Enhancement of Thermostability of Aspergillus flavus Urate Oxidase by Immobilization on the Ni-Based Magnetic Metal–Organic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Recombinant Uox
2.3. Synthesis of Ni-Based Magnetic MOF (NimMOF) Nanomaterial
2.4. Characterization of the Synthesized NimMOF
2.5. Measurement of the Uox Activity
2.6. Immobilization of Uox on NimMOF
2.7. Analysis of the Optimum Temperature for Uox Activity
2.8. Thermal Inactivation and Thermal Stability Studies
2.9. Analysis of Kinetics and Thermodynamic Parameters
2.10. The Optimum pH and pH Stability of Uox Preparations
2.11. Intrinsic and Extrinsic Fluorescent Measurements
3. Results and Discussion
3.1. Preparation of Recombinant Uox
3.2. Characterization of the Magnetic MOF Nanoparticles (Ni-MOF)
3.2.1. FTIR Spectra of the Ni-MOF Particles
3.2.2. XRD Patterns
3.2.3. VSM and SEM Analysis of NimMOF
3.3. Analysis of the Performances of Uox and Uox@NimMOF
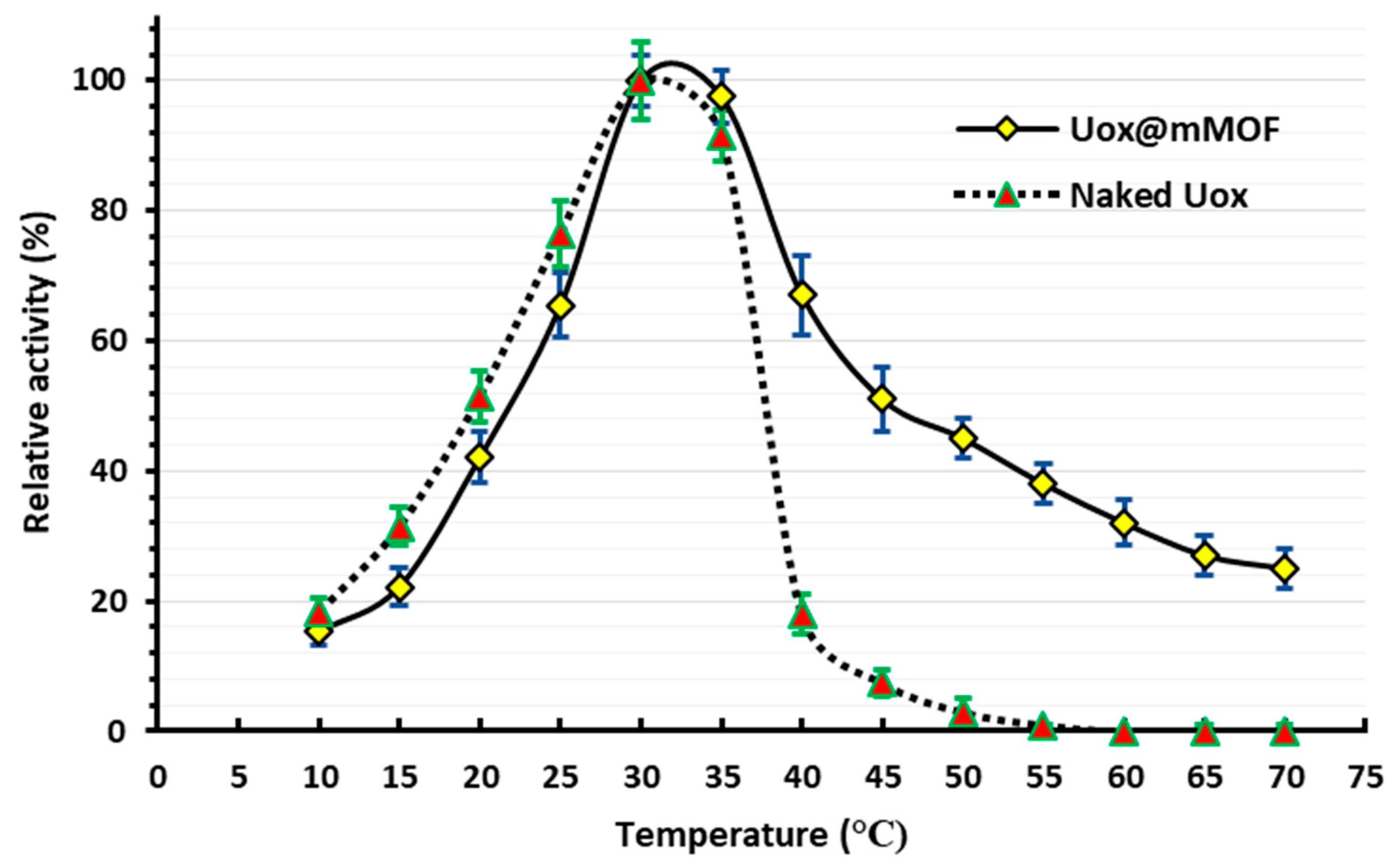
3.3.1. The Optimum Temperature for the Uox and Uox@NimMOF Activity
3.3.2. Thermal Inactivation and Thermal Stability Studies
3.3.3. Analysis of Enzyme Kinetic and Thermodynamics Parameters
3.3.4. Optimum pH and pH Stability for Uox Preparations
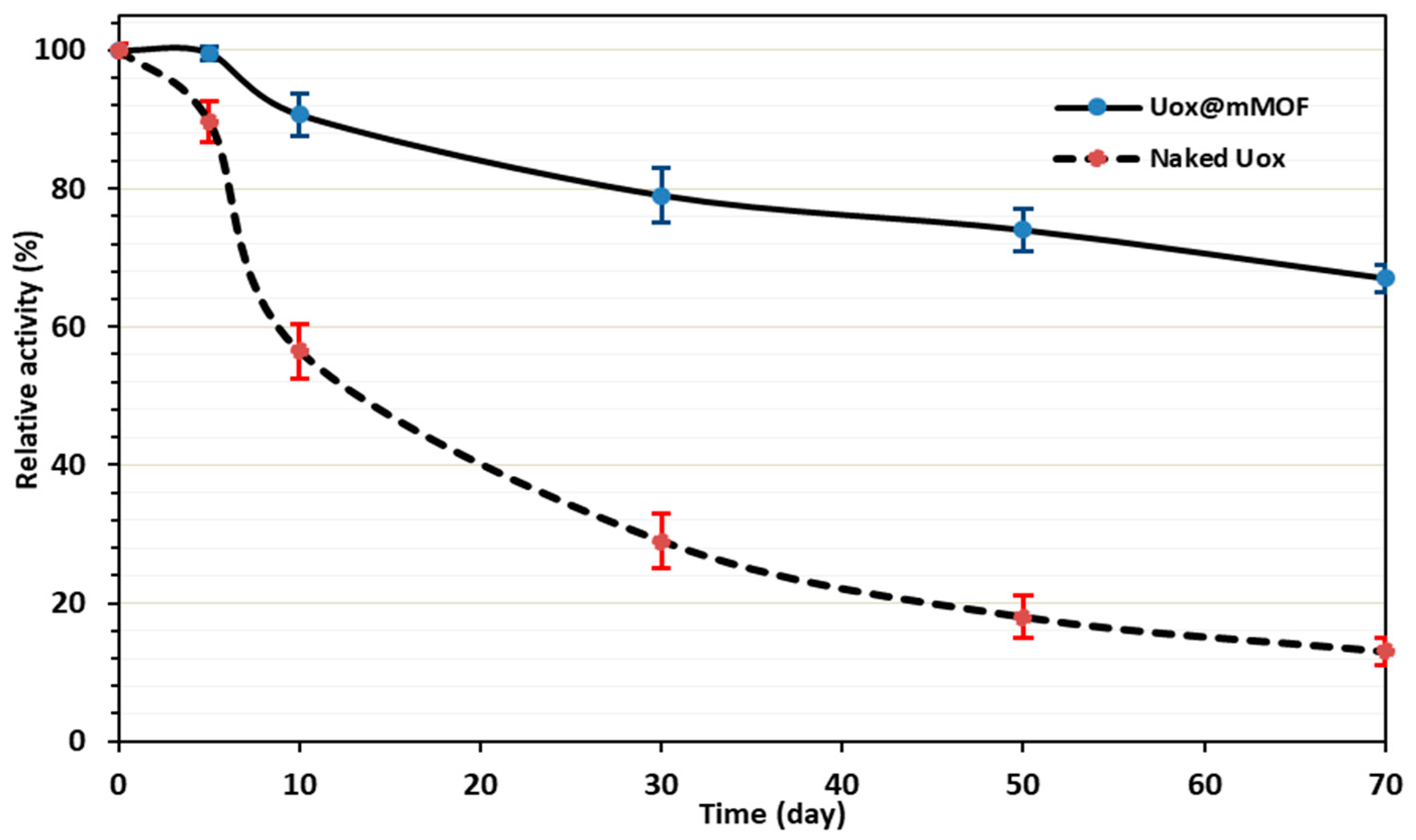
3.3.5. Storage Stability Studies
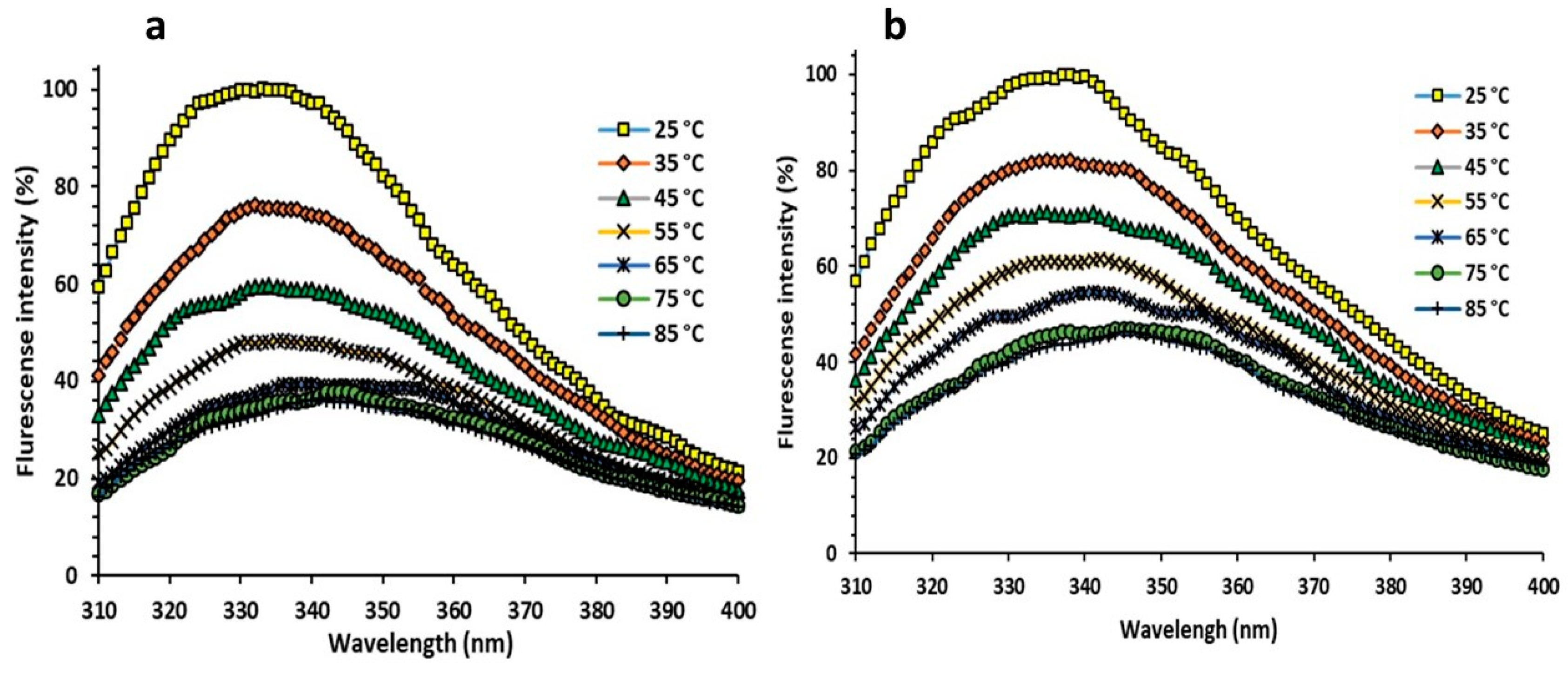
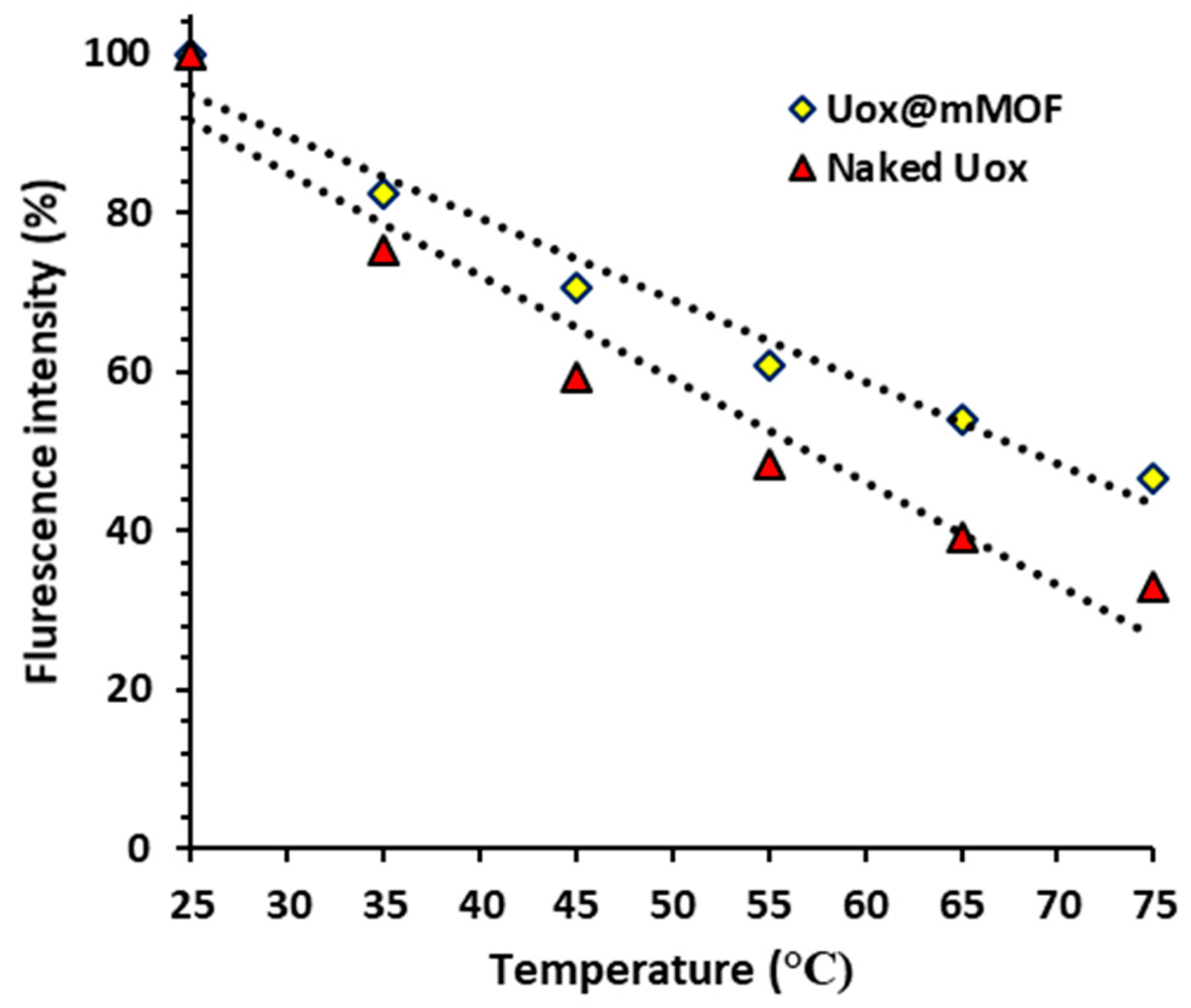
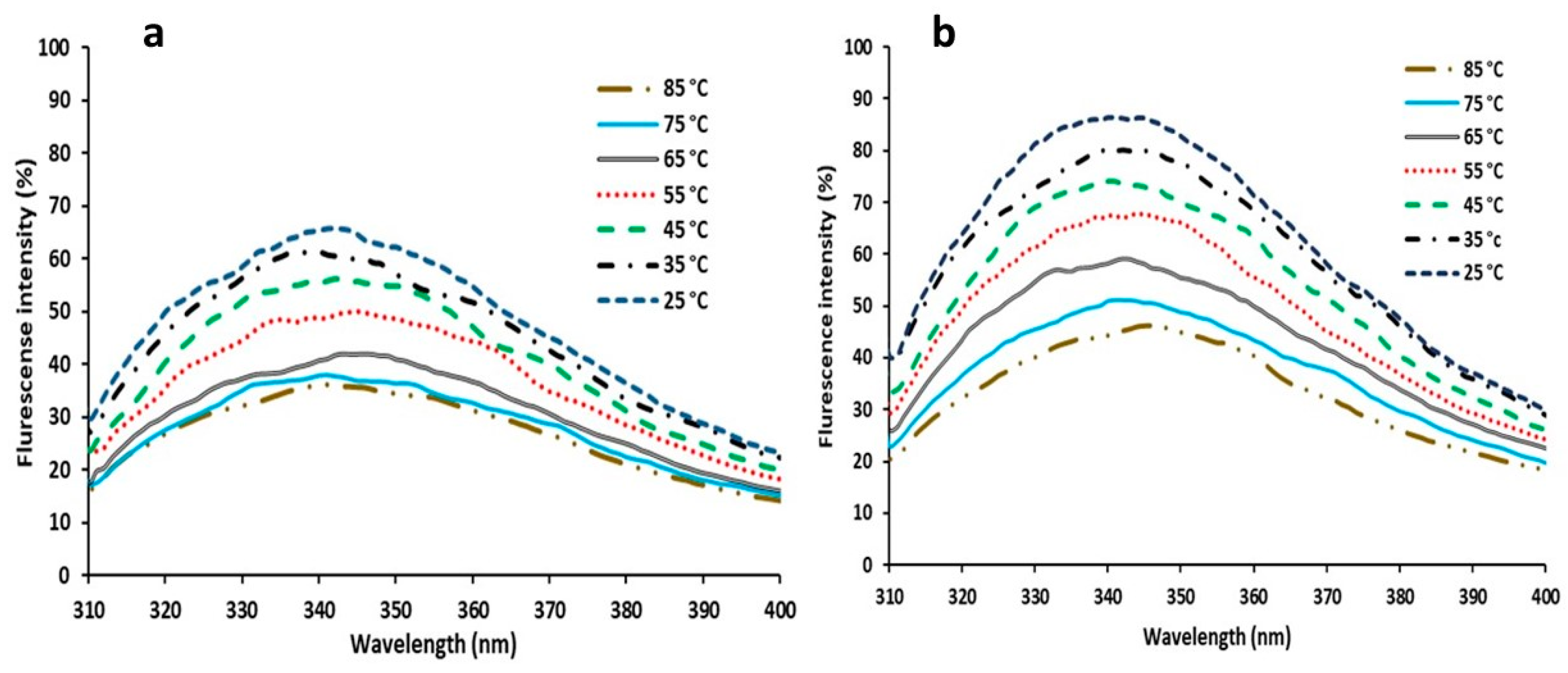
3.3.6. Florescent Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oda, M.; Satta, Y.; Takenaka, O.; Takahata, N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol. Biol. Evol. 2002, 19, 640–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terkeltaub, R. Update on gout: New therapeutic strategies and options. Nat. Rev. Rheumatol. 2010, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Altman, A.; Pui, C.-H.; Younes, A.; Cairo, M.S. guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review. J. Clin. Oncol. 2008, 26, 2767–2778. [Google Scholar] [CrossRef] [Green Version]
- Pui, C.-H.; Mahmoud, H.H.; Wiley, J.M.; Woods, G.M.; Leverger, G.; Camitta, B.; Hastings, C.; Blaney, S.M.; Relling, M.V.; Reaman, G.H. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients with leukemia or lymphoma. J. Clin. Oncol. 2001, 19, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Pleskacova, A.; Brejcha, S.; Pacal, L.; Kankova, K.; Tomandl, J. Simultaneous determination of uric acid, xanthine and hypoxanthine in human plasma and serum by hplc–uv: Uric acid metabolism tracking. Chromatographia 2017, 80, 529–536. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Chauhan, N.; Dahiya, T.; Pundir, C. Construction of amperometric uric acid biosensor based on uricase immobilized on (pbnps/cmwcnt/pani/au com) posite. Int. J. Biol. Macromol. 2012, 50, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gabison, L.; Chiadmi, M.; El Hajji, M.; Castro, B.; Colloc’h, N.; Prangé, T. Near-atomic resolution structures of urate oxidase complexed with its substrate and analogues: The protonation state of the ligand. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Collings, I.; Watier, Y.; Giffard, M.; Dagogo, S.; Kahn, R.; Bonneté, F.; Wright, J.P.; Fitch, A.N.; Margiolaki, I. Polymorphism of microcrystalline urate oxidase from Aspergillus flavus. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 539–548. [Google Scholar] [CrossRef]
- Gabison, L.; Prangé, T.; Colloc’h, N.; El Hajji, M.; Castro, B.; Chiadmi, M. Structural analysis of urate oxidase in complex with its natural substrate inhibited by cyanide: Mechanistic implications. BMC Struct. Biol. 2008, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Caves, M.S.; Derham, B.K.; Jezek, J.; Freedman, R.B. Thermal inactivation of uricase (urate oxidase): Mechanism and effects of additives. Biochemistry 2013, 52, 497–507. [Google Scholar] [CrossRef]
- Conley, T.G.; Priest, D.G. Thermodynamics and stoicheiometry of the binding of substrate analogues to uricase. Biochem. J. 1980, 187, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayol, A.; Capdevielle, J.; Malazzi, P.; Buzy, A.; Bonnet, M.C.; Colloc’h, N.; Mornon, J.P.; Loyaux, D.; Ferrara, P. Modification of a reactive cysteine explains differences between rasburicase and uricozyme®, a natural Aspergillus flavus uricase. Biotechnol. Appl. Biochem. 2002, 36, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Bomalaski, J.S.; Holtsberg, F.W.; Ensor, C.M.; Clark, M.A. Uricase formulated with polyethylene glycol (uricase-peg 20): Biochemical rationale and preclinical studies. J. Rheumatol. 2002, 29, 1942–1949. [Google Scholar] [PubMed]
- Liu, Z.; Lu, D.; Li, J.; Chen, W.; Liu, Z. Strengthening intersubunit hydrogen bonds for enhanced stability of recombinant urate oxidase from Aspergillus flavus: Molecular simulations and experimental validation. Phys. Chem. Chem. Phys. 2009, 11, 333–340. [Google Scholar] [CrossRef]
- Bohinc, K.; Bajuk, J.; Jukić, J.; Abram, A.; Oder, M.; Torkar, K.G.; Raspor, P.; Kovačević, D. Bacterial adhesion capacity of protein-terminating polyelectrolyte multilayers. Int. J. Adhes. Adhes. 2020, 103, 102687. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Chen, Z.; Liu, D.; Yu, E.; Zhang, X.; Fu, H.; Fu, J.; Zhang, J.; Du, H. Amorphous molybdenum sulfide nanocatalysts simultaneously realizing efficient upgrading of residue and synergistic synthesis of 2D mos 2 nanosheets/carbon hierarchical structures. Green Chem. 2020, 22, 44–53. [Google Scholar] [CrossRef]
- Tong, X.; Ou, X.; Wu, N.; Wang, H.; Li, J.; Tang, Y. High oxidation potential≈ 6.0 V of concentrated electrolyte toward high-performance dual-ion battery. Adv. Energy Mater. 2021, 2100151. [Google Scholar] [CrossRef]
- Sargazi, S.; Hajinezhad, M.R.; Rahdar, A.; Zafar, M.N.; Awan, A.; Baino, F. Assessment of snfe2o4 nanoparticles for potential application in theranostics: Synthesis, characterization, in vitro, and in vivo toxicity. Materials 2021, 14, 825. [Google Scholar] [CrossRef]
- Sheervalilou, R.; Shirvaliloo, M.; Sargazi, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Recent advances in iron oxide nanoparticles for brain cancer theranostics: From in vitro to clinical applications. Expert Opin. Drug Deliv. 2021, 18, 949–977. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Dehaghani, A.H.S.; Pirouzfar, V.; Akhondi, E. Mixed matrix membranes comprising pmp polymer with dispersed alumina nanoparticle fillers to separate CO2/N2. Macromol. Res. 2016, 24, 782–792. [Google Scholar] [CrossRef]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [Green Version]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [Green Version]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badoei-Dalfard, A.; Sohrabi, N.; Karami, Z.; Sargazi, G. Fabrication of an efficient and sensitive colorimetric biosensor based on uricase/th-mof for uric acid sensing in biological samples. Biosens. Bioelectron. 2019, 141, 111420. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, Y.; Riahi-Madvar, A.; Mirzaee, R.; Torkzadeh-Mahani, M.; Asadikaram, G.; Sargazi, G. Optimization of in vitro refolding conditions of recombinant lepidium draba peroxidase using design of experiments. Int. J. Biol. Macromol. 2018, 118, 1369–1376. [Google Scholar] [CrossRef]
- Sargazi, G.; Afzali, D.; Ebrahimi, A.K.; Badoei-Dalfard, A.; Malekabadi, S.; Karami, Z. Ultrasound assisted reverse micelle efficient synthesis of new ta-mof@Fe3O4 core/shell nanostructures as a novel candidate for lipase immobilization. Mater. Sci. Eng. C 2018, 93, 768–775. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Recent progress in biocatalysis with enzymes immobilized on mesoporous hosts. Top. Catal. 2012, 55, 1081–1100. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.; Zhang, Y.; Jia, D.; Zhang, X.; Hou, Y.; Li, R.; Wang, J. Maximum undeformed equivalent chip thickness for ductile-brittle transition of zirconia ceramics under different lubrication conditions. Int. J. Mach. Tools Manuf. 2017, 122, 55–65. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (mofs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Ebrahimi, A.K.; Barani, M.; Sheikhshoaie, I. Fabrication of a new superparamagnetic metal-organic framework with core-shell nanocomposite structures: Characterization, biocompatibility, and drug release study. Mater. Sci. Eng. C 2018, 92, 349–355. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Facile synthesis of glucoamylase embedded metal-organic frameworks (glucoamylase-mof) with enhanced stability. Int. J. Biol. Macromol. 2017, 95, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-step fabrication of biomass-derived hierarchically porous carbon/mno nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526, 146696. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; He, X.; Fan, G.; Li, X.; He, Z.; Wang, G.; Zhang, I. Fabrication of cellulosic paper containing zeolitic imidazolate framework and its application in removal of anionic dye from aqueous solution. Bioresources 2021, 16, 2644–2654. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–organic frameworks (mofs) based electrochemical biosensors for early cancer diagnosis in vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of mofs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, C.-A.; Chen, R.; Wu, L.; Zou, X.; Wang, J. Ultrafast synthesis of ni-mof in one minute by ball milling. Nanomaterials 2018, 8, 1067. [Google Scholar] [CrossRef] [Green Version]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincӑ, M. Grand challenges and future opportunities for metal–organic frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef]
- Alijani, H.Q.; Pourseyedi, S.; Torkzadeh-Mahani, M.; Seifalian, A.; Khatami, M. Bimetallic nickel-ferrite nanorod particles: Greener synthesis using rosemary and its biomedical efficiency. Artif. Cells Nanomed. Biotechnol. 2020, 48, 242–251. [Google Scholar] [CrossRef]
- Ghazal, S.; Akbari, A.; Hosseini, H.A.; Sabouri, Z.; Forouzanfar, F.; Khatami, M.; Darroudi, M. Sol-gel biosynthesis of nickel oxide nanoparticles using cydonia oblonga extract and evaluation of their cytotoxicity and photocatalytic activities. J. Mol. Struct. 2020, 1217, 128378. [Google Scholar]
- Ghazal, S.; Akbari, A.; Hosseini, H.A.; Sabouri, Z.; Khatami, M.; Darroudi, M. Sol-gel synthesis of selenium-doped nickel oxide nanoparticles and evaluation of their cytotoxic and photocatalytic properties. Inorg. Chem. Res. 2021, 5, 37–49. [Google Scholar]
- Mazzei, L.; Musiani, F.; Ciurli, S. The structure-based reaction mechanism of urease, a nickel dependent enzyme: Tale of a long debate. JBIC J. Biol. Inorg. Chem. 2020, 25, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Armenia, I.; Bonavia, M.V.G.; De Matteis, L.; Ivanchenko, P.; Martra, G.; Gornati, R.; Jesus, M.; Bernardini, G. Enzyme activation by alternating magnetic field: Importance of the bioconjugation methodology. J. Colloid Interface Sci. 2019, 537, 615–628. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Heat transfer and flow characteristics of Fe3O4-water nanofluids under magnetic excitation. Int. J. Therm. Sci. 2021, 163, 106826. [Google Scholar]
- Zhang, X.; Zhang, Y. Experimental study on enhanced heat transfer and flow performance of magnetic nanofluids under alternating magnetic field. Int. J. Therm. Sci. 2021, 164, 106897. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Zhang, D.; Zhang, X. Experimental evaluation of mos2 nanoparticles in jet mql grinding with different types of vegetable oil as base oil. J. Clean. Prod. 2015, 87, 930–940. [Google Scholar]
- Zhang, H.; Guan, W.; Zhang, L.; Guan, X.; Wang, S. Degradation of an organic dye by bisulfite catalytically activated with iron manganese oxides: The role of superoxide radicals. ACS Omega 2020, 5, 18007–18012. [Google Scholar] [CrossRef]
- Han, Y.; Li, H.; Feng, H.; Tian, Y.; Jiang, Z.; He, T. Mechanism of dislocation evolution during plastic deformation of nitrogen-doped Cocrfemnni high-entropy alloy. Mater. Sci. Eng. A 2021, 814, 141235. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Paradinas, M.; Ocal, C.; Schüpbach, B.; Terfort, A.; Zacher, D.; Fischer, R.A.; Wöll, C. Controlling interpenetration in metal–organic frameworks by liquid-phase epitaxy. Nat. Mater. 2009, 8, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; He, J.; Deng, L.; Gao, X. Fabrication of cyclodextrin-functionalized superparamagnetic fe3o4/amino-silane core–shell nanoparticles via layer-by-layer method. Appl. Surf. Sci. 2009, 255, 7974–7980. [Google Scholar] [CrossRef]
- Mahler, H.; Hübscher, G.; Baum, H. Studies on uricase i. Preparation, purification, and properties of a cuproprotein. J. Biol. Chem. 1955, 216, 625–642. [Google Scholar] [CrossRef]
- Lohrasbi-nejad, A.; Torkzadeh-Mahani, M.; Hosseinkhani, S. Hydrophobin−1 promotes thermostability of firefly luciferase. FEBS J. 2016, 283, 2494–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.-J.; He, H.-P.; Liu, Q.; She, K.; Zhang, B.-Q.; Wang, H.-S.; Tang, H.-T.; Pan, Y.-M. Photocatalyst-controlled and visible light-enabled selective oxidation of pyridinium salts. Sci. China Chem. 2021, 64, 753–760. [Google Scholar] [CrossRef]
- Ji, C.; Nguyen, L.N.; Hou, J.; Hai, F.I.; Chen, V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep. Purif. Technol. 2017, 178, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Kopka, B.; Diener, M.; Wirtz, A.; Pohl, M.; Jaeger, K.E.; Krauss, U. Purification and simultaneous immobilization of arabidopsis thaliana hydroxynitrile lyase using a family 2 carbohydrate–binding module. Biotechnol. J. 2015, 10, 811–819. [Google Scholar] [CrossRef]
- Mahesh, M.; Arivizhivendhan, K.; Maharaja, P.; Boopathy, R.; Hamsavathani, V.; Sekaran, G. Production, purification and immobilization of pectinase from Aspergillus ibericus onto functionalized nanoporous activated carbon (fnac) and its application on treatment of pectin containing wastewater. J. Mol. Catal. B Enzym. 2016, 133, 43–54. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Ai, S.; Sun, Z.; Wan, Q.; Zhu, Z.; Xian, Y.; Jin, L.; Yamamoto, K. Immobilization of uricase on zno nanorods for a reagentless uric acid biosensor. Anal. Chim. Acta 2004, 519, 155–160. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Hou, L.; Fan, H.; Weng, S.; Xu, C.E.; Ren, J.; Li, B.; Chen, W. High-level expression, purification, and characterization of non-tagged Aspergillus flavus urate oxidase in Escherichia coli. Protein Expr. Purif. 2006, 49, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Legoux, R.; Delpech, B.; Dumont, X.; Guillemot, J.-C.; Ramond, P.; Shire, D.; Caput, D.; Ferrara, P.; Loison, G. Cloning and expression in Escherichia coli of the gene encoding Aspergillus flavus urate oxidase. J. Biol. Chem. 1992, 267, 8565–8570. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.; Zhang, X.; Liu, H.; Sun, B.; Guo, S. Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Bioresour. Technol. 2021, 332, 125120. [Google Scholar] [CrossRef]
- Retailleau, P.; Colloc’h, N.; Vivares, D.; Bonneté, F.; Castro, B.; El Hajji, M.; Prangé, T. Urate oxidase from Aspergillus flavus: New crystal-packing contacts in relation to the content of the active site. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005, 61, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. magnetic-metal organic framework (magnetic-mof): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, Z.; Mao, X.; Chen, X.-L.; Li, H.-H.; Wang, Y.-Y. Multifunctional textiles/metal− organic frameworks composites for efficient ultraviolet radiation blocking and noise reduction. ACS Appl. Mater. Interfaces 2020, 12, 55316–55323. [Google Scholar] [CrossRef]
- Zhang, K.; Huo, Q.; Zhou, Y.-Y.; Wang, H.-H.; Li, G.-P.; Wang, Y.-W.; Wang, Y.-Y. Textiles/metal–organic frameworks composites as flexible air filters for efficient particulate matter removal. ACS Appl. Mater. Interfaces 2019, 11, 17368–17374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, X.; Deng, C. Rational synthesis of novel recyclable Fe3O4 mof nanocomposites for enzymatic digestion. Chem. Commun. 2015, 51, 8116–8119. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, W.; Chen, H.; Shi, J. A simple one-pot self-assembly route to nanoporous and monodispersed Fe3O4 particles with oriented attachment structure and magnetic property. J. Phys. Chem. C 2007, 111, 5281–5285. [Google Scholar] [CrossRef]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme encapsulation in metal–organic frameworks for applications in catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Atalla, M.; Farag, M.; Eman, R.; El-lataif, A.; Nehad, E. Optimum conditions for uricase enzyme production by gliomastix gueg. Malays. J. Microbiol. 2009, 5, 45–50. [Google Scholar]
- Bayol, A.; Dupin, P.; Boe, J.; Claudy, P.; Létoffé, J. Study of ph and temperature-induced transitions in urate oxidase (uox-ec1. 7.3. 3) by microcalorimetry (dsc), size exclusion chromatography (sec) and enzymatic activity experiments. Biophys. Chem. 1995, 54, 229–235. [Google Scholar] [CrossRef]
- Imani, M.; Shahmohamadnejad, S. Recombinant production of Aspergillus flavus uricase and investigation of its thermal stability in the presence of raffinose and lactose. 3 Biotech 2017, 7, 201. [Google Scholar] [CrossRef]
- Da Silva Freitas, D.; Spencer, P.J.; Vassão, R.C.; Abrahão-Neto, J. Biochemical and biopharmaceutical properties of pegylated uricase. Int. J. Pharm. 2010, 387, 215–222. [Google Scholar] [CrossRef]
- Schiavon, O.; Caliceti, P.; Ferruti, P.; Veronese, F. Therapeutic proteins: A comparison of chemical and biological properties of uricase conjugated to linear or branched poly (ethylene glycol) and poly (N-acryloylmorpholine). Il Farmaco 2000, 55, 264–269. [Google Scholar] [CrossRef]
- Retailleau, P.; Colloc’h, N.; Vivarès, D.; Bonnete, F.; Castro, B.; El Hajji, M.; Mornon, J.-P.; Monard, G.; Prangé, T. Complexed and ligand-free high-resolution structures of urate oxidase (uox) from Aspergillus flavus: A reassignment of the active-site binding mode. ACTA Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colloc’h, N.; Prangé, T. Functional relevance of the internal hydrophobic cavity of urate oxidase. FEBS Lett. 2014, 588, 1715–1719. [Google Scholar] [CrossRef] [Green Version]

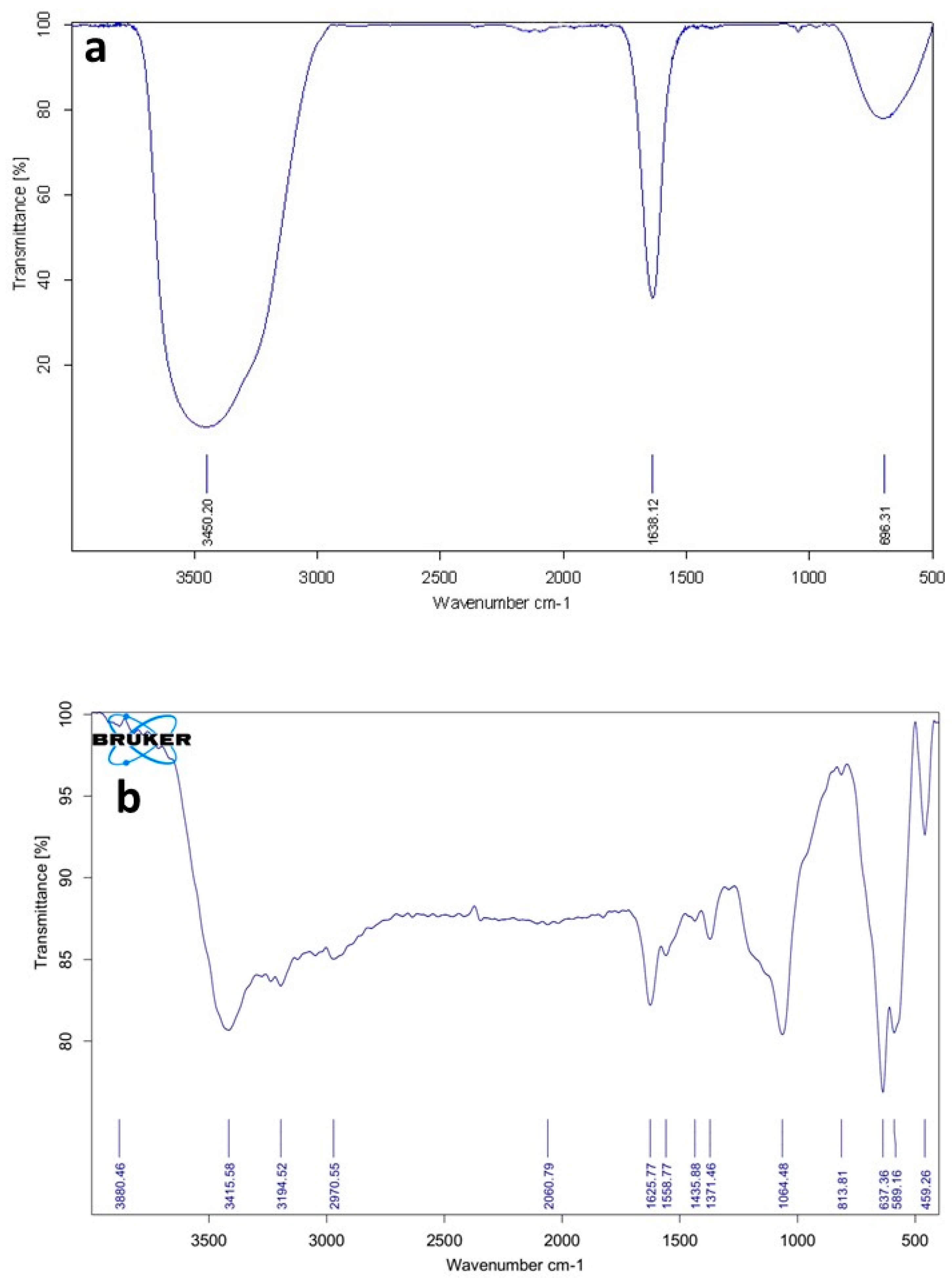
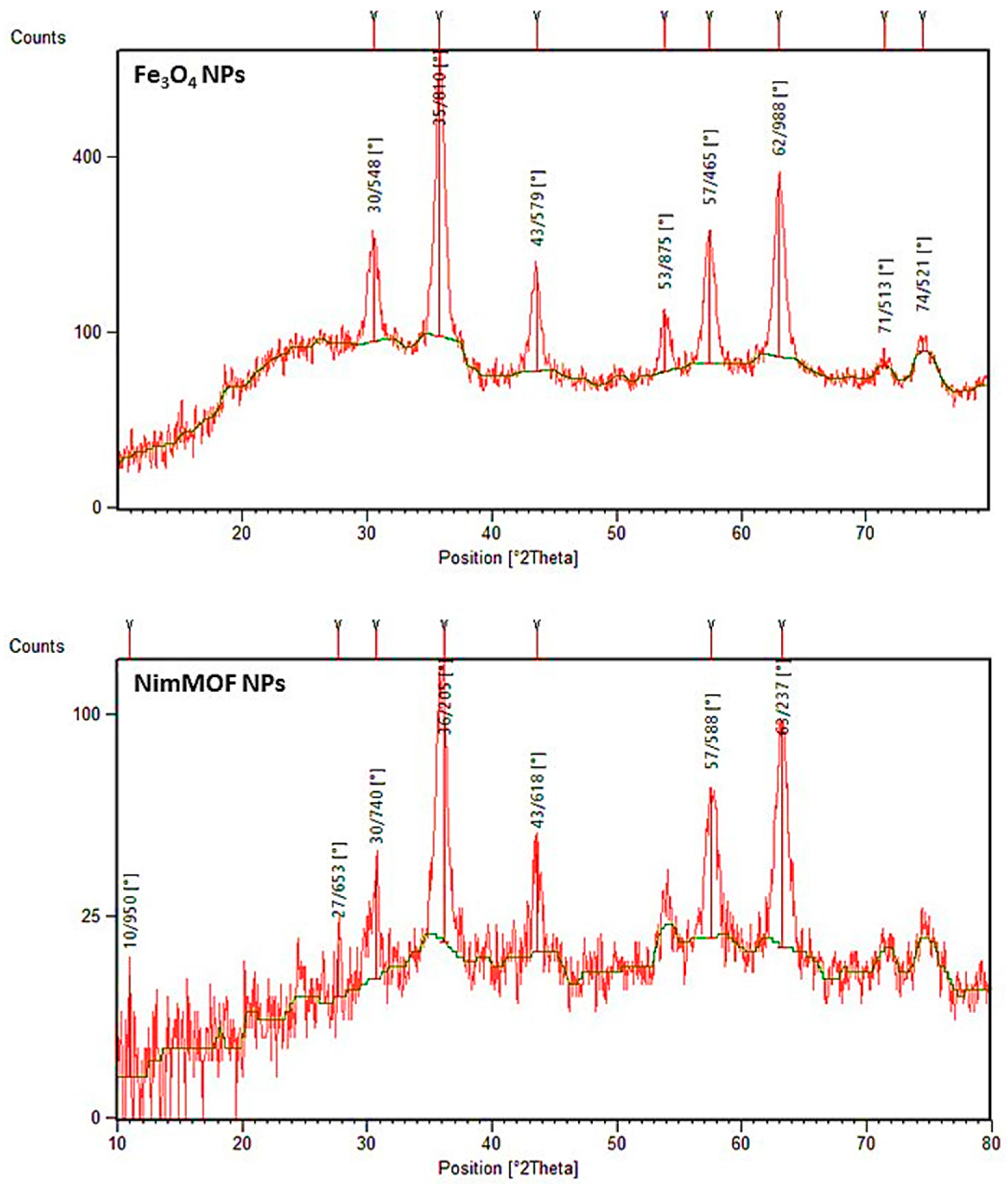
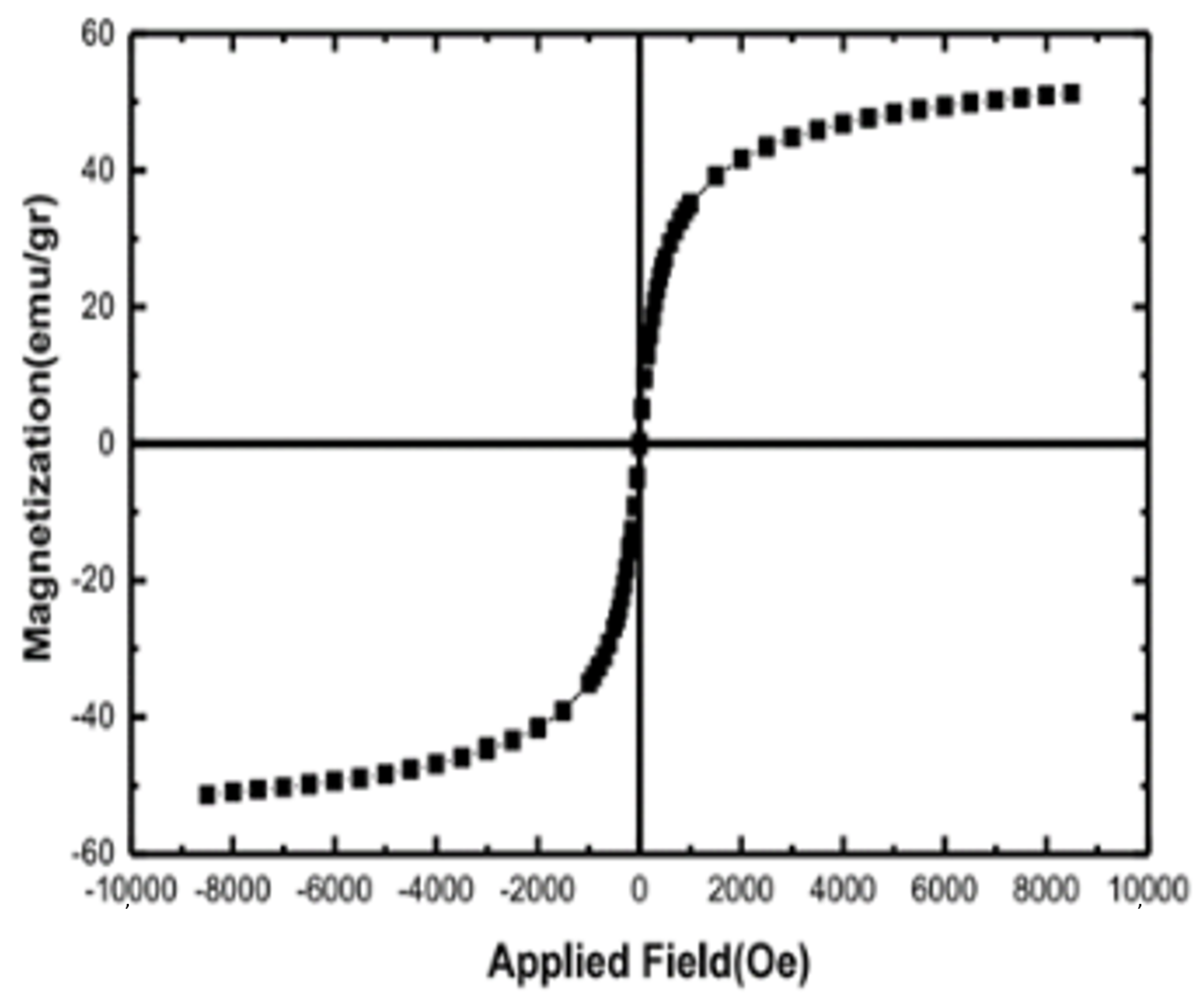
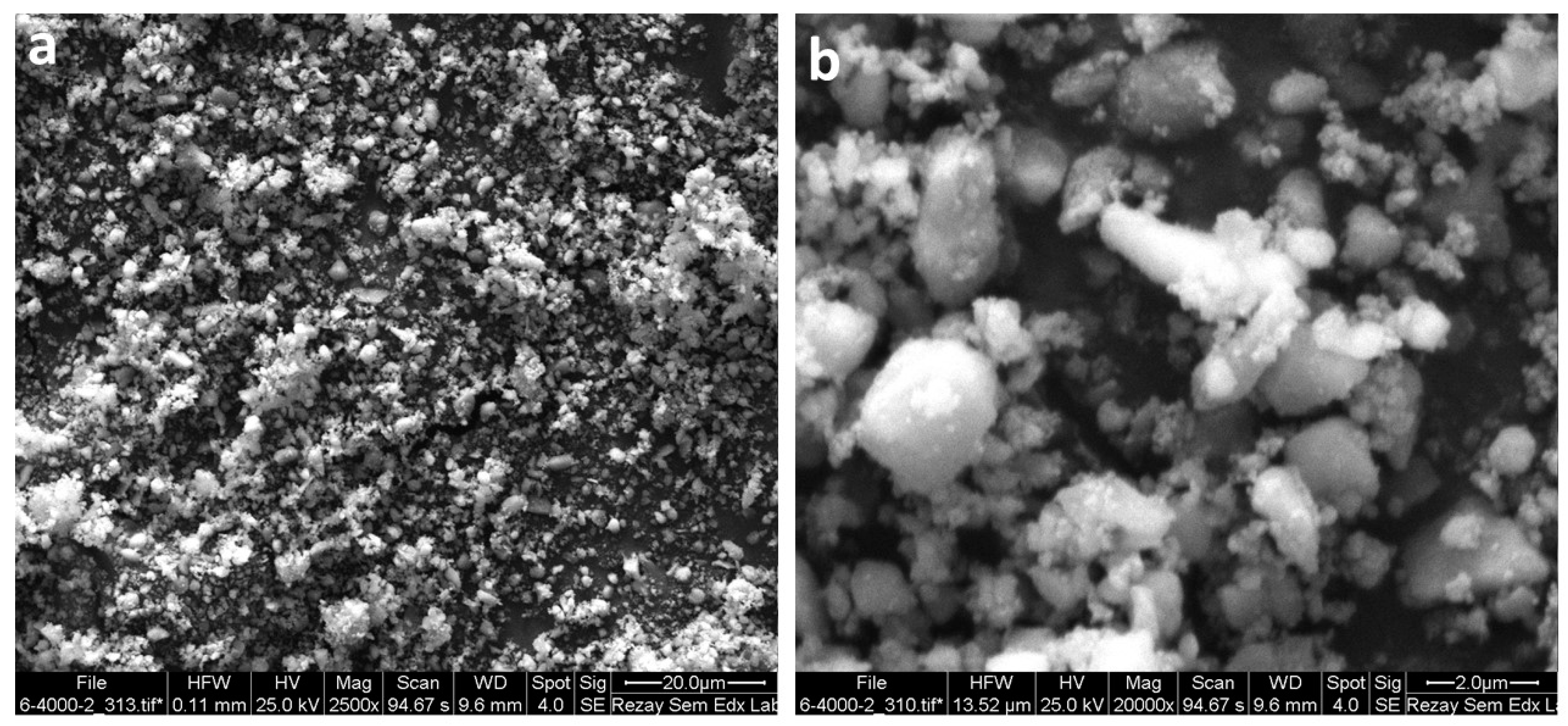




| Temperature (K) | kinact (min−1) | T1/2 (min) | |
|---|---|---|---|
| Naked Uox | 313 | 0.051 | 13.55 |
| 318 | 0.067 | 10.23 | |
| 323 | 0.103 | 6.74 | |
| 328 | 0.313 | 2.21 | |
| Uox@NimMOF | 313 | 0.019 | 36.55 |
| 318 | 0.027 | 25.31 | |
| 323 | 0.035 | 19.58 | |
| 328 | 0.055 | 12.5 |
| Km (µM) | Kcat (min−1) | |
|---|---|---|
| Naked UOX | 50.5 ± 3.2 | 2.24 × 10−6 |
| Uox@NimMOF | 52.2 ± 4.1 | 1.92 × 10−6 |
| T (°K) | Ea (KJ·mol−1) | ∆H (KJ·mol−1) | ∆G (KJ·mol−1) | ∆S (KJ·mol−1 K) | |
|---|---|---|---|---|---|
| Naked Uox | 313 | 99.80 | 97.19 | 59.35 | 0.121 |
| 318 | 97.16 | 61.06 | 0.113 | ||
| 323 | 97.11 | 63.20 | 0.105 | ||
| 328 | 97.07 | 67.26 | 0.091 | ||
| Uox@NimMOF | 313 | 58.81 | 56.20 | 56.76 | −0.011 |
| 318 | 56.16 | 58.68 | −0.008 | ||
| 323 | 56.11 | 60.34 | −0.013 | ||
| 328 | 56.07 | 62.54 | −0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motamedi, N.; Barani, M.; Lohrasbi-Nejad, A.; Mortazavi, M.; Riahi-Medvar, A.; Varma, R.S.; Torkzadeh-Mahani, M. Enhancement of Thermostability of Aspergillus flavus Urate Oxidase by Immobilization on the Ni-Based Magnetic Metal–Organic Framework. Nanomaterials 2021, 11, 1759. https://doi.org/10.3390/nano11071759
Motamedi N, Barani M, Lohrasbi-Nejad A, Mortazavi M, Riahi-Medvar A, Varma RS, Torkzadeh-Mahani M. Enhancement of Thermostability of Aspergillus flavus Urate Oxidase by Immobilization on the Ni-Based Magnetic Metal–Organic Framework. Nanomaterials. 2021; 11(7):1759. https://doi.org/10.3390/nano11071759
Chicago/Turabian StyleMotamedi, Neda, Mahmood Barani, Azadeh Lohrasbi-Nejad, Mojtaba Mortazavi, Ali Riahi-Medvar, Rajender S. Varma, and Masoud Torkzadeh-Mahani. 2021. "Enhancement of Thermostability of Aspergillus flavus Urate Oxidase by Immobilization on the Ni-Based Magnetic Metal–Organic Framework" Nanomaterials 11, no. 7: 1759. https://doi.org/10.3390/nano11071759







