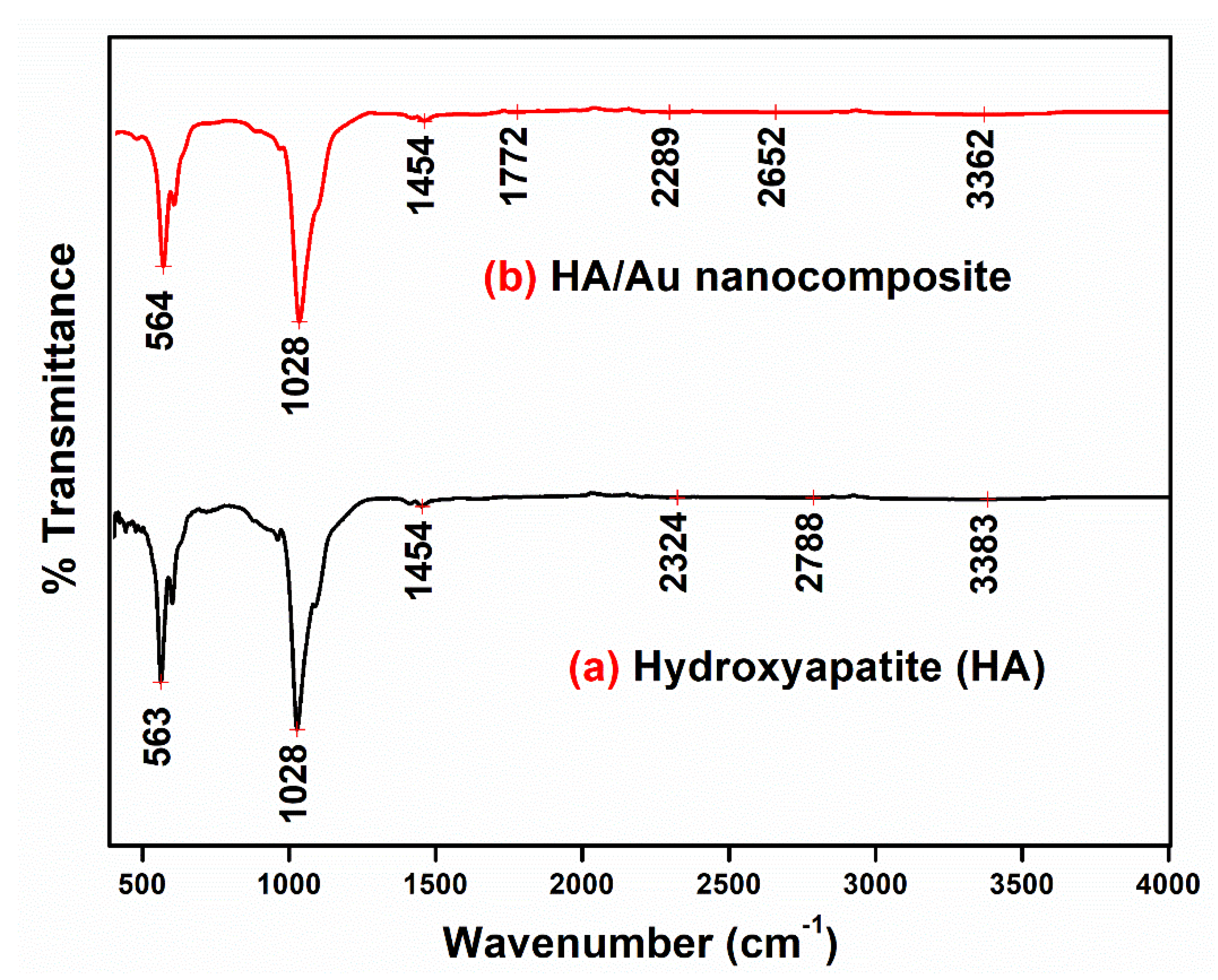
3.4. FTIR Spectra Analysis
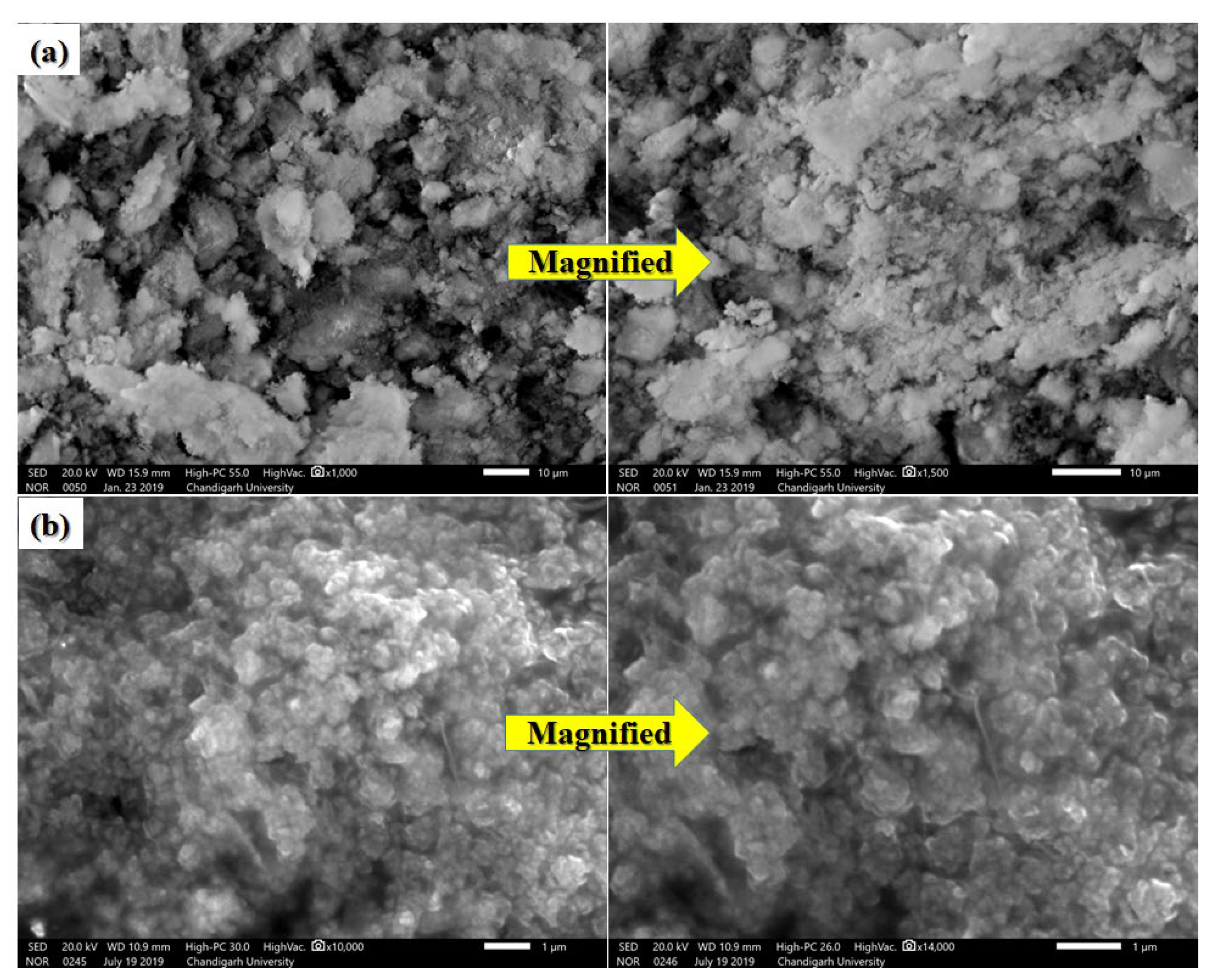
3.5. SEM Results
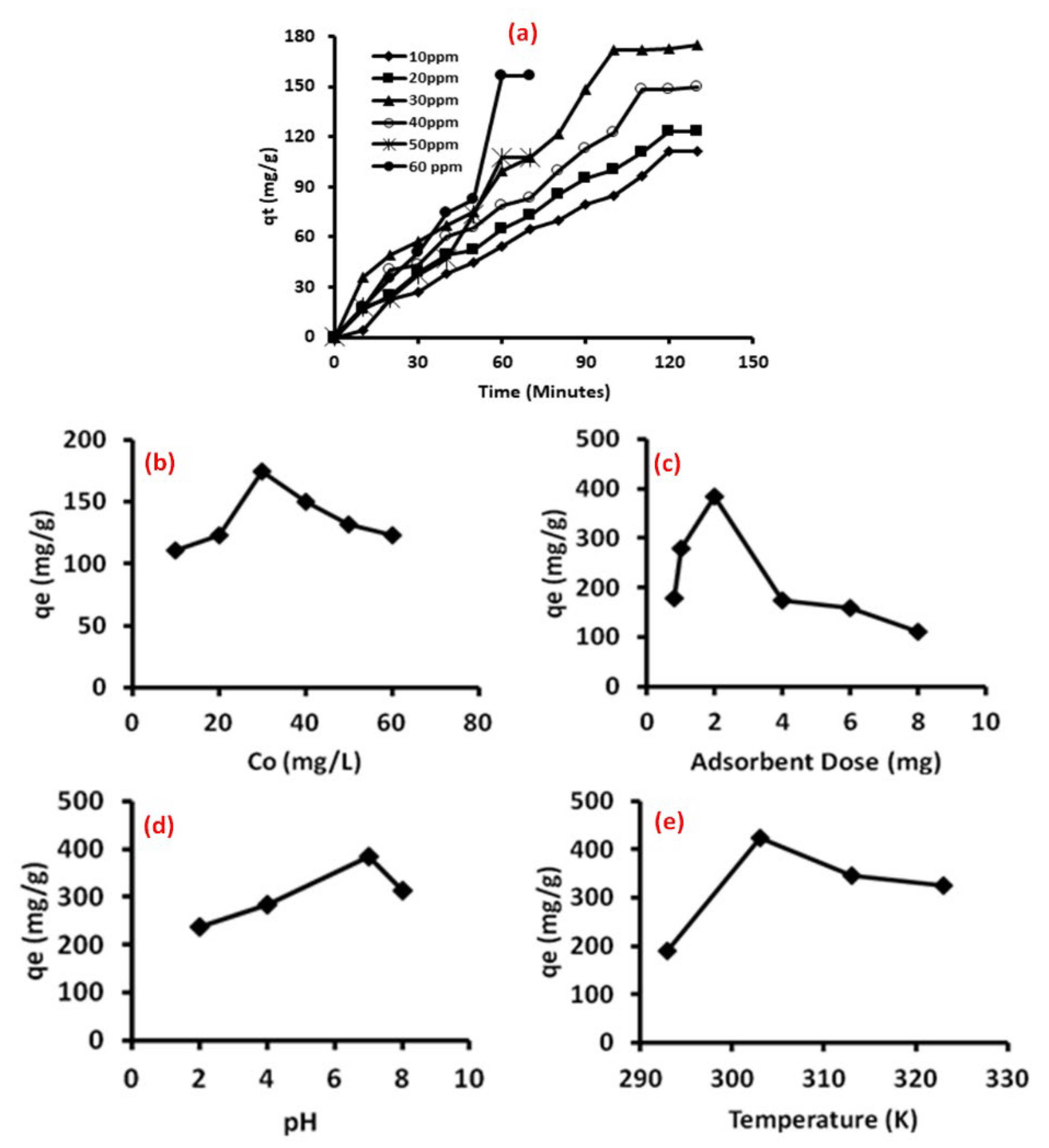
3.6. Adsorption Studies
3.7. Adsorption Kinetics
3.8. Validity of Kinetic Models
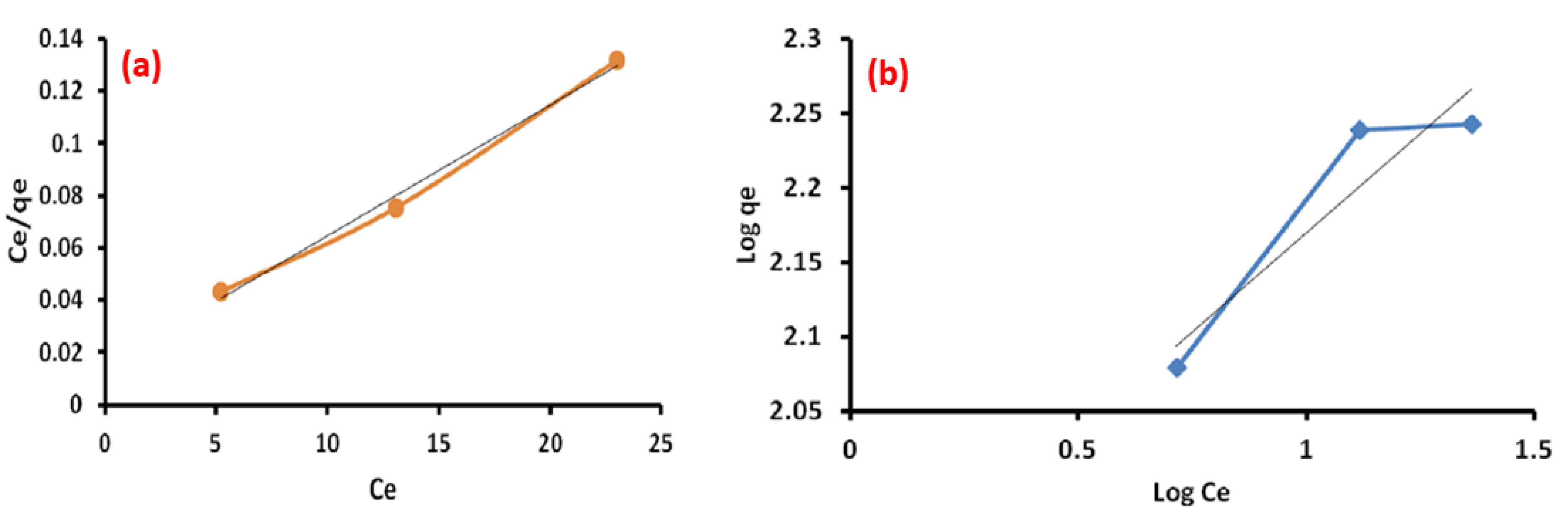
3.9. Adsorption Isotherm
3.10. Thermodynamic Studies
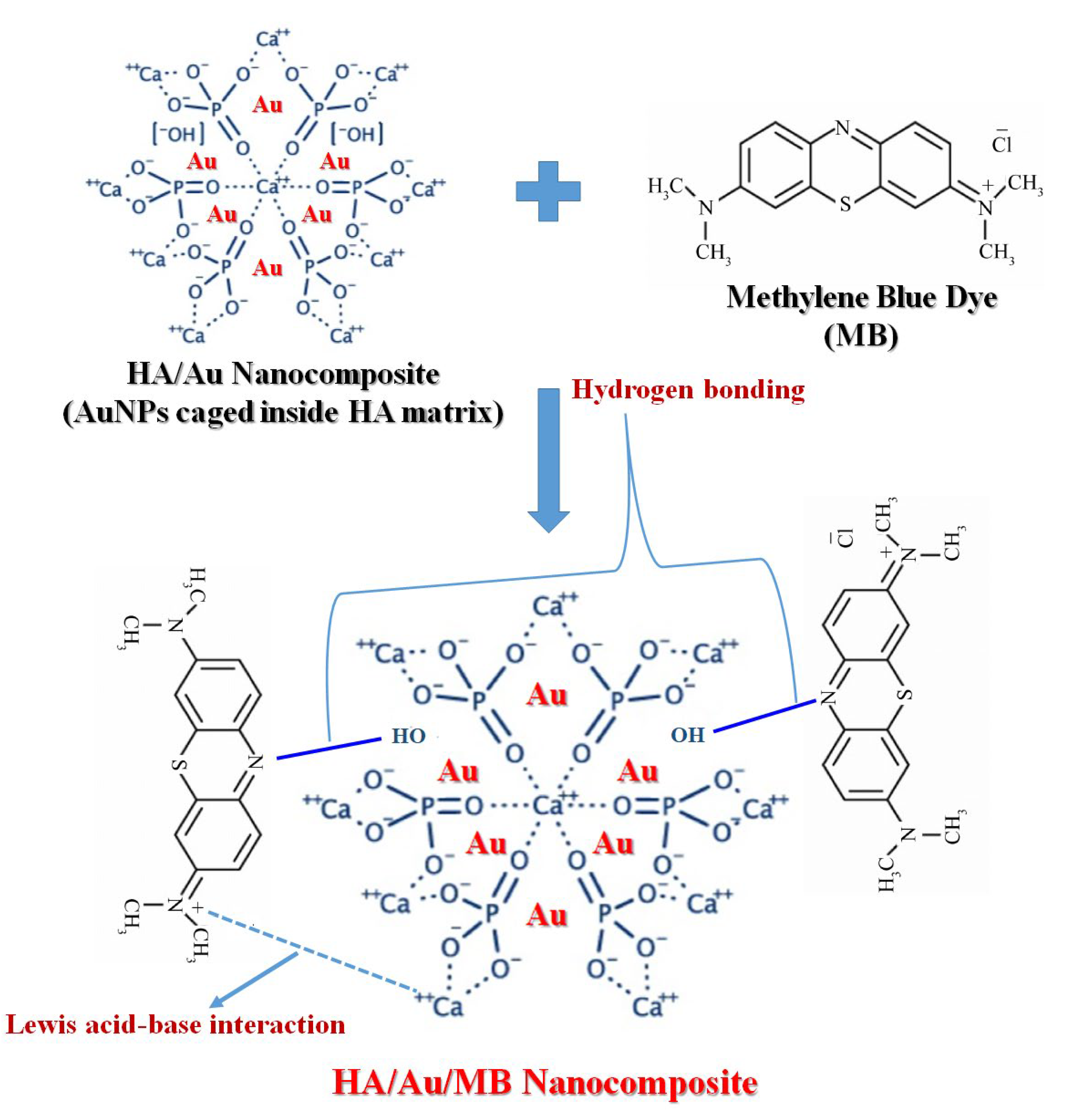
3.11. Mechanism of Dye Adsorption
3.12. Antibacterial Activity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Zhao, L.; Tang, Y.; Liu, Y.; Pu, H.; Gan, N.; Liu, Y.; Li, H. A simple and green method to construct cyclodextrin polymer for the effective and simultaneous estrogen pollutant and metal removal. Chem. Eng. J. 2019, 366, 598–607. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q.; Tang, Y.; Yan, X.; Li, B. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Sci. Rep. 2015, 511, 873. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.D.; Naji, A.L.; Faisal, H.A.A.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020, 709, 135895. [Google Scholar] [CrossRef] [PubMed]
- Naushad, N.; Alqadami, A.A.; Alothma, A.Z.; Alsohaimi, H.I.; Algamdi, S.M.; Aldawsari, M.A. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Meng, L.; Li, C.; Liu, X.; Lu, J.; Cheng, Y.; Xio, L.P.; Wang, H. Preparation of magnetic hydrogel microspheres of lignin derivate for application in water. Sci. Total Environ. 2019, 685, 847–855. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, A.N.; Ngadi, N.; Razmi, A.F.; Inuwa, M.I.; Mat, R.; Amin, S.A.N. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Tech. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Yagub, T.M.; Sen, K.T.; Afroze, S.; Ang, M.H. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Pirkarami, A.; Olya, M.E. Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism. J. Saudi Chem. Soc. 2017, 21, S179–S186. [Google Scholar] [CrossRef] [Green Version]
- Zamel, D.; Hassanin, H.A.; Ellethy, R.; Singer, G.; Abdelmoneim, A. Novel Bacteria-Immobilized Cellulose Acetate/Poly(ethylene oxide) Nanofibrous Membrane for Wastewater Treatment. Sci. Rep. 2019, 9, 18994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.-S.; Chiang, C.-C.; Hsu, Y.-C. Sorption Kinetics for Dye Removal from Aqueous Solution Using Activated Clay. Sep. Sci. Technol. 2001, 36, 2473–2488. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Paulino, A.T.; Guilherme, R.M.; Reis, V.A.; Campese, M.G.; Muniz, C.E.; Nozaki, J. Removal of methylene blue dye from an aqueous media using superabsorbent hydrogel supported on modified polysaccharide. J. Colloid Interface Sci. 2006, 301, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, J.; He, B.; Zhang, F.; Li, H. Peach gum for efficient removal of methylene blue and methyl violet dyes from aqueous solution. Carbohydr. Polym. 2014, 101, 574–581. [Google Scholar] [CrossRef]
- Banat, M.I.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Mohd-Setapar, H.S.; Chuong, S.C.; Khatoon, A.; Wani, A.W.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Banerjee, P.; Gupta, D.S.; De, S. Removal of dye from aqueous solution using a combination of advanced oxidation process and nanofiltration. J. Hazard. Mater. 2007, 140, 95–103. [Google Scholar] [CrossRef]
- Lin, H.S.; Peng, F.C. Treatment of textile wastewater by electrochemical method. Water Res. 1994, 28, 277–282. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002, 36, 4717–4724. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Porter, J.C.; Cheng, W.; Zhang, X.; Wang, L.; Elimelech, M. Concentration and Recovery of Dyes from Textile Wastewater Using a Self-Standing, Support-Free Forward Osmosis Membrane. Environ. Sci. Technol. 2019, 53, 3078–3086. [Google Scholar] [CrossRef]
- Van Thamaraisel, C.; Noel, M. Membrane Processes for Dye Wastewater Treatment: Recent Progress in Fouling Control. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1007–1040. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Alothman, Z.A.; Al-Muhtaseb, H.A. Green and eco-friendly nanocomposite for the removal of toxic Hg(II) metal ion from aqueous environment: Adsorption kinetics & isotherm modelling. J. Mol. Liq. 2019, 279, 1–8. [Google Scholar]
- Wang, H.; Ji, X.; Ahmed, M.; Huang, F.; Sessler, L.J. Hydrogels for anion removal from water. J. Mater. Chem. A 2019, 7, 1394–1403. [Google Scholar] [CrossRef]
- Varaprasad, K.; Nunez, D.; Yallapu, M.M.; Jayaramudu, T.; Elgueta, E.; Oyarzun, P. Nano-hydroxyapatite polymeric hydrogels for dye Removal. RSC Adv. 2018, 8, 18118–18127. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Gisi, D.S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar]
- Sobczak-Kupiec, A.; Pluta, A.; Drabczyk, A.; Wlos, M.; Tyliszczak, B. Synthesis and characterization of ceramic—Polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram. Int. 2018, 44, 13630–13638. [Google Scholar] [CrossRef]
- Bundela, H.; Bajpai, K.A. Designing of hydroxyapatite-gelatin based porous matrix as bone substitute: Correlation with biocompatibility aspects. Express Polym. Lett. 2008, 2, 201–213. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, R.; Wu, P.; Wu, L. Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem. Eng. J. 2012, 211–212, 336–342. [Google Scholar] [CrossRef]
- Mousa, M.S.; Ammar, S.N.; Ibrahim, A.H. Removal of lead ions using hydroxyapatite nano-material prepared from phosphogypsum waste. J. Saudi Chem. Soc. 2016, 20, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Gong, J.-L.; Zeng, G.-M.; Niua, Q.-Y.; Zhang, H.-Y.; Niu, C.-G.; Deng, J.-H.; Yan, M. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010, 162, 487–494. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Li, J.; Luo, J.-H.; Jin, Y.; Zheng, D. Removal of cadmium (II) from aqueous solution by a new adsorbent of fluor-hydroxyapatite composites. J. Taiwan Inst. Chem. E 2017, 70, 200–208. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mohammad, M.A.; Salaheldin, A.T.; El-Anadouli, E.B. A promising hydroxyapatite/graphene hybrid nanocomposite for methylene blue dye’s removal in wastewater treatment. Int. J. Electrochem. Sci. 2018, 13, 8222–8240. [Google Scholar] [CrossRef]
- Adeogun, A.I.; Ofudje, E.A.; Idowu, M.A.; Kareem, S.O.; Vahidhabanu, S.; Babu, B.R. Biowaste-Derived Hydroxyapatite for Effective Removal of Reactive Yellow 4 Dye: Equilibrium, Kinetic, and Thermodynamic Studies. ACS Omega 2018, 3, 1991–2000. [Google Scholar] [CrossRef]
- Guan, Y.; Cao, W.; Wang, X.; Marchetti, A.; Tu, Y. Hydroxyapatite nano-rods for the fast removal of congo red dye from aqueous solution. Mater. Res. Exp. 2018, 5, 065053. [Google Scholar] [CrossRef]
- Kumar, P.V.; Kala, S.M.J.; Prakash, K.S. Green synthesis of gold nanoparticles using Croton Caudatus Geisel Leaf extract and their biological studies. Mater. Lett. 2018, 236, 19–22. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.; Thapa, S.; Bhagat, M.; Kumar, V.; Sharma, V. Nanohydroxyapatite-, Gelatin-, and Acrylic Acid-Based Novel Dental Restorative Material. ACS Omega 2020, 5, 27886–27895. [Google Scholar] [CrossRef]
- Yelten-Yilmaz, A.; Yilmaz, S. Wet chemical precipitation synthesis of hydroxyapatite (HA) powders. Ceram. Int. 2018, 44, 9703–9710. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Kumar, V.; Bhatia, J.K.; Sharma, S.; Sharma, V. Microwave-assisted synthesis of gum gellan-cl-poly(acrylic-co- methacrylic acid) hydrogel for cationic dyes removal. Polym. Bull. 2020, 77, 4917–4935. [Google Scholar] [CrossRef]
- Sharma, S.; Virk, K.; Sharma, S.; Bose, S.K.; Kumar, V.; Sharma, V.; Focarete, M.L.; Kalia, S. Preparation of gum acacia-poly(acrylamide-IPN-acrylic acid) based nanocomposite hydrogels via polymerization methods for antimicrobial applications. J. Mol. Str. 2020, 1215, 128298. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, A.S.; Yudha, S.S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. B 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Green synthesis of stabilized spherical shaped gold nanoparticles using novel aqueous Elaeis guineensis (oil palm) leaves extract. J. Mol. Struct. 2018, 1159, 167–173. [Google Scholar] [CrossRef]
- Smitha, S.L.; Philip, D.; Gopchandran, K.G. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 735–739. [Google Scholar] [CrossRef]
- Santos dos, C.F.; Gomes, P.S.; Almeida, M.M.; Willinger, M.-G.; Franke, R.-P.; Fernandes, M.H. Costa ME Gold-dotted hydroxyapatite nanoparticles as multifunctional platforms for medical applications. RSC Adv. 2015, 5, 69184–69195. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, M.; Chen, F.; Wei, Y.; Chen, X.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway. Int. J. Nanomed. 2019, 14, 7987–8000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.P.S.L.; Mantilaka, M.M.M.G.P.G.; Rajapakse, R.M.G.; Pitawala, H.M.T.G.A.; Premachandra, T.N.; Herath, H.M.T.U.; Rajapakse, R.P.V.J.; Upul Wijayantha, K.G. Urea-assisted synthesis of hydroxyapatite nanorods from naturally occurring impure apatite rocks for biomedical applications. RSC Adv. 2017, 7, 24806. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Singh, P.; Pyare, R. Synthesis, characterization, mechanical and biological properties of biocomposite based on zirconia containing 1393 bioactive glass with hydroxyapatite. Ceram. Int. 2020, 46, 10442–10451. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Dhumale, V.A.; Gosavi, S.W.; Sharma, R.B.; Datar, S.S. Catalytic activity of allamanda mediated phytosynthesized anisotropic gold nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045005. [Google Scholar] [CrossRef] [Green Version]
- Rodrıguez-Lugo, V.; Karthik, T.V.K.; Mendoza-Anaya, D.; Rubio-Rosas, E.; Villasenor Ceron, L.S.; Reyes-Valderrama, M.I.; Salinas-Rodrıguez, E. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 15, 180962. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Qian, G.; Fen, M. Using polyacrylamide to control particle size and synthesize porous nano hydroxyapatite. Results Phys. 2020, 16, 102991. [Google Scholar] [CrossRef]
- Reddy, B.; Madhusudhan, G.; Ramakrishan, A. Catalytic reduction of methylene blue and congo red dyes using green synthesized gold nanoparticles capped by salmalia malabarica gum. Int. Nano Lett. 2015, 5, 215–222. [Google Scholar]
- Indira, T.K.; Lakshmi, P.K. Magnetic Nanoparticles: A review. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 1035–1042. [Google Scholar]
- Malik, P.K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dyes Pigm. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Arshadi, M.; Salimi Vahid, F.; Salvacion, J.W.L.; Soleymanzadeh, M. Adsorption studies of methyl orange on an immobilized Mn-nanoparticle: Kinetic and thermodynamic. RSC Adv. 2014, 4, 16005–16017. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arabian J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Misran, E.; Bani, O.; Situmeang, E.M.; Purba, A.S. Removal efficiency of methylene blue using activated carbon from waste banana stem: Study on pH influence. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012085. [Google Scholar] [CrossRef]
- Tharaneedhar, V.; Kumar, P.S.; Saravanan, A.; Ravikumar, C.; Jaikumar, V. Prediction and interpretation of adsorption parameters for the sequestration of methylene blue dye from aqueous solution using microwave assisted corncob activated carbon. Sustain. Mater. Technol. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Ragab, A.; Ahmed, I.; Bader, D. The Removal of Brilliant Green Dye from Aqueous Solution Using Nano Hydroxyapatite/Chitosan Composite as a Sorbent. Molecules 2019, 24, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M Peydayesh, A.R. Kelishami, Adsorption of methylene blue onto Platanus orientalis leaf powder: Kinetic, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 1014–1019. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Huang, Y.; Huang, N.; Ren, C.; Zhang, W. Adsorption removal of Congo red from aqueous solution bypolyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J. Alloy. Compd. 2015, 633, 338–346. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Po, Q.H. Preparation of chitosan coated magnetic hydroxyapatite nanoparticles and application for adsorption of reactive Blue 19 and Ni2+ ions. Sci. World J. 2014, 2014, 273082. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Le, T.P.T.; Do, H.T.; Vo, H.T.; Pham, N.T.; Nguyen, T.T.; Cao, H.T.; Nguyen, P.T.; Dinh, T.M.T.; Le, H.V.; et al. Fabrication of Porous Hydroxyapatite Granules as an Effective Adsorbent for the Removal of Aqueous Pb(II) Ions. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Sricharoen, P.; Kongsri, S.; Kukusamude, C.; Areerob, Y.; Nuengmatcha, P.; Chanthai, S.; Limchoowong, N. Ultrasound-irradiated synthesis of 3-mercaptopropyl trimethoxysilane-modified hydroxyapatite derived from fish-scale residues followed by ultrasound-assisted organic dyes removal. Sci. Rep. 2018, 11, 5560. [Google Scholar] [CrossRef]
- Joudi, M.; Nasserlah, H.; Hafdi, H.; Mouldar, J.; Hatimi, B.; Mhammedi, M.E.; Bakasse, M. Synthesis of an efficient hydroxyapatite–chitosan–montmorillonite thin film for the adsorption of anionic and cationic dyes, adsorption isotherm, kinetic and thermodynamic study. Sn Appl. Sci. 2020, 2, 1078. [Google Scholar] [CrossRef]
- Barka, N.; Qouzal, S.; Assabbane, A.; Nounhan, A.; Ichou, Y.A. Removal of reactive yellow 84 from aqueous solutions by adsorption onto hydroxyapatite. J. Saudi Chem. Soc. 2011, 15, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Badruddoza, A.Z.M.; Goh, S.S.H.; Hidajat, K.; Uddin, M.S. Synthesis of Carboxymethyl-β-Cyclodextrin Conjugated Magnetic Nano-Adsorbent for Removal of Methylene Blue. Colloids Surf. Physicochem. Eng. Asp. 2010, 367, 85–95. [Google Scholar] [CrossRef]
- Paska, O.M.; Pacurariua, C.; Muntean, S.G. Kinetic and thermodynamic studies on methylene blue biosorption using corn-husk. RSC Adv. 2014, 4, 62621–62630. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauff, M. Catalytic activity of faceted gold nanoparticles studied by a model reaction: Evidence for substrate-induced surface restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Tiozzo, C.; Guidotti, M. Niobium-Containing Hydroxyapatites as Amphoteric Catalysts: Synthesis, Properties, and Activity. ACS Catal. 2014, 4, 469–479. [Google Scholar] [CrossRef]
- Bouyarmane, H.; El Asri, S.; Rami, A.; Roux, C.; Mahly, M.A.; Saoiabi, A.; Coradinc, T.; Laghzizil, A. Pyridine and Phenol Removal Using Natural and Synthetic Apatites as Low Costsorbents: Influence of Porosity and Surface Interactions. J. Hazard Mater. 2010, 181, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, F. Current and emerging serious Gram-positive infections. Clin. Microbiol. Infect. 2005, 11, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriolog. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar]
- Yan, B.; Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control 2006, 34, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kaith, B.S.; Shanker, U.; Gupta, B. γ-radiation induced synthesis of antibacterial silver nanocomposite scaffolds derived from natural gum Boswellia serrata. J. Drug Del Sci. Technol. 2020, 56, 101550. [Google Scholar] [CrossRef]







| Conc. (ppm) | Pseudo First Order Kinetics | Pseudo Second Order Kinetics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe(exp) (mg/g) | k1 (min−1) | qe(cal) (mg/g) | R2 | Δq (%) | k2 (g mg −1 min −1) | qe(cal) (mg/g) | R2 | Δq (%) | |
| 10 | 120 | 1.28 × 10−2 | 127.2 | 0.991 | 1.27 | 117871.2 | 312.5 | 0.7926 | 15.0 |
| 20 | 173.2 | 0.85 × 10−2 | 171.1 | 0.981 | 1.41 | 1026259 | 909.0 | 0.1171 | 18.5 |
| 30 | 174.7 | 1.68 × 10−2 | 190.6 | 0.967 | 2.79 | 35.31249 | −15.528 | 0.3179 | 12.4 |
| 40 | 149.8 | 1.66 × 10−2 | 157.7 | 0.984 | 4.14 | 19655.94 | −166.6 | 0.2816 | 16.9 |
| 50 | 122.6 | 2.34 × 10−2 | 131.6 | 0.976 | 6.72 | 843870.5 | −714.28 | 0.1513 | 19.3 |
| Langmuir Isotherm | Freundlich Isotherm | ||
|---|---|---|---|
| Qm (mg/g) | 200 | Kf | 79.9 |
| b (L/mg) | 0.337 | n | 3.75 |
| R2 | 0.992 | R2 | 0.871 |
| Adsorbent | Adsorption Capacity (Qmax) (mg/g) | Reference |
|---|---|---|
| Poultry eggshell derived apatite | 127.9 | [39] |
| Carboxymethyl cellulose/acrylamide/nano-hydroxyapatite composite hydrogels | 29.52 | [29] |
| Nano Hydroxyapatite/Chitosan Composite | 30.2 | [65] |
| Hydroxyapatite nano-rods | 337.330 | [40] |
| Porous hydroxyapatite (HAp) granules | 6.32 | [69] |
| 3-mercaptopropyl trimethoxysilane-modified hydroxyapatite | 625 | [70] |
| Hydroxyapatite–chitosan–Montmorillonite thin film | 243.18 | [71] |
| Hydroxyapatite | 50.25 | [72] |
| HA/Au nanocomposite | 200 | Present work |
| Temp (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| ∆GO (KJ/mol) | ∆HO (KJ/mol) | ∆SO (J/molK) | |
| 293 | −6.05 | 33.3 | 134.37 |
| 303 | −7.50 | ||
| 313 | −8.73 | ||
| Sample Code | Sample Name | Zone of Inhibition (mm) | ||
|---|---|---|---|---|
| Microc occus luteus (A) | Staphylococcus aureus (B) | Pseudomonas aeruginosa (C) | ||
| 1 | AuNPs | 3 | 4 | 2 |
| 2 | HA/Au nanocomposite | 7 | 23 | 4 |
| 3 | dye adsorbed waste HA/Au nanocomposite | 5 | 21 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, K.; Sharma, S.; Sharma, V.; Mishra, P.K.; Ekielski, A.; Sharma, V.; Kumar, V. Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies. Nanomaterials 2021, 11, 1403. https://doi.org/10.3390/nano11061403
Sharma K, Sharma S, Sharma V, Mishra PK, Ekielski A, Sharma V, Kumar V. Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies. Nanomaterials. 2021; 11(6):1403. https://doi.org/10.3390/nano11061403
Chicago/Turabian StyleSharma, Kashma, Shreya Sharma, Vipasha Sharma, Pawan Kumar Mishra, Adam Ekielski, Vishal Sharma, and Vijay Kumar. 2021. "Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies" Nanomaterials 11, no. 6: 1403. https://doi.org/10.3390/nano11061403
APA StyleSharma, K., Sharma, S., Sharma, V., Mishra, P. K., Ekielski, A., Sharma, V., & Kumar, V. (2021). Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies. Nanomaterials, 11(6), 1403. https://doi.org/10.3390/nano11061403








