Theoretical Investigation of the Prospect to Tailor ZnO Electronic Properties with VP Thin Films
Abstract
1. Introduction
2. Computational Methods
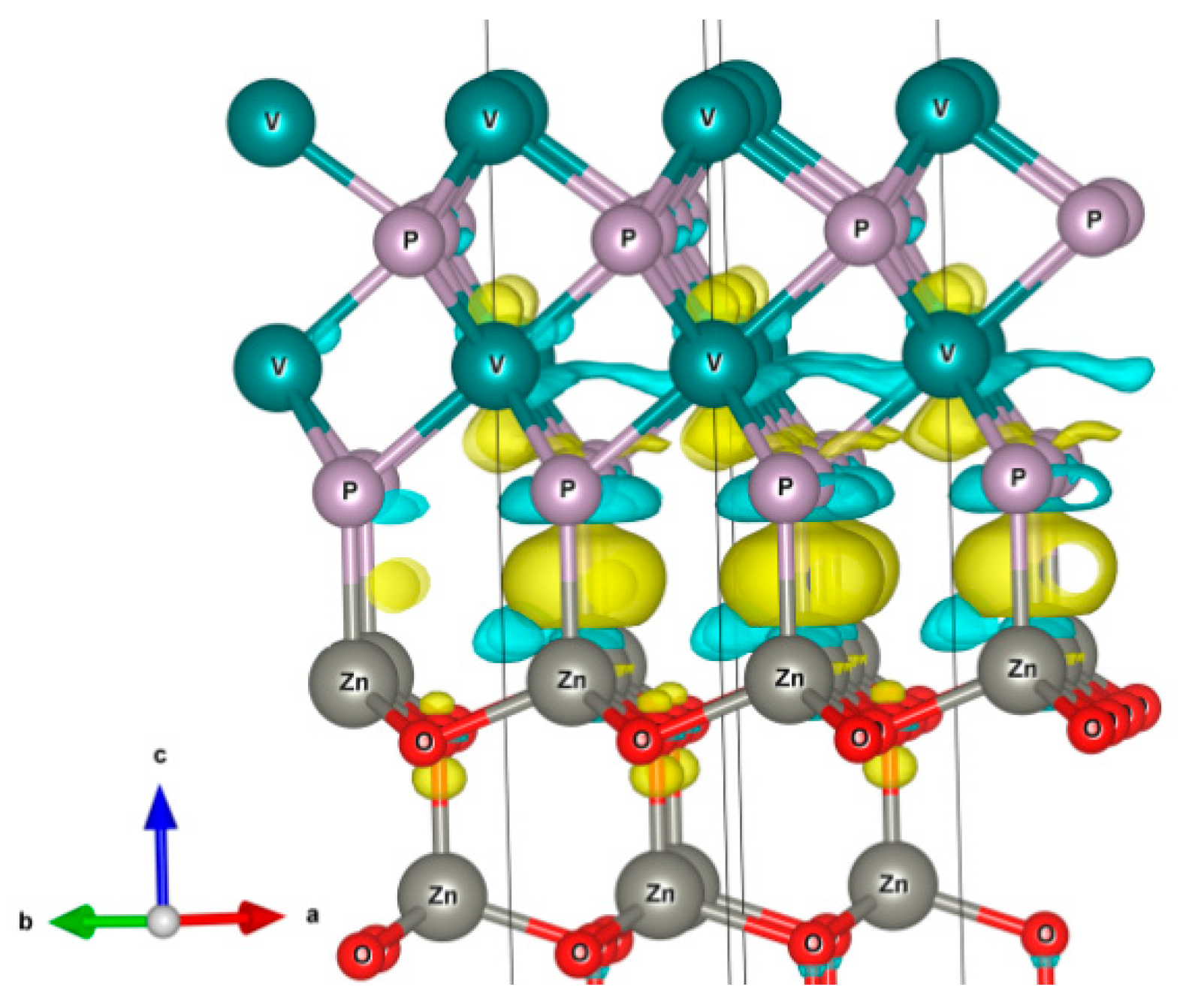
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Boujnah, M.; Boumdyan, M.; Naji, S.; Benyoussef, A.; El Kenz, A.; Loulidi, M. High efficiency of transmittance and electrical conductivity of V doped ZnO used in solar cells applications. J. Alloys Compd. 2016, 671, 560–565. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, S.; Li, C. Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Arshi, N.; Dwivedi, S.; Koo, B.H.; Azam, A.; Alsharaeh, E. Low temperature growth of ZnO nanotubes for fluorescence quenching detection of DNA. J. Mater. Sci. Mater. Med. 2016, 27, 189. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Farooq, W.A.; Khan, M.I.; Akhtar, M.N.; Rehman, S.U.; Ahmad, N.; Khalid, M.; Atif, M.; AlMutairi, M.A.; Irfan, M. Effect of ZnO Nanoparticles Coating Layers on Top of ZnO Nanowires for Morphological, Optical, and Photovoltaic Properties of Dye-Sensitized Solar Cells. Micromachines 2019, 10, 819. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yi, Z.; Zhou, Z.; Tang, Y.; Yi, Y. Fabriction of ZnO Nanorods with Strong UV Absorption and Different Hydrophobicity on Foamed Nickel under Different Hydrothermal Conditions. Micromachines 2019, 10, 164. [Google Scholar] [CrossRef]
- Lamson, T.L.; Khan, S.; Wang, Z.; Zhang, Y.K.; Yu, Y.; Chen, Z.S.; Xu, H. Patterned Synthesis of ZnO Nanorod Arrays for Nanoplasmonic Waveguide Applications. Opt. Commun. 2018, 411, 53–58. [Google Scholar] [CrossRef]
- Lupan, O.; Pauporté, T.; Viana, B. Low-voltage UV-electroluminescence from ZnO-nanowire Array/p-GaN light-emitting diodes. Adv. Mater. 2010, 22, 3298–3302. [Google Scholar] [CrossRef]
- Pauporté, T.; Lupan, O.; Zhang, J.; Tugsuz, T.; Ciofini, I.; Labat, F.; Viana, B. Low-Temperature Preparation of Ag-Doped ZnO Nanowire Arrays, DFT Study, and Application to Light-Emitting Diode. ACS Appl. Mater. Interfaces 2015, 7, 11871–11880. [Google Scholar] [CrossRef]
- Lupan, O.; Pauporté, T.; Le Bahers, T.; Ciofini, I.; Viana, B. High Aspect Ratio Ternary Zn1−xCdxO Nanowires by Electrodeposition for Light-Emitting Diode Applications. J. Phys. Chem. C 2011, 115, 14548–14558. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Yan, L.; Jiang, J.; Han, X.; Deng, G.; Chi, C.; Song, J. n-ZnO/p-GaN heterojunction light-emitting diodes featuring a buried polarization-induced tunneling junction. AIP Adv. 2016, 6, 125204. [Google Scholar] [CrossRef]
- Rahman, M.A.; Scott, J.A.; Gentle, A.; Phillips, M.R.; Ton-That, C. A facile method for bright, colour-tunable light-emitting diodes based on Ga-doped ZnO nanorods. Nanotechnology 2018, 29, 425707. [Google Scholar] [CrossRef]
- Davydova, M.; Laposa, A.; Smarhak, J.; Kromka, A.; Neykova, N.; Nahlik, J.; Kroutil, J.; Drahokoupil, J.; Voves, J. Gas-sensing behaviour of ZnO/diamond nanostructures. Beilstein J. Nanotechnol. 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Do, T.A.T.; Ho, T.G.; Bui, T.H.; Pham, Q.N.; Giang, H.T.; Do, T.T.; Nguyen, D.V.; Tran, D.L. Surface-plasmon-enhanced ultraviolet emission of Au-decorated ZnO structures for gas sensing and photocatalytic devices. Beilstein J. Nanotechnol. 2018, 9, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Nugraha; Saputro, A.G.; Agusta, M.K.; Yuliarto, B.; Dipojono, H.K.; Rusydi, F.; Maezono, R. Selectivity of CO and NO adsorption on ZnO (0002) surfaces: A DFT investigation. Appl. Surf. Sci. 2017, 410, 373–382. [Google Scholar] [CrossRef]
- Lin, C.F.; Kao, C.H.; Lin, C.Y.; Chen, K.L.; Lin, Y.H. NH3 Plasma-Treated Magnesium Doped Zinc Oxide in Biomedical Sensors with Electrolyte–Insulator–Semiconductor (EIS) Structure for Urea and Glucose Applications. Nanomaterials 2020, 10, 583. [Google Scholar] [CrossRef]
- Bai, Z.Q.; Liu, Z.W. A broadband photodetector based on Rhodamine B-sensitized ZnO nanowires film. Sci Rep. 2017, 7, 11384. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, P.; Wang, N.; Ma, Y.; San, H. UV-Assisted Photochemical Synthesis of Reduced Graphene Oxide/ZnO Nanowires Composite for Photoresponse Enhancement in UV Photodetectors. Nanomaterials 2018, 8, 26. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Taher, M.A.; Tamaddon, A.M. Facile synthesis and characterization of magnetic nanocomposite ZnO/CoFe2O4 hetero-structure for rapid photocatalytic degradation of imidacloprid. Heliyon 2019, 5, e02870. [Google Scholar] [CrossRef]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Luo, S.; Xiang, L.; Li, W.; Xie, D. Unraveling photoexcited electron transfer pathway of oxygen vacancy-enriched ZnO/Pd hybrid toward visible light-enhanced methane detection at a relatively low temperature. Appl. Catal. B Environ. 2020, 264, 118554. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Xia, Y.; Zhang, B. Mesoporous ZnO nanosheets with rich surface oxygen vacancies for UV-activated methane gas sensing at room temperature. Sens. Actuators B Chem. 2021, 333, 129547. [Google Scholar] [CrossRef]
- Khai, T.V.; Thu, L.V.; Ha, L.T.T.; Thanh, V.M.; Lam, T.D. Structural, optical and gas sensing properties of vertically well-aligned ZnO nanowires grown on graphene/Si substrate by thermal evaporation method. Mater. Charact. 2018, 141, 296–317. [Google Scholar] [CrossRef]
- Mondal, P.; Appani, S.K.; Sutar, D.S.; Major, S.S. Effect of oxygen partial pressure on the behavior of Ga-doped ZnO/p-Si heterojunction diodes fabricated by reactive sputtering. J. Mater. Sci. Mater. Electron. 2021, 32, 4248–4257. [Google Scholar] [CrossRef]
- Pham, A.T.T.; Hoang, D.V.; Nguyen, T.H.; Le, O.K.T.; Wong, D.P.; Kuo, J.L.; Chen, K.H.; Phan, T.B.; Tran, V.C. Hydrogen enhancing Ga doping efficiency and electron mobility in high-performance transparent conducting Ga-doped ZnO films. J. Alloys Compd. 2021, 860, 158518. [Google Scholar] [CrossRef]
- Simanjuntak, F.M.; Prasad, O.K.; Panda, D.; Lin, C.A.; Tsai, T.L.; Wei, K.H.; Tseng, T.Y. Impacts of Co doping on ZnO transparent switching memory device characteristics. Appl. Phys. Lett. 2016, 108, 183506. [Google Scholar] [CrossRef]
- Drewelow, G.; Reed, A.; Stone, C.; Roh, K.; Jiang, Z.T.; Truc, L.N.T.; No, K.; Park, H.; Lee, S. Work function investigations of Al-doped ZnO for band-alignment in electronic and optoelectronic applications. Appl. Surf. Sci. 2019, 484, 990–998. [Google Scholar] [CrossRef]
- Gao, Q.; Dai, Y.; Han, B.; Zhu, W.; Li, X.; Li, C. Enhanced gas-sensitivity and ferromagnetism performances by the Ni-doping induced oxygen vacancies in (Mn, Ni) codoped ZnO nanorods. Appl. Surf. Sci. 2019, 490, 178–187. [Google Scholar] [CrossRef]
- Ali, N.; Vijaya, A.R.; Khan, Z.A.; Tarafder, K.; Kumar, A.; Wadhwa, M.K.; Singh, B.; Ghosh, S. Ferromagnetism from non-magnetic ions: Ag-doped ZnO. Sci. Rep. 2019, 9, 20039. [Google Scholar] [CrossRef]
- Hurma, T. Effect of boron doping concentration on structural optical electrical properties of nanostructured ZnO films. J. Mol. Struct. 2019, 1189, 1–7. [Google Scholar] [CrossRef]
- Sankar ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Ajala, F.; Hamrouni, A.; Houas, A.; Lachheb, H.; Megna, B.; Palmisano, L.; Parrino, F. The influence of Al doping on the photocatalytic activity of nanostructured ZnO: The role of adsorbed water. Appl. Surf. Sci. 2018, 445, 376–382. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Ren, G.K.; Tan, X.; Liu, R.; Liu, C.; Lin, Y.H.; Nan, C.W. Enhancing the thermoelectric performance of ZnO epitaxial films by Ga doping and thermal tuning. J. Mater. Chem. A 2018, 6, 24128–24135. [Google Scholar] [CrossRef]
- Jiang, M.; He, G.; Chen, H.; Zhang, Z.; Zheng, L.; Shan, C.; Shen, D.; Fang, X. Wavelength-Tunable Electroluminescent Light Sources from Individual Ga-Doped ZnO Microwires. Small 2017, 13, 1604034. [Google Scholar] [CrossRef]
- Bharath, S.P.; Bangera, K.V.; Shivakumar, G.K. Enhanced gas sensing properties of indium doped ZnO thin films. Superlattices Microstruct. 2018, 124, 72–78. [Google Scholar] [CrossRef]
- Chang, L.W.; Sung, Y.C.; Yeh, J.W.; Shih, H.C. Enhanced optoelectronic performance from the Ti-doped ZnO nanowires. J. Appl. Phys. 2011, 109, 074318. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Nogueira, A.E.; Gonçalves, M.C.P.; Paris, E.C.; Ribeiro, C.; Poirier, G.Y.; Giraldi, T.R. Photoactivity of N-doped ZnO nanoparticles in oxidative and reductive reactions. Appl. Surf. Sci. 2018, 433, 879–886. [Google Scholar] [CrossRef]
- Kumari, V.; Mittal, A.; Jindal, J.; Yadav, S.; Kumar, N. S-, N- and C-doped ZnO as semiconductor photocatalysts: A review. Front. Mater. Sci. 2019, 13, 1–22. [Google Scholar] [CrossRef]
- Gayen, R.N.; Paul, R. Phosphorous doping in vertically aligned ZnO nanorods grown by wet-chemical method. Nano-Struct. Nano-Objects 2018, 13, 163–169. [Google Scholar] [CrossRef]
- Murkute, P.; Sushama, S.; Ghadi, H.; Saha, S.; Chakrabarti, S. Effects of phosphorus implantation time on the optical, structural, and elemental properties of ZnO thin films and its correlation with the 3.31-eV peak. J. Alloys Compd. 2018, 768, 800–809. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H. Effect of Sb doping on the electrical and dielectric properties of ZnO nanocrystals. Ceram. Int. 2019, 45, 8000–8007. [Google Scholar] [CrossRef]
- Joshi, B.N.; Yoon, H.; Na, S.-H.; Choi, J.-Y.; Yoon, S.S. Enhanced photocatalytic performance of graphene-ZnO nanoplatelet composite thin films prepared by electrostatic spray deposition. Ceram. Int. 2014, 40, 3647–3654. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.; Abdala, A. Solvent-free synthesis of ZnO-graphene nanocomposite with superior photocatalytic activity. Appl. Surf. Sci. 2019, 465, 1107–1113. [Google Scholar] [CrossRef]
- Xue, B.; Zou, Y. High photocatalytic activity of ZnO–graphene composite. J. Colloid Interface Sci. 2018, 529, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Tran, D.T.; Nguyen, M.T.; Le, T.T.T.; Ha, M.N.; Nguyen, M.V.; Pham, T.D. Enhanced photocatalytic degradation of methyl orange using ZnO/graphene oxide nanocomposites. Res. Chem. Intermed. 2018, 44, 3081–3095. [Google Scholar] [CrossRef]
- Guo, J.; Legum, B.; Anasori, B.; Wang, K.; Lelyukh, P.; Gogotsi, Y.; Randall, C.A. Cold Sintered Ceramic Nanocomposites of 2D MXene and Zinc Oxide. Adv. Mater. 2018, 30, 1801846. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Lu, P.; Wu, J.; Shen, X.; Gao, X.; Shi, Z.; Lu, M.; Yu, W.W.; Zhang, Y. ZnO–Ti3C2 MXene Electron Transport Layer for High External Quantum Efficiency Perovskite Nanocrystal Light-Emitting Diodes. Adv. Sci. 2020, 7, 2001562. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, R.; Zhang, J.; Shia, Y.; Zhang, B. Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 2013, 49, 6656–6658. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Tian, L.; Yan, X.; Chen, X. Electrochemical Activity of Iron Phosphide Nanoparticles in Hydrogen Evolution Reaction. ACS Catal. 2016, 6, 5441–5448. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Crompton, J.C.; Callejas, J.F.; Popczun, E.J.; Read, C.G.; Lewis, N.S.; Schaak, R.E. Electrocatalytic hydrogen evolution using amorphous tungsten phosphide nanoparticles. Chem. Commun. 2014, 50, 11026–11028. [Google Scholar] [CrossRef] [PubMed]
- Callejas, J.F.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Synthesis, Characterization, and Properties of Metal Phosphide Catalysts for the Hydrogen-Evolution Reaction. Chem. Mater. 2016, 28, 6017–6044. [Google Scholar] [CrossRef]
- Li, C.; Gao, H.; Wan, W.; Mueller, T. Mechanisms for hydrogen evolution on transition metal phosphide catalysts and a comparison to Pt (111). Phys. Chem. Chem. Phys. 2019, 21, 24489–24498. [Google Scholar] [CrossRef]
- Shao, Y.; Shi, X.; Pan, H. Electronic, Magnetic, and Catalytic Properties of Thermodynamically Stable Two-Dimensional Transition-Metal Phosphides. Chem. Mater. 2017, 29, 8892–8900. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Zhang, H.; Liu, H.; Yu, X.; Dai, X.; Liu, G.; Chen, G. Ti2P monolayer as a high performance 2-D electrode material for ion batteries. Phys. Chem. Chem. Phys. 2020, 22, 18480–18487. [Google Scholar] [CrossRef]
- Liu, Q.; Xing, J.; Jiang, Z.; Jiang, X.; Wang, Y.; Zhao, J. 2D tetragonal transition-metal phosphides: An ideal platform to screen metal shrouded crystals for multifunctional applications. Nanoscale 2020, 12, 6776–6784. [Google Scholar] [CrossRef]
- Kadioglu, Y. Ballistic transport and optical properties of a new half-metallic monolayer: Vanadium phosphide. Mater. Sci. Eng. B 2021, 268, 115111. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Sowa, H.; Ahsbahs, H. High-pressure X-ray investigation of zincite ZnO single crystals using diamond anvils with an improved shape. J. Appl. Cryst. 2006, 39, 169–175. [Google Scholar] [CrossRef]
- Schoenberg, N. An X-ray investigation of transition metal phosphides. Acta Chem. Scand. 1954, 8, 226–239. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Biz, C.; Fianchini, M.; Gracia, J. Catalysis Meets Spintronics; Spin Potentials Associated with Open-Shell Orbital Configurations Enhance the Activity of Pt3Co Nanostructures for Oxygen Reduction: A Density Functional Theory Study. ACS Appl. Nano Mater. 2020, 3, 506–515. [Google Scholar] [CrossRef]
- Campbell, V.E.; Tonelli, M.; Cimatti, I.; Moussy, J.-B.; Tortech, L.; Dappe, Y.J.; Rivière, E.; Guillot, R.; Delprat, S.; Mattana, R.; et al. Engineering the magnetic coupling and anisotropy at the molecule–magnetic surface interface in molecular spintronic devices. Nat. Commun. 2016, 7, 13646. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Comput. Mater. 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. An improved grid-based algorithm for Bader charge allocation. J. Comp. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 254–360. [Google Scholar] [CrossRef]
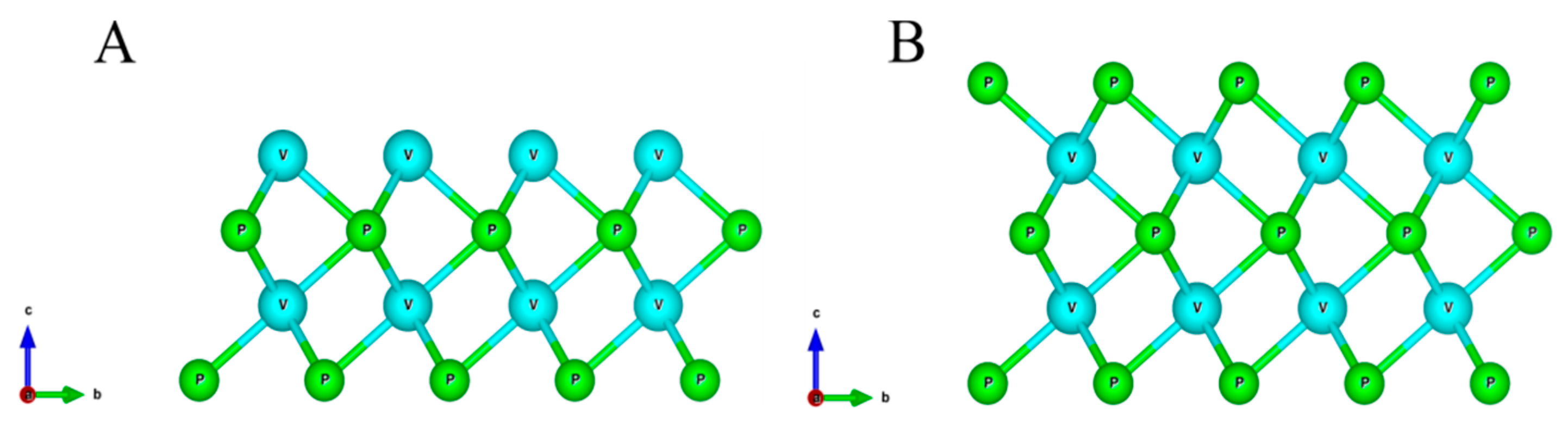
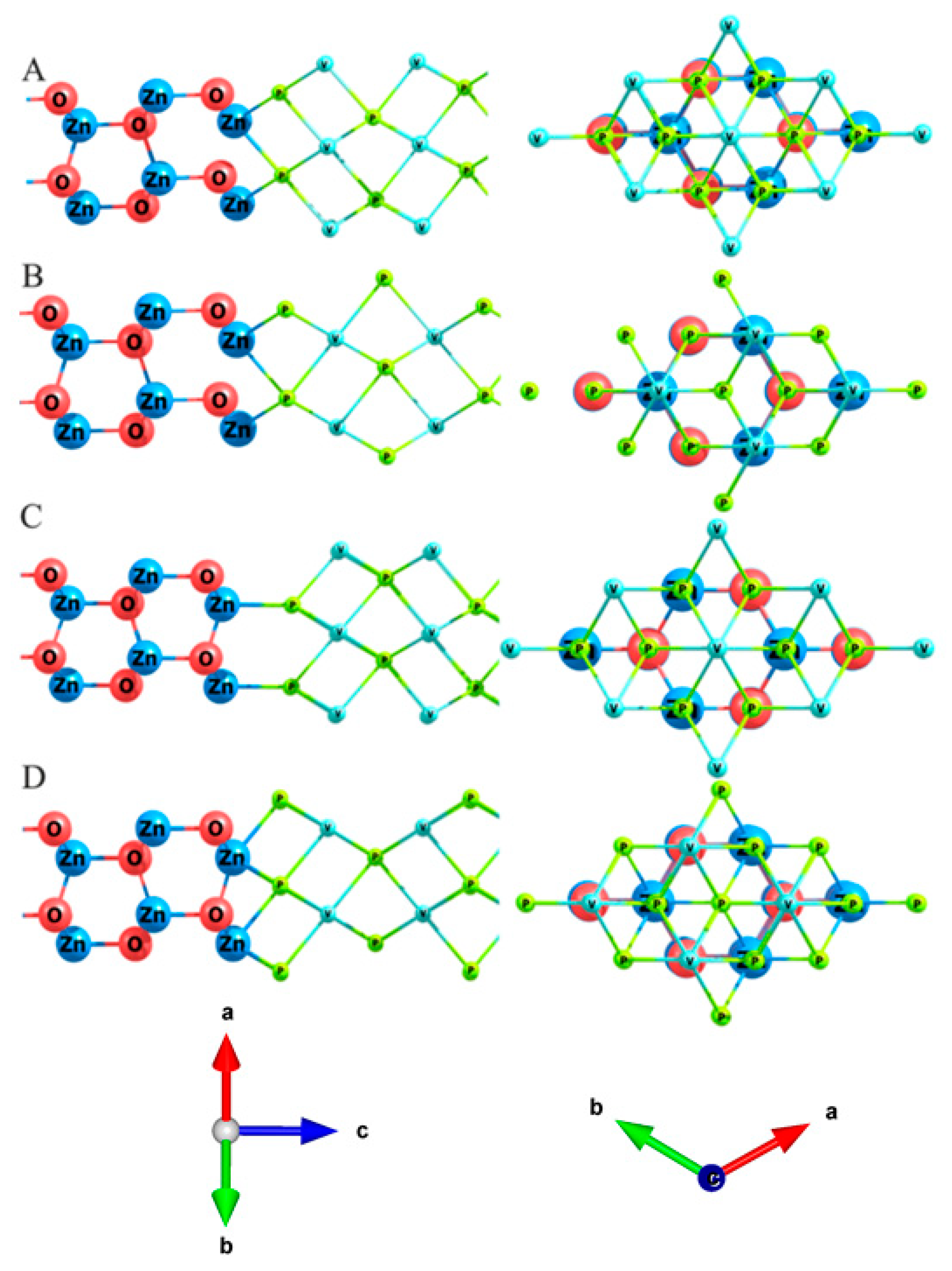
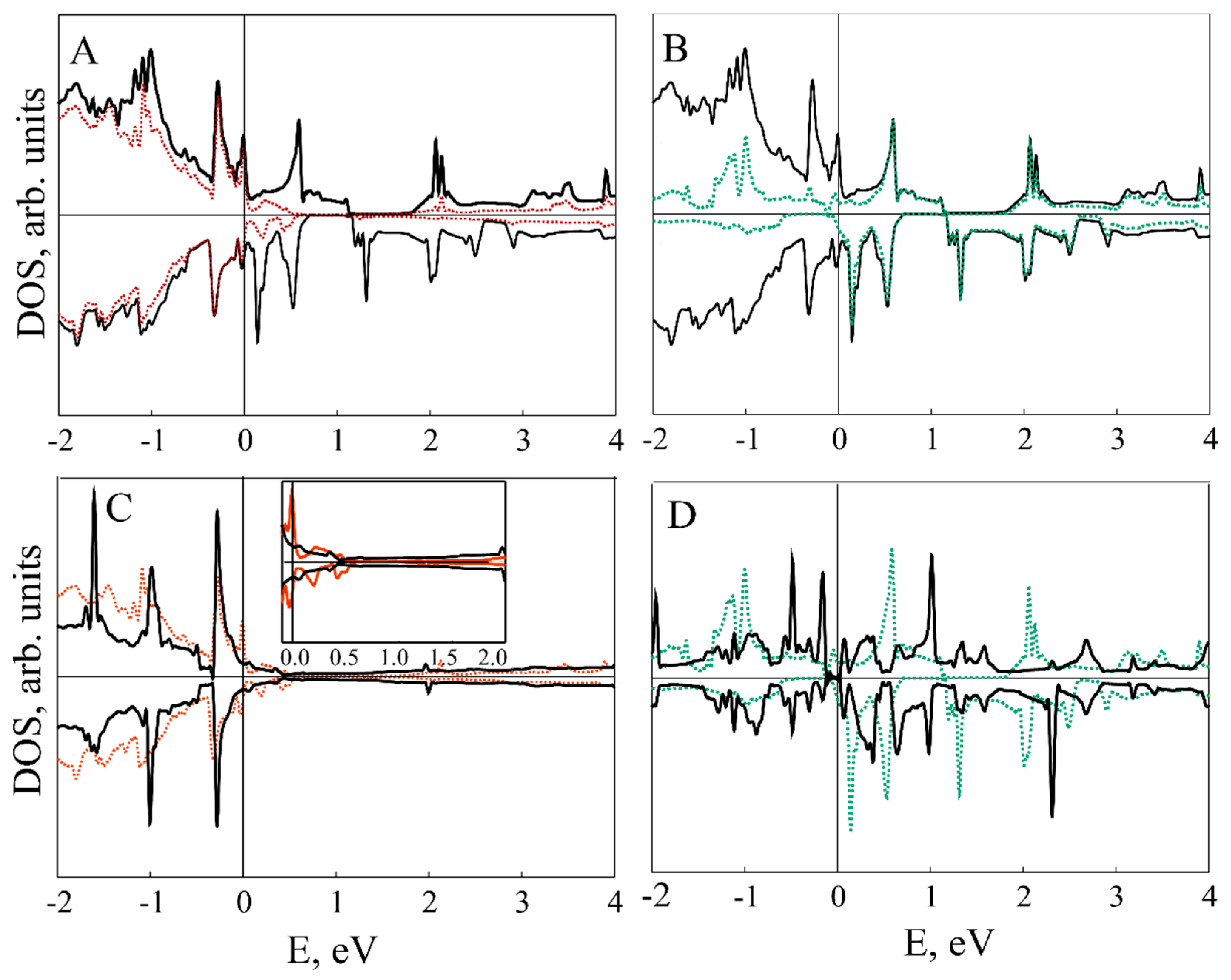

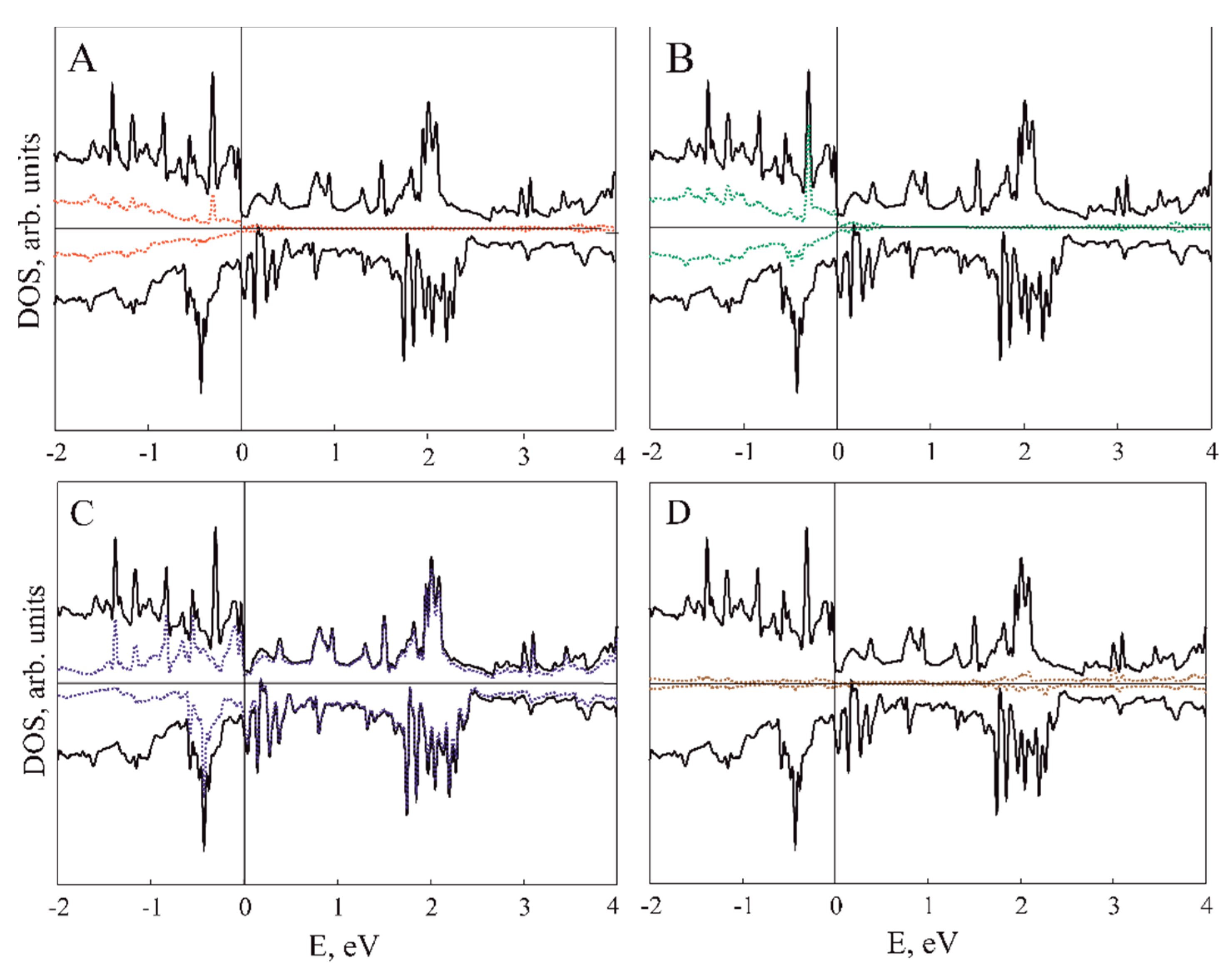


| Configuration | Magnetic Moment, μB | a, Å |
|---|---|---|
| Bulk VP | 0.000 | 3.130 |
| VP monolayer | 2.000 | 2.962 |
| VP2 | 0.756 | 3.214 |
| V2P2 | 1.636 | 3.058 |
| V2P3 | 0.000 | 3.058 |
| V3P3 | 1.511 | 3.078 |
| V3P4 | 0.147 | 3.111 |
| V4P4 | 1.791 | 3.086 |
| V4P5 | 0.025 | 3.115 |
| Structure | V4P4 | VP Monolayer | ||
|---|---|---|---|---|
| Estack, eV | µ, µB | Estack, eV | µ, µB | |
| [P_top_O:V_hex] | −0.637 | 1.159 | −0.465 | 1.390 |
| [P_top_O:P_hex] | −0.644 | 1.206 | −0.655 | 2.163 |
| [P_top_Zn:V_hex] | −1.273 | 1.242 | −1.167 | 2.285 |
| [V_top_O:P_hex] | −0.705 | 1.250 | −0.772 | |
| Composite Structure | VP2 | V3P4 |
|---|---|---|
| [P_top_O:V_hex] | −1.861 | −3.975 |
| [P_top_O:P_hex] | −1.651 | −3.781 |
| [P_top_Zn:V_hex] | −2.204 | −4.388 |
| [V_top_O:P_hex] | −1.662 | −3.781 |
| Number of Layers | QVP, e− | µVP, µB |
|---|---|---|
| 4 | −0.117 | 1.896 |
| 3 | −0.186 | 1.355 |
| 2 | −0.177 | 1.842 |
| 1 | −0.079 | 2.254 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholtobina, A.S.; Kovaleva, E.A.; Melchakova, J.; Ovchinnikov, S.G.; Kuzubov, A.A. Theoretical Investigation of the Prospect to Tailor ZnO Electronic Properties with VP Thin Films. Nanomaterials 2021, 11, 1412. https://doi.org/10.3390/nano11061412
Kholtobina AS, Kovaleva EA, Melchakova J, Ovchinnikov SG, Kuzubov AA. Theoretical Investigation of the Prospect to Tailor ZnO Electronic Properties with VP Thin Films. Nanomaterials. 2021; 11(6):1412. https://doi.org/10.3390/nano11061412
Chicago/Turabian StyleKholtobina, Anastasiia S., Evgenia A. Kovaleva, Julia Melchakova, Sergey G. Ovchinnikov, and Alexander A. Kuzubov. 2021. "Theoretical Investigation of the Prospect to Tailor ZnO Electronic Properties with VP Thin Films" Nanomaterials 11, no. 6: 1412. https://doi.org/10.3390/nano11061412
APA StyleKholtobina, A. S., Kovaleva, E. A., Melchakova, J., Ovchinnikov, S. G., & Kuzubov, A. A. (2021). Theoretical Investigation of the Prospect to Tailor ZnO Electronic Properties with VP Thin Films. Nanomaterials, 11(6), 1412. https://doi.org/10.3390/nano11061412







