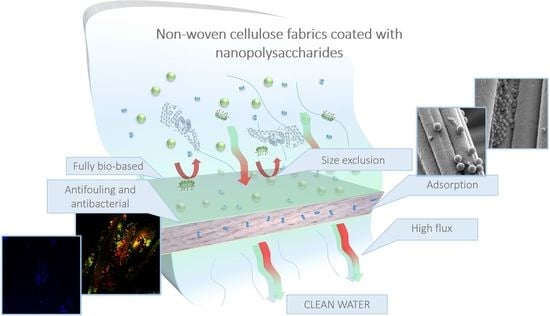
Water Filtration Membranes Based on Non-Woven Cellulose Fabrics: Effect of Nanopolysaccharide Coatings on Selective Particle Rejection, Antifouling, and Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing Method
2.3. Characterization
2.3.1. Morphology and Surface Characterization
2.3.2. Membrane Performance
2.3.3. Antifouling Performance
Determination of Bovine Serum Albumin Protein Adsorption
Bacterial Colonization and Viability
3. Results and Discussion
3.1. Surface Morphology
3.2. Membrane Performance
3.3. Antifouling Performance
3.3.1. Bovine Serum Albumin Adsorption
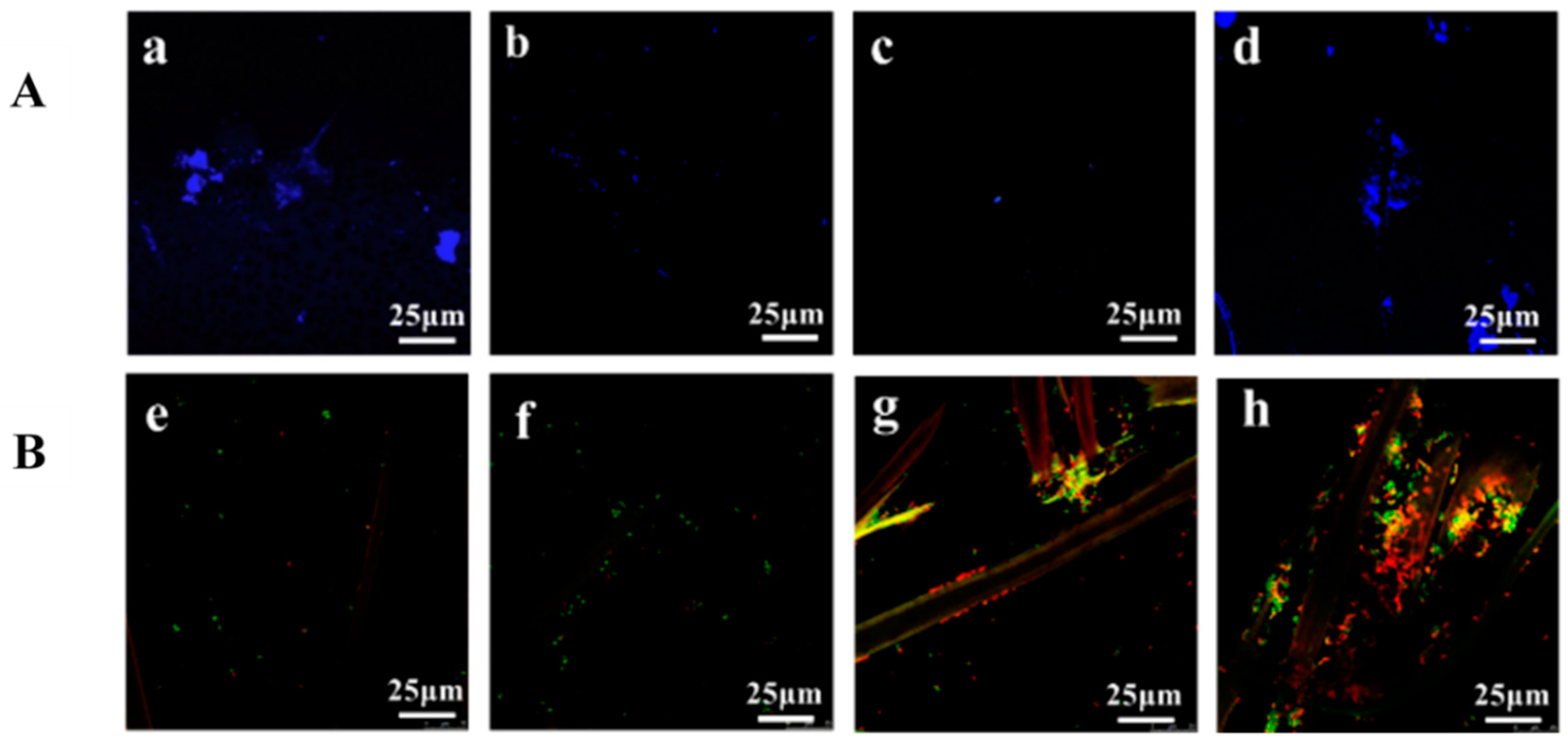
3.3.2. Escherichia coli Colonization and Cellular Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mays, T.J. A new classification of pore sizes. Stud. Surf. Sci. Catal. 2007, 160, 57–62. [Google Scholar]
- Zobel, S.; Gries, T. The use of nonwovens as filtration materials. In Applications of Nonwovens in Technical Textiles; Chapman, R.A., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 160–183. ISBN 9781845694371. [Google Scholar]
- Singh, J.; Shrivastava, A.; Mukophadhyaya, K.; Prasad, D.; Sharma, V. Design and Development of Composite Nonwoven Filter for Pre-filtration of Textile Effluents Using Nano-technology. J. Mater. Sci. Eng. 2017, 6. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultrafine polysaccharide nanofibrous membranes for water purification. Biomacromolecules 2011, 12, 970–976. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Peniche, C.; Arguelles-Monal, W.; Goycoolea, M. Chitin and chitosan: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water fi ltration. J. Memb. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.-Y.; Tammelin, T.; Mathew, A.P.; Nedoma, A.J.; Li, K.; Bismarck, A. Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 2015, 86, 209–214. [Google Scholar] [CrossRef]
- Aguilar-Sanchez, A.; Jalvo, B.; Mautner, A.; Nameer, S.; Pöhler, T.; Tammelin, T.; Mathew, A.P. Waterborne nanocellulose coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. J. Memb. Sci. 2021, 620, 118842. [Google Scholar] [CrossRef]
- Aguilar-Sanchez, A.; Jalvo, B.; Mautner, A.; Rissanen, V.; Kontturi, K.S.; Abdelhamid, H.N.; Tammelin, T.; Mathew, A.P. Charged ultrafiltration membranes based on TEMPO-oxidized cellulose nanofibrils/poly(vinyl alcohol) antifouling coating. RSC Adv. 2021, 11, 6859–6868. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Kokol, V.; Wei, J.; Grahn, M. High-flux affinity membranes based on cellulose nanocomposites for removal of heavy metal ions from industrial effluents. RCS Adv. 2016, 6, 20644–20653. [Google Scholar] [CrossRef] [Green Version]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef] [Green Version]
- Georgouvelas, D.; Abdelhamid, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y.; Narain, R. Fabrication of antifouling and antibacterial polyethersulfone (PES) /cellulose nanocrystals (CNC) nanocomposite membranes. J. Membr. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- Naseri, N.; Mathew, A.P.; Girandon, L.; Fröhlich, M.; Oksman, K. Porous electrospun nanocomposite mats based on chitosan—cellulose nanocrystals for wound dressing: Effect of surface characteristics of nanocrystals. Cellulose 2015, 22, 521–534. [Google Scholar] [CrossRef]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Preparation and characterization of nano-chitin whisker reinforced PVDF membrane with excellent antifouling property. J. Membr. Sci. 2015, 480, 1–10. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutra conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking cellulose nanocrystals: From the laboratory to industrial production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous microfiltration membrane based on cellulose nanowhiskers. Biomacromolecules 2012, 13, 180–186. [Google Scholar] [CrossRef]
- Qu, P.; Tang, H.; Gao, Y.; Zhang, L.; Wang, S. Polyethersulfone composite membrane blended with cellulose fibrils. BioResources 2010, 5, 2323–2363. [Google Scholar]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012, 392–393, 167–174. [Google Scholar] [CrossRef]
- Stewart, M.; Arnold, K. Water Injection systems. In Emulsions and Oil Treating Equipment; Stewart, M., Arnold, K., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2009; pp. 213–265. [Google Scholar]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean. 2020, 125. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes: A review. Angew. Chem. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chu, R.; Wu, R.; Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 2011, 12, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Meng, J.; Xu, M.; Yuan, T.; Yang, N.; Sun, T.; Zhang, Y.; Feng, X.; Cheng, B. A simple but efficient zwitterionization method towards cellulose membrane with superior antifouling property and biocompatibility. J. Membr. Sci. 2015, 492, 547–558. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.M.; Bartelt-Hunt, S.; Rodenhausen, K.B.; Schubert, M.; Bartz, J.C. Investigation of bovine serum albumin (BSA) attachment onto self-assembled monolayers (SAMs) using combinatorial quartz crystal microbalance with dissipation (QCM-D) and spectroscopic ellipsometry (SE). PLoS ONE 2015, 10, e0141282. [Google Scholar] [CrossRef]
- Shirahama, H.; Takeda, K.; Suzawa, T. Adsorption of bovine serum albumin onto polystyrene latex: Effects of coexistent electrolyte anions. J. Colloid Interface Sci. 1986, 109, 552–556. [Google Scholar] [CrossRef]
- Hughes Wassell, D.T.; Embery, G. Adsorption of bovine serum albumin on to titanium powder. Biomaterials 1996, 17, 859–864. [Google Scholar] [CrossRef]
- Kudelski, A. Influence of electrostatically bound proteins on the structure of linkage monolayers: Adsorption of bovine serum albumin on silver and gold substrates coated with monolayers of 2-mercaptoethanesulphonate. Vib. Spectrosc. 2003, 33, 197–204. [Google Scholar] [CrossRef]
- Bajpai, A.K. Adsorption of bovine serum albumin onto glass powder surfaces coated with polyvinyl alcohol. J. Appl. Polym. Sci. 2000, 78, 933–940. [Google Scholar] [CrossRef]
- Kopac, T.; Bozgeyik, K.; Yener, J. Effect of pH and temperature on the adsorption of bovine serum albumin onto titanium dioxide. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 322, 19–28. [Google Scholar] [CrossRef]
- Servagent-Noinville, S.; Revault, M.; Quiquampoix, H.; Baron, M.H. Conformational changes of bovine serum albumin induced by adsorption on different clay surfaces: FTIR analysis. J. Colloid Interface Sci. 2000, 221, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Anzai, J.I.; Guo, B.; Osa, T. Quartz-crystal microbalance and cyclic voltammetric studies of the adsorption behaviour of serum albumin on self-assembled thiol monolayers possessing different hydrophobicity and polarity. Bioelectrochem. Bioenerg. 1996, 40, 35–40. [Google Scholar] [CrossRef]
- Contreras, A.E.; Steiner, Z.; Miao, J.; Kasher, R.; Li, Q. Studying the role of common membrane surface functionalities on adsorption and cleaning of organic foulants using QCM-D. Environ. Sci. Technol. 2011, 45, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, V.S.; Garnier, G. Cellulose Nano-Films as Bio-Interfaces. Front. Chem. 2019, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Missirlis, Y.F.; Harris, L.; Douglas, J. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Lasa, I. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 2006, 9, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Oss, C.J. Hydrophobicity and hydrophilicity of biosurfaces. Curr. Opin. Colloid Interface Sci. 1997, 2, 503–512. [Google Scholar] [CrossRef]
- Prakash, B.; Veeregowda, B.M.; Krishnappa, G. Biofilms: A survival strategy of bacteria. Curr. Sci. 2003, 85, 1299–1307. [Google Scholar]
- Ong, Y.L.; Razatos, A.; Georgiou, G.; Sharma, M.M. Adhesion forces between E. coli bacteria and biomaterial surfaces. Langmuir 1999, 15, 2719–2725. [Google Scholar] [CrossRef]
- Jalvo, B.; Mathew, A.P.; Rosal, R. Coaxial poly(lactic acid) electrospun composite membranes incorporating cellulose and chitin nanocrystals. J. Memb. Sci. 2017, 544, 261–271. [Google Scholar] [CrossRef]
- Jalvo, B.; Santiago-Morales, J.; Romero, P.; Guzman de Villoria, R.; Rosal, R. Microbial colonisation of transparent glass-like carbon films triggered by a reversible radiation-induced hyydrophobic to hydrophilic transition. RCS Adv. 2016, 6, 50278–50287. [Google Scholar]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Kostenbauder, H. Physical factors influencing the activity of antimicrobial agents. In Desinfection, Sterilization and Preservation; Block, S., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1991; pp. 59–71. [Google Scholar]
- Zi, Y.; Zhu, M.; Li, X.; Xu, Y.; Wei, H.; Li, D.; Mu, C. Effects of carboxyl and aldehyde groups on the antibacterial activity of oxidized amylose. Carbohydr. Polym. 2018, 192, 118–125. [Google Scholar] [CrossRef]
- Tachaboonyakiat, W.; Sukpaiboon, E.; Pinyakong, O. Development of an antibacterial chitin betainate wound dressing. Polym. J. 2014, 46, 505–510. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of Chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Mera, A.; Araki, J.; Ohtsuki, T. Chitin nanowhiskers mediate transformation of Escherichia coli by exogenous plasmid DNA. J. Biotechnol. Biomater. 2011, 1, 1–6. [Google Scholar] [CrossRef]



| Sample | Water Contact Angle (°) | Surface ζ-Potential (pH 7, mV) | Maximum Pore size (µm) | Water Permeance (L |
|---|---|---|---|---|
| Cellulose unmodified membrane | 106.37 ± 2.7 | −2.6 ± 1.3 | 2.11 ± 0.18 | 13,154.3 ± 2092 |
| CNC impregnated membrane | 68.24 ± 3.7 | −5.9 ± 2.8 | 2.00 ± 0.15 | 14,363.8 ± 1228.4 |
| T-CNF impregnated membrane | 49.24 ± 1.1 | −9.2 ± 2.5 | 1.96 ± 0.13 | 7498.4 ± 211.1 |
| ChNC impregnated membrane | 0 a | 7.2 ± 3.2 | 1.98 ± 0.10 | 13,417.6 ± 1229.5 |
| Conditions | Sample Type | Maximum Tensile strength (MPa) | E-Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| Dry | Unmodified cellulose fabric | 4.3 ± 0.5 | 9.0 ± 1.6 | 51 ± 2 |
| CNC impregnated fabric | 4.8 ± 0.2 | 46.9 ± 0.5 | 44 ± 1 | |
| T-CNF impregnated fabric | 7.2 ± 0.2 | 194.8 ± 0.5 | 39 ± 3 | |
| ChNC impregnated fabric | 5.1 ± 0.4 | 12.3± 1.0 | 45 ± 2 | |
| Wet | Unmodified cellulose fabric | 2.2 ± 0.1 | 8.2 ± 0.4 | 44 ± 2 |
| CNC impregnated fabric | 2.6 ± 0.0 | 8.5 ± 0.4 | 48 ± 2 | |
| T-CNF impregnated fabric | 3.00 ± 0.1 | 8.8 ± 0.6 | 44 ± 1 | |
| ChNC impregnated fabric | 2.2 ± 0.1 | 7.6 ± 0.3 | 49 ± 1 |
| Sample | UV-vis Absorbance at 280 nm |
|---|---|
| Unmodified cellulose fabric | 0.04 ± 0.04 |
| CNC impregnated fabric | 0.12 ± 0.12 |
| T-CNF impregnated fabric | 0.30 ± 0.17 |
| ChNC impregnated fabric | 0.014 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalvo, B.; Aguilar-Sanchez, A.; Ruiz-Caldas, M.-X.; Mathew, A.P. Water Filtration Membranes Based on Non-Woven Cellulose Fabrics: Effect of Nanopolysaccharide Coatings on Selective Particle Rejection, Antifouling, and Antibacterial Properties. Nanomaterials 2021, 11, 1752. https://doi.org/10.3390/nano11071752
Jalvo B, Aguilar-Sanchez A, Ruiz-Caldas M-X, Mathew AP. Water Filtration Membranes Based on Non-Woven Cellulose Fabrics: Effect of Nanopolysaccharide Coatings on Selective Particle Rejection, Antifouling, and Antibacterial Properties. Nanomaterials. 2021; 11(7):1752. https://doi.org/10.3390/nano11071752
Chicago/Turabian StyleJalvo, Blanca, Andrea Aguilar-Sanchez, Maria-Ximena Ruiz-Caldas, and Aji P. Mathew. 2021. "Water Filtration Membranes Based on Non-Woven Cellulose Fabrics: Effect of Nanopolysaccharide Coatings on Selective Particle Rejection, Antifouling, and Antibacterial Properties" Nanomaterials 11, no. 7: 1752. https://doi.org/10.3390/nano11071752