Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation Method
2.2. MWs Analysis
2.3. Particle Size Analysis
2.4. Microstructure Analysis
2.5. Cell Culture
2.6. Cell-Based Antioxidant Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. MWs Distributions
3.2. Particle Size Analysis
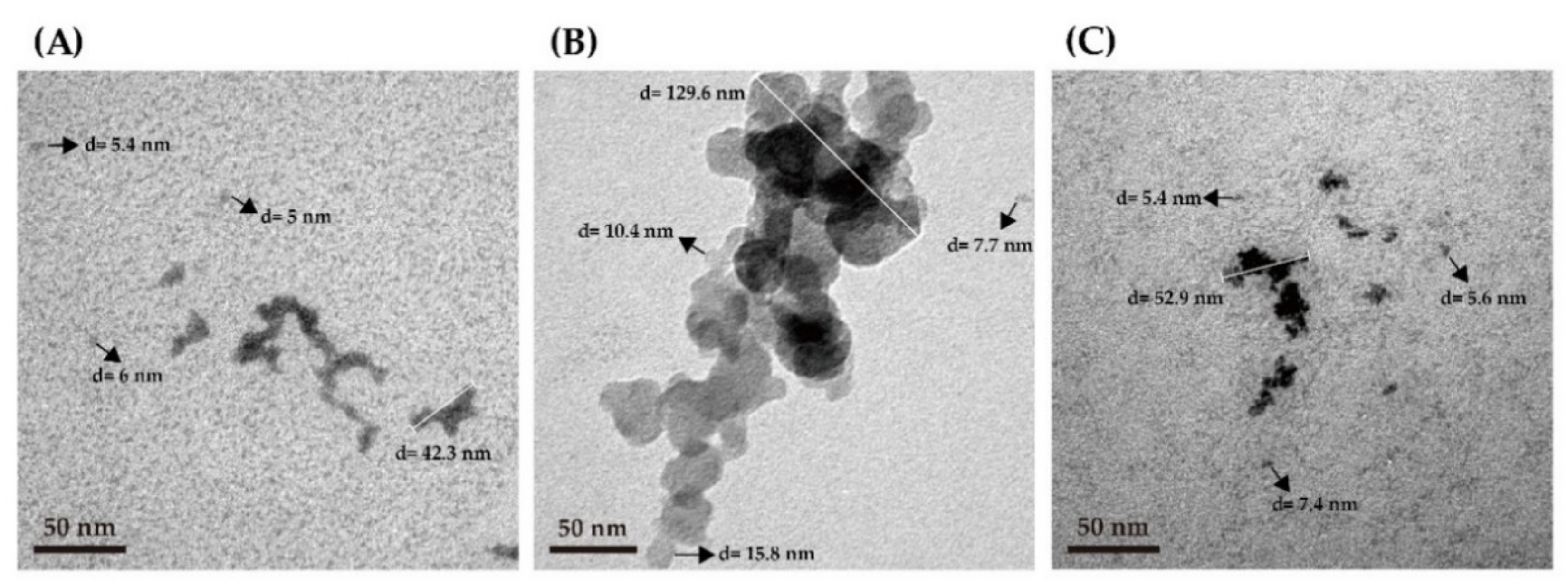
3.3. Microstructure Analysis
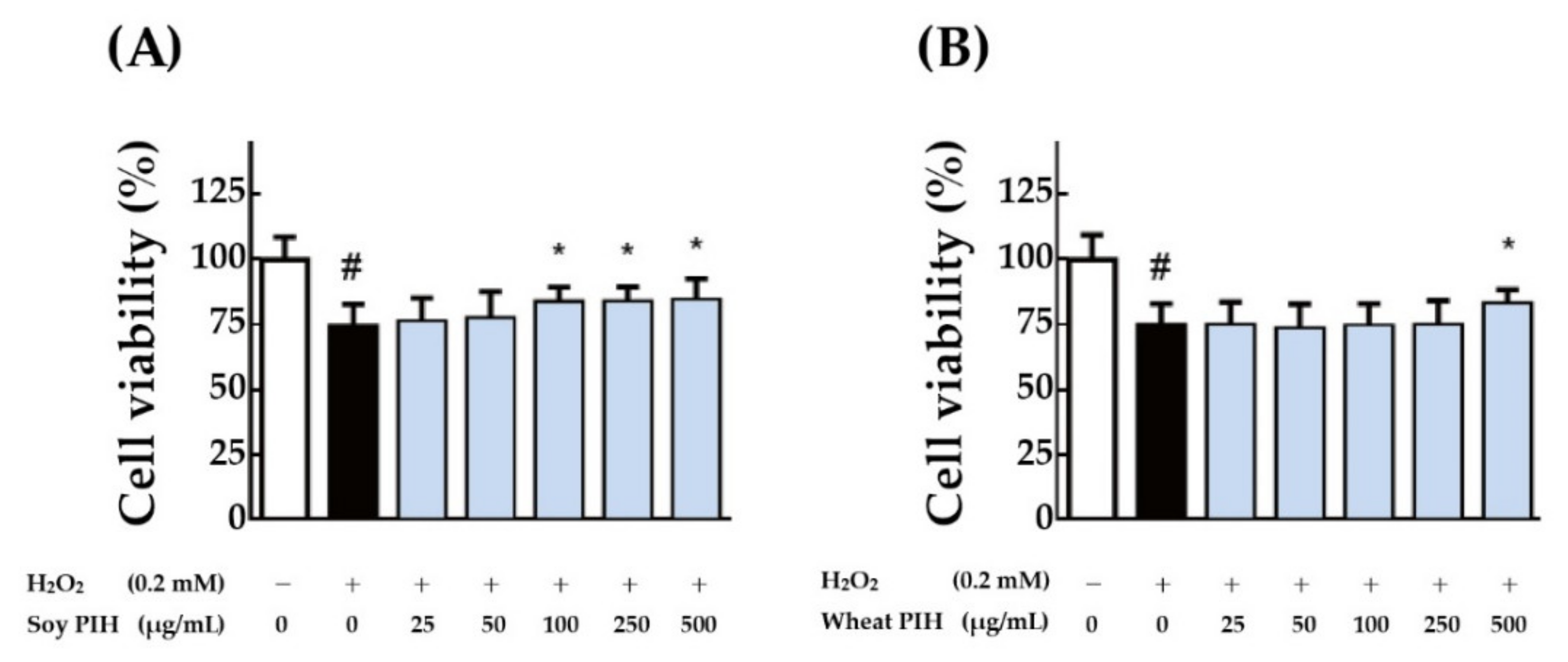
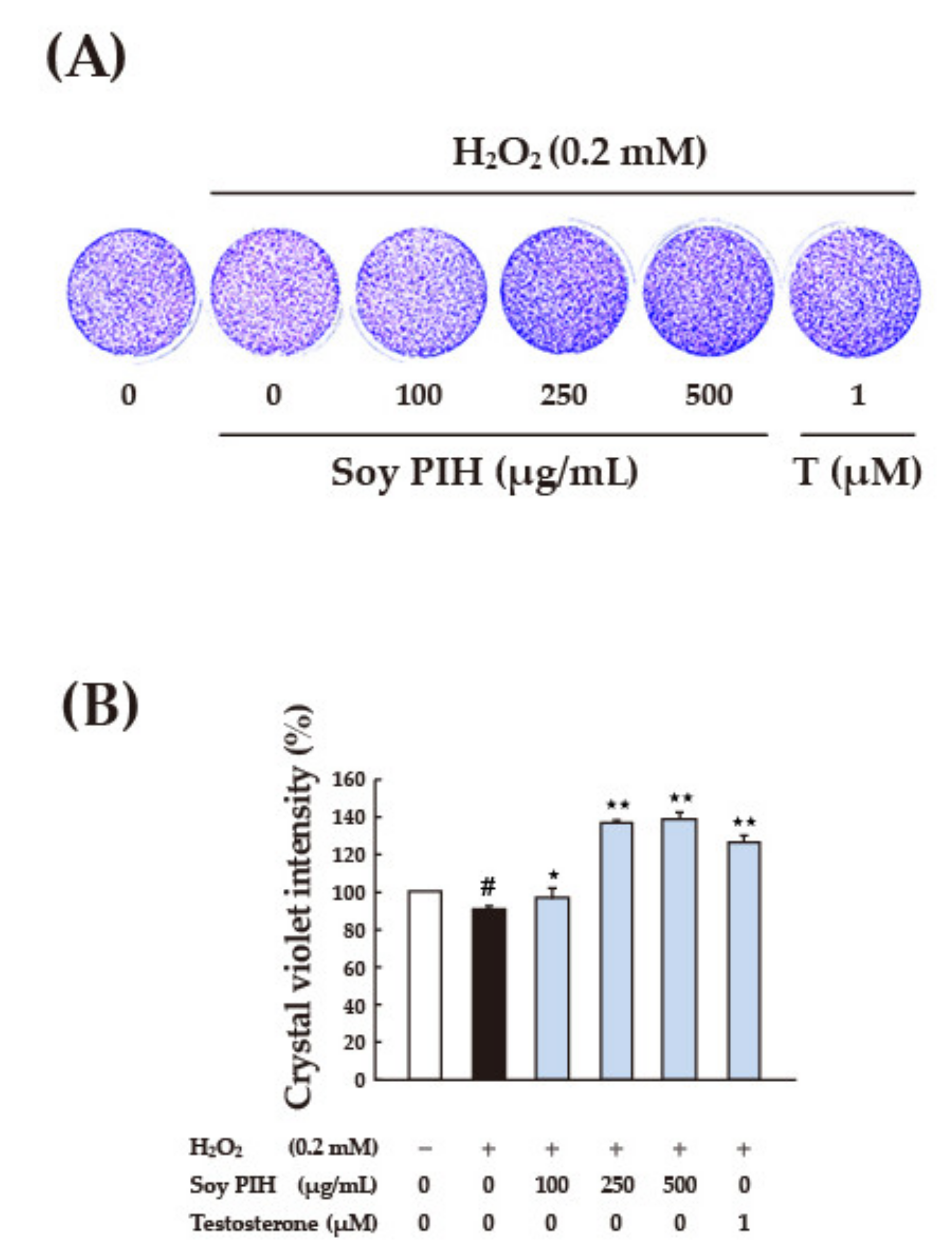
3.4. Cell-Based Antioxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F. Animal and plant protein sources and cardiometabolic health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Stewart, S.E.; Jayalath, V.H.; Ng, A.P.; Mirrahimi, A.; de Souza, R.J.; Hanley, A.J.; Bazinet, R.P.; Mejia, S.B.; Leiter, L.A.; et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2015, 7, 9804–9824. [Google Scholar] [CrossRef] [PubMed]
- Eshel, G.; Stainier, P.; Shepon, A.; Swaminathan, A. Environmentally optimal, nutritionally sound, protein and energy conserving plant based alternatives to U.S. meat. Sci. Rep. 2019, 9, 10345. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Akbari, N.; Milani, J.M.; Biparva, P. Functional and conformational properties of proteolytic enzyme-modified potato protein isolate. J. Sci. Food Agric. 2020, 100, 1320–1327. [Google Scholar] [CrossRef]
- Asokan, S.M.; Wang, T.; Wang, M.F.; Lin, W.T. A novel dipeptide from potato protein hydrolysate augments the effects of exercise training against high-fat diet-induced damages in senescence-accelerated mouse-prone 8 by boosting pAMPK / SIRT1/ PGC-1α/ pFOXO3 pathway. Aging 2020, 12, 7334–7349. [Google Scholar] [CrossRef]
- Do Evangelho, J.A.; Vanier, N.L.; Pinto, V.Z.; De Berrios, J.J.; Dias, A.R.G.; da Rosa Zavareze, E. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef]
- Azócar, M.I.; Alarcón, R.; Castillo, A.; Blamey, J.M.; Walter, M.; Paez, M. Capping of silver nanoparticles by anti-inflammatory ligands: Antibacterial activity and superoxide anion generation. J. Photochem. Photobiol. B 2019, 193, 100–108. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Saffari, A.; Jacobs, J.; Baek, K.I.; Hough, G.; Larauche, M.H.; Ma, J.; Jen, N.; Moussaoui, N.; et al. Ambient ultrafine particle ingestion alters gut microbiota in association with increased atherogenic lipid metabolites. Sci. Rep. 2017, 7, 42906. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Tanidjaja, I.; Damodaran, S. Nanostructure and functionality of enzymatically repolymerized whey protein hydrolysate. Food Chem. 2018, 256, 405–412. [Google Scholar] [CrossRef]
- Danquah, M.K.; Agyei, D. Pharmaceutical applications of bioactive peptides. OA Biotechnol. 2012, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Noreen, S.; Siddiqa, A.; Fatima, R.; Anwar, F.; Adnan, M.; Raza, A. Protease production and purification from agro industrial waste by utilizing Penicillium digitatum. Int. J. Appl. Biol. Forensic 2017, 1, 119–129. [Google Scholar]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Mikiashvili, N. Effectiveness of different proteases in reducing allergen content and IgE-binding of raw peanuts. Food Chem. 2020, 307, 125565. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an enzyme preparation with industrial relevance: Automated nine-step purification and partial characterization of eight enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Tipbunjong, C.; Sookbangnop, P.; Ajavakom, V.; Suksamrarn, A.; Kitiyanant, Y.; Pholpramool, C. Synthetic curcuminoid analogues abrogate oxidationinduced cell death and promote myogenic differentiation of C2C12 mouse myoblasts. Trop. J. Pharm. Res. 2018, 17, 1483–1489. [Google Scholar] [CrossRef]
- Salucci, S.; Battistelli, M.; Burattini, S.; Squillace, C.; Canonico, B.; Gobbi, P.; Papa, S.; Falcieri, E. C2C12 myoblast sensitivity to different apoptotic chemical triggers. Micron 2010, 41, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ahn, C.W.; Kim, H.J.; Lee, K.A.; Park, O.J.; Kwon, D.Y. Black soybean peptide mixture purified from Rhynchosia volubilis exerts antioxidant activity against H2O2-induced cytotoxicity and improves thrombosis. J. Med. Plants Res. 2011, 5, 6477–6483. [Google Scholar]
- Wali, A.; Mijiti, Y.; Yanhua, G.; Yili, A.; Aisa, H.A.; Kawuli, A. Isolation and identification of a novel antioxidant peptide from chickpea (Cicer arietinum L.) sprout protein hydrolysates. Int. J. Pept. Res. Ther. 2021, 27, 219–227. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Kudo, K.; Onodera, S.; Takeda, Y.; Benkeblia, N.; Shiomi, N. Antioxidative activities of some peptides isolated from hydrolyzed potato protein extract. J. Funct. Foods 2009, 1, 170–176. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, T.S.; Mu, T.H. Production and characterisation of antioxidant peptides from sweet potato protein by enzymatic hydrolysis with radio frequency pretreatment. Int. J. Food Sci. Technol. 2020, 55, 2352–2358. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Wang, L.S.; Wu, L.C.; Lu, S.Y.; Chang, L.L.; Teng, I.T.; Yang, C.M.; Ho, J.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Vucelic-Radovic, B.V. Assessment of soy genotype and processing method on quality of soybean tofu. J. Agric. Food Chem. 2011, 59, 7368–7376. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Hsia, S.-Y.; Chan, Y.-C.; Hsieh, J.-F. Complex coacervation of soy proteins, isoflavones and chitosan. Molecules 2017, 22, 1022. [Google Scholar] [CrossRef] [Green Version]
- DuPont, F.M.; Samoil, V.; Chan, R. Extraction of up to 95% of wheat (Triticum aestivum) flour protein using warm sodium dodecyl sulfate (SDS) without reduction or sonication. J. Agric. Food Chem. 2008, 56, 7431–7438. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [Green Version]
- Opazo-Navarrete, M.; Altenburg, M.D.; Boom, R.M.; Janssen, A.E.M. The effect of gel microstructure on simulated gastric digestion of protein gels. Food Biophys. 2018, 13, 124–138. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Karamać, M.; Rybarczyk, A. Chymotryptic hydrolysis of lentil meal proteins and characteristics of the resulting hydrolysates. Pol. J. Food Nutr. Sci. 2008, 58, 351–357. [Google Scholar]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Chang, C.Y.; Jin, J.D.; Chang, H.L.; Huang, K.C.; Chiang, Y.F.; Hsia, S.M. Physicochemical and antioxidative characteristics of potato protein isolate hydrolysate. Molecules 2020, 25, 4450. [Google Scholar] [CrossRef]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17beta-estradiol-hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef]
- Midelfort, K.S.; Wittrup, K.D. Context-dependent mutations predominate in an engineered high-affinity single chain antibody fragment. Protein Sci. 2006, 15, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.J.; Pardeshi, N.N.; Mulder, A.M.; Mulligan, S.K.; Quispe, J.; On, K.; Carragher, B.; Potter, C.S.; Carpenter, J.F.; Schneemann, A. Transmission electron microscopy as an orthogonal method to characterize protein aggregates. J. Pharm. Sci. 2015, 104, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Wang, L.L.; Xiong, Y.L. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability †. J. Agric. Food Chem. 2005, 53, 9186–9192. [Google Scholar] [CrossRef]
- Hood, D.A.; Uguccioni, G.; Vainshtein, A.; D’souza, D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: Implications for health and disease. Compr. Physiol. 2011, 1, 1119–1134. [Google Scholar]
- Nishida, H.; Ichikawa, H.; Konishi, T. Shengmai-san enhances antioxidant potential in C2C12 myoblasts through the induction of intracellular glutathione peroxidase. J. Pharmacol. Sci. 2007, 105, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [Green Version]
- Pronsato, L.; Boland, R.; Milanesi, L. Testosterone exerts antiapoptotic effects against H2O2 in C2C12 skeletal muscle cells through the apoptotic intrinsic pathway. J. Endocrinol. 2012, 212, 371–381. [Google Scholar] [CrossRef] [Green Version]
- La Colla, A.B.; Pronsato, L.; Ronda, A.C.; Milanesi, L.M.; Vasconsuelo, A.A.; Boland, R.L. 17β-estradiol and testosterone protect mitochondria against oxidative stress in skeletal muscle cells. Actual. Osteol. 2014, 10, 122–135. [Google Scholar]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Nindl, B.C. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J. Appl. Physiol. 2017, 122, 549–558. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Jin, J.-D.; Chang, H.-L.; Huang, K.-C.; Chiang, Y.-F.; Ali, M.; Hsia, S.-M. Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis. Nanomaterials 2021, 11, 1509. https://doi.org/10.3390/nano11061509
Chang C-Y, Jin J-D, Chang H-L, Huang K-C, Chiang Y-F, Ali M, Hsia S-M. Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis. Nanomaterials. 2021; 11(6):1509. https://doi.org/10.3390/nano11061509
Chicago/Turabian StyleChang, Chiung-Yueh, Jinn-Der Jin, Hsiao-Li Chang, Ko-Chieh Huang, Yi-Fen Chiang, Mohamed Ali, and Shih-Min Hsia. 2021. "Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis" Nanomaterials 11, no. 6: 1509. https://doi.org/10.3390/nano11061509
APA StyleChang, C.-Y., Jin, J.-D., Chang, H.-L., Huang, K.-C., Chiang, Y.-F., Ali, M., & Hsia, S.-M. (2021). Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis. Nanomaterials, 11(6), 1509. https://doi.org/10.3390/nano11061509