Temperature-Dependent Excitonic Photoluminescence and Nonlinear Absorption of CdTe Nanocrystal/Polyvinyl Alcohol Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of CdTe NC/PVA Films
2.2. Optical Experimental Setup
2.2.1. Temperature-Dependent Steady-State and Time-Resolved PL Measurement
2.2.2. Femtosecond Z-scan Measurements
3. Results and Discussions
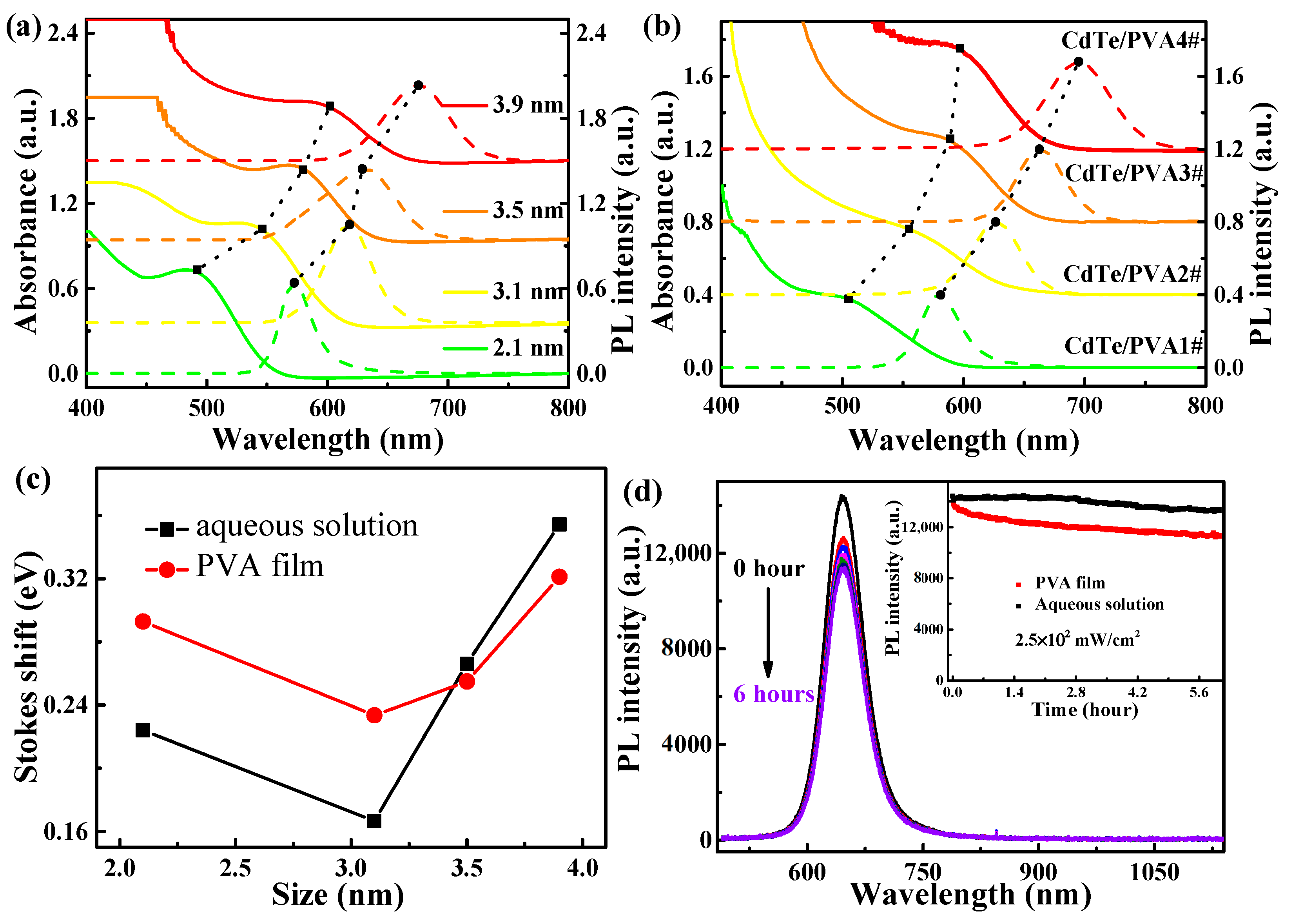
3.1. Basic Structural and Optical Properites
3.2. Steady-State PL Spectrum of CdTe NC/PVA Film at the Temperature from 80 to 300 K
3.3. Time-Resolved PL Spectra of CdTe NC/PVA Films at Temperatures from 80 to 320 K
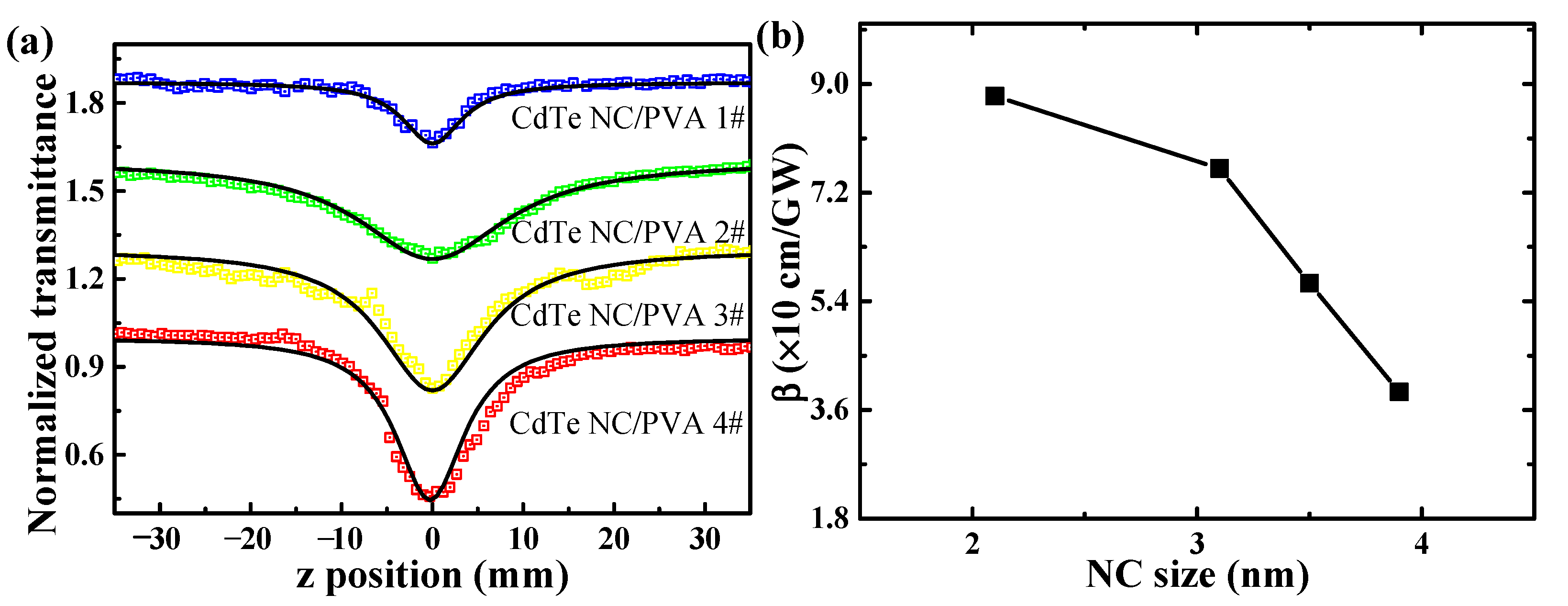
3.4. Nonlinear Absorption by Femtosecond Z-scan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shenhar, R.; Norsten, T.B.; Rotello, V.M. Polymer-Mediated Nanoparticle Assembly: Structural Control and Applications. Adv. Mater. 2005, 17, 657–669. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Zheng, C.; Yang, B. Crosslink-Enhanced Emission Effect on Luminescence in Polymers: Advances and Perspectives. Angew. Chem. Int. Ed. 2020, 59, 9826–9840. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, H.; Zhang, J.; Wei, H.; Ju, J.; Li, M.; Yang, B. White-light emission nanofibers obtained from assembling aqueous single-colored CdTe NCs into a PPV precursor and PVA matrix. J. Mater. Chem. 2009, 19, 6740–6744. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, K.; Pan, A.; Zhu, Y.; Hong, J.; Liang, P. Use of CdTe Quantum Dots as Heat Resistant Temperature Sensor for Bearing Rotating Elements Monitoring. IEEE J. Sel. Areas Commun. 2020, 38, 463–470. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, Y.; Wu, W.; Luo, K.; Rong, Z.; Xie, S.; Zuo, W.; Yu, J.; Zhang, R.; Qin, D.; et al. Hole Transfer Layer Engineering for CdTe Nanocrystal Photovoltaics with Improved Efficiency. Nanomaterials 2020, 10, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, J.; Bishop, D.M.; Todorov, T.K.; Gunawan, O.; Rath, J.; Nekovei, R.; Artegiani, E.; Romeo, A. Flexible CIGS. CdTe and a-Si:H based thin film solar cells: A review. Prog. Mater. Sci. 2020, 110, 100619–100638. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Vsetickova, M.; Stankova, M.; Uhlirova, D.; Ruttkay-Nedecky, B.; Ofomaja, A.; Fernandez, C.; Kepinska, M.; Baron, M.; Duong, N.B.; et al. Study of Physico-Chemical Changes of CdTe QDs after Their Exposure to Environmental Conditions. Nanomaterials 2020, 10, 10050865–10050884. [Google Scholar] [CrossRef]
- Protasenko, V.; Bacinello, D.; Kuno, M. Experimental determination of the absorption cross-section and molar extinction coefficient of CdSe and CdTe nanowires. J. Phys. Chem. B 2006, 110, 25322–25331. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Hoeppener, S.; van den Berg, A.M.J.; Susha, A.S.; Rogach, A.L.; Feldmann, J.; Schubert, U.S. Inkjet printing of luminescent CdTe nanocrystal-polymer composites. Adv. Funct. Mater. 2007, 17, 23–28. [Google Scholar] [CrossRef]
- Olvera-Felix, C.; Ramirez-Bon, R.; Ochoa-Landin, R.; Ruvalcaba-Manzo, S.G.; Castillo, S.J. Optical and Structural Characterization of CdTe Nanoparticles Synthesized Using Chemical Bath Deposition Technique. J. Electron. Mater. 2020, 49, 1257–1265. [Google Scholar] [CrossRef]
- Balakhayeva, R.; Akilbekov, A.; Baimukhanov, Z.; Usseinov, A.; Giniyatova, S.; Zdorovets, M.; Vlasukova, L.; Popov, A.I.; Dauletbekova, A. CdTe Nanocrystal Synthesis in SiO2/Si Ion-Track Template: The Study of Electronic and Structural Properties. Phys. Status Solidi A Appl. Mater. Sci. 2021, 218, 2000231–2000235. [Google Scholar] [CrossRef]
- Dzhagan, V.; Stroyuk, O.; Raievska, O.; Isaieva, O.; Kapush, O.; Selyshchev, O.; Yukhymchuk, V.; Valakh, M.; Zahn, D.R.T. Photoinduced Enhancement of Photoluminescence of Colloidal II-VI Nanocrystals in Polymer Matrices. Nanomaterials 2020, 10, 10122565–10122581. [Google Scholar] [CrossRef] [PubMed]
- Phukan, P.; Saikia, D. Optical and Structural Investigation of CdSe Quantum Dots Dispersed in PVA Matrix and Photovoltaic Applications. Int. J. Photoenergy 2013, 2013, 728280–728284. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhang, J.; Song, Y.; Zhang, G.; Zhang, H.; Yang, B. Fluorescent Nanocomposite Based on PVA Polymer Dots. Acta Chim. Sin. 2012, 70, 2311–2315. [Google Scholar] [CrossRef] [Green Version]
- Jeeju, P.P.; Jayalekshmi, S. On the Interesting Optical Properties of Highly Transparent, Thermally Stable, Spin-Coated Polystyrene/Zinc Oxide Nanocomposite Films. J. Appl. Polym. Sci. 2011, 120, 1361–1366. [Google Scholar] [CrossRef]
- Jeeju, P.P.; Jayalekshmi, S.; Chandrasekharan, K.; Sudheesh, P. Size dependent nonlinear optical properties of spin coated zinc oxide-polystyrene nanocomposite films. Opt. Commun. 2012, 285, 5433–5439. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef]
- Qian, H.; Dong, C.; Weng, J.; Ren, J. Facile one-pot synthesis of luminescent, water-soluble, and biocompatible glutathione-coated CdTe nanocrystals. Small 2006, 2, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.A.; Wu, H.; Williams, K.R.; Cao, Y.C. Synthesis of CdSe and CdTe nanocrystals without precursor injection. Angew. Chem. Int. Ed. 2005, 44, 6712–6715. [Google Scholar] [CrossRef]
- He, Y.; Lu, H.-T.; Sai, L.-M.; Lai, W.-Y.; Fan, Q.-L.; Wang, L.-H.; Huang, W. Synthesis of CdTe nanocrystals through program process of microwave irradiation. J. Phys. Chem. B 2006, 110, 13352–13356. [Google Scholar] [CrossRef]
- Xu, C.; Kong, A.; Ding, H.; Shan, Y. Non-organometallic phosphine-free synthesis of high quality CdTe nanocrystals. Mater. Lett. 2012, 82, 45–47. [Google Scholar] [CrossRef]
- Gaponik, N.; Talapin, D.V.; Rogach, A.L.; Hoppe, K.; Shevchenko, E.V.; Kornowski, A.; Eychmüller, A.; Weller, H. Thiol-Capping of CdTe Nanocrystals: An Alternative to Organometallic Synthetic Routes. J. Phys. Chem. B 2002, 106, 7177–7185. [Google Scholar] [CrossRef]
- Rogach, A.L.; Franzl, T.; Klar, T.A.; Feldmann, J.; Gaponik, N.; Lesnyak, V.; Shavel, A.; Eychmueller, A.; Rakovich, Y.P.; Donegan, J.F. Aqueous synthesis of thiol-capped CdTe nanocrystals: State-of-the-art. J. Phys. Chem. C 2007, 111, 14628–14637. [Google Scholar] [CrossRef]
- Chai, Z.; Gao, Y.; Kong, D.; Wu, W. Nonlinear Absorptions of CdSeTe Quantum Dots under Ultrafast Laser Radiation. J. Nanomater. 2016, 2016, 9138059–9138063. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.; Hagan, D.J.; Stryland, E.W.V. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Q.; Zhang, Y.-Y.; Zhang, F.; Li, W.-Y. One-pot synthesis of thermal responsive QDs-PNIPAM hybrid fluorescent microspheres by controlling the polymerization temperature at two different polymerization stages. J. Mater. Chem. 2011, 21, 6556–6562. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Kupchak, I.; Korbutyak, D.; Kalytchuk, S.; Kryuchenko, Y.; Chkrebtii, A. Stokes shift in CdTe quantum dots. J. Phys. Stud. 2010, 14, 2701. [Google Scholar] [CrossRef]
- Chai, Z.; Wu, W.; Kong, D.; Gao, Y.; Chang, Q. Size-dependent electronic decays and coherent phonon of CdSeTe quantum dots in glass matrix. J. Non-Cryst. Solids 2013, 382, 121–124. [Google Scholar] [CrossRef]
- Wu, W.; Yu, D.; Ye, H.-a.; Gao, Y.; Chang, Q. Temperature and composition dependent excitonic luminescence and exciton-phonon coupling in CdSeS nanocrystals. Nanoscale Res. Lett. 2012, 7, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Ravindra, N.; Srivastava, V. Temperature dependence of the energy gap in semiconductors. J. Phys. Chem. Solids 1979, 40, 791–793. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Hellwege, K.H. Numerical Data and Functional Relationship in Science and Technology; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Vyhnan, N.; Khalavka, Y. Size-dependent temperature sensitivity of photoluminescence peak position of CdTe quantum dots. Luminescence 2014, 29, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Comsa, G.; Heitkamp, D.; Räde, H. Effect of size on the vibrational specific heat of ultrafine palladium particles. Solid State Commun. 1977, 24, 547–550. [Google Scholar] [CrossRef]
- De, A.; Mondal, N.; Samanta, A. Luminescence tuning and exciton dynamics of Mn-doped CsPbCl3 nanocrystals. Nanoscale 2017, 9, 16722–16727. [Google Scholar] [CrossRef]
- Rudin, S.; Reinecke, T.L.; Segall, B. Temperature-dependent exciton linewidths in semiconductors. Phys. Rev. B 1990, 42, 11218–11231. [Google Scholar] [CrossRef]
- al Salman, A.; Tortschanoff, A.; Mohamed, M.B.; Tonti, D.; van Mourik, F.; Chergui, M. Temperature effects on the spectral properties of colloidal CdSe nanodots, nanorods, and tetrapods. Appl. Phys. Lett. 2007, 90, 093104–093106. [Google Scholar] [CrossRef]
- Wu, K.; Bera, A.; Ma, C.; Du, Y.; Yang, Y.; Li, L.; Wu, T. Temperature-dependent excitonic photoluminescence of hybrid organometal halide perovskite films. Phys. Chem. Chem. Phys. 2014, 16, 22476–22481. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y. Ultrafast carrier dynamics and coherent acoustic phonons in bulk CdSe. Opt. Lett. 2015, 40, 64–67. [Google Scholar] [CrossRef]
- Shinde, A.; Gahlaut, R.; Mahamuni, S. Low-Temperature Photoluminescence Studies of CsPbBr3 Quantum Dots. J. Phys. Chem. C 2017, 121, 14872–14878. [Google Scholar] [CrossRef]
- Jin, Q.; Wu, W.; Zheng, Z.; Yan, Y.; Liu, W.; Li, A.; Yang, Y.; Su, W. The third-order optical nonlinearity and upconversion luminescence of CdTe quantum dots under femtosecond laser excitation. J. Nanopart. Res. 2009, 11, 665–670. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; He, Z. Large off-resonant and intensity-dependent third-order optical nonlinearities of CdSeS quantum dots/polystyrene composite film. Mater. Lett. 2013, 93, 366–369. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Hutchings, D.C.; Hagan, D.J.; Stryland, E.W.V. Dispersion of bound electron nonlinear refraction in solids. IEEE J. Quantum Electron. 1991, 27, 1296–1309. [Google Scholar] [CrossRef]
- Wu, W.; Chai, Z.; Gao, Y.; Kong, D.; He, F.; Meng, X.; Wang, Y. Carrier dynamics and optical nonlinearity of alloyed CdSeTe quantum dots in glass matrix. Opt. Mater. Express 2017, 7, 1547–1556. [Google Scholar] [CrossRef]
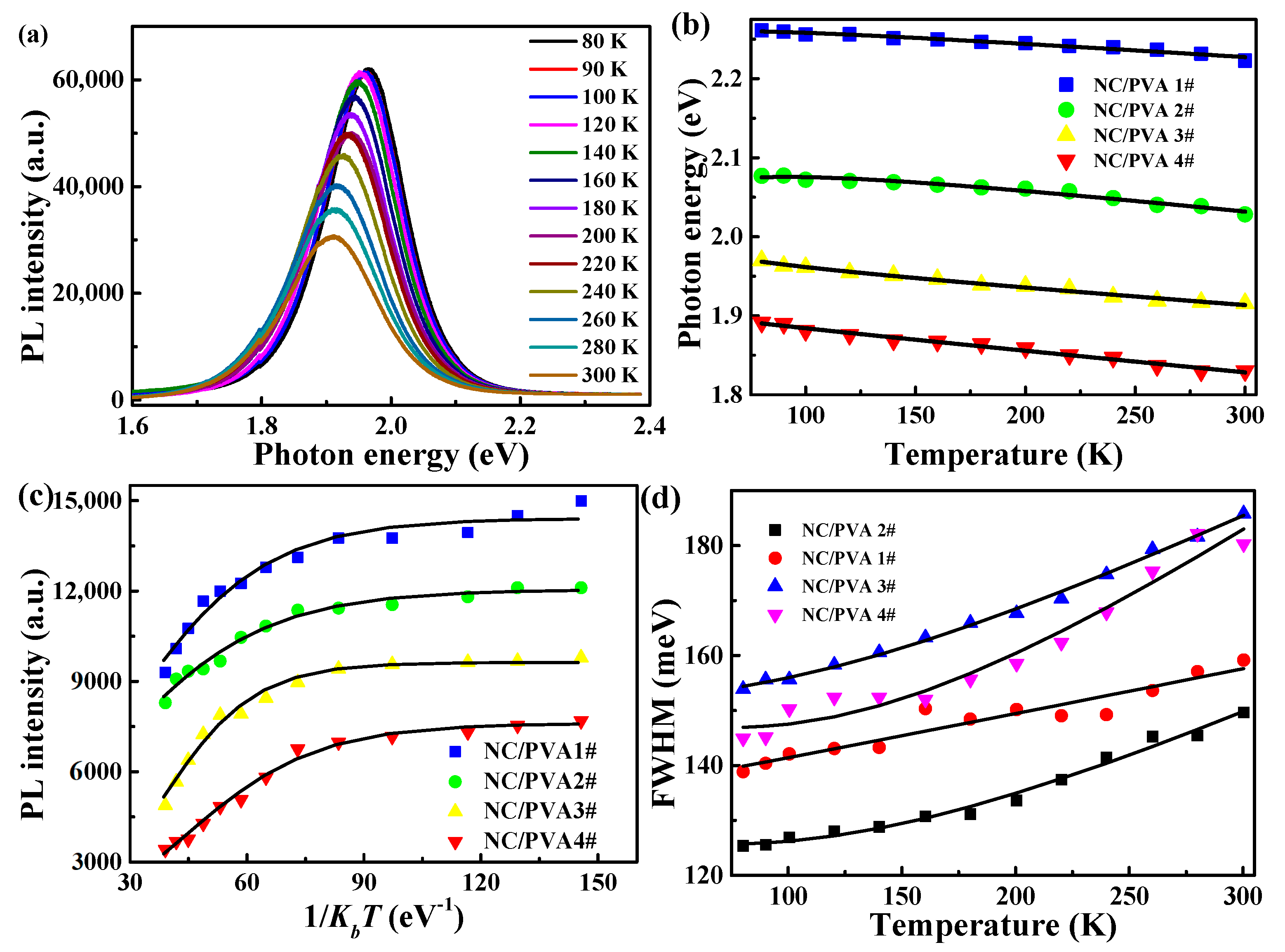

| Films | Eg(0) (eV) | α (eV/K) | β (K) | EB (meV) | Γ0 (meV) | Γop (meV) | ћωop (meV) |
|---|---|---|---|---|---|---|---|
| CdTe NC/PVA 1# | 2.26 | 2.9 × 10−4 | 312 | 62.7 | 16 | 16 | 20 |
| CdTe NC/PVA 2# | 2.08 | 3.2 × 10−4 | 244 | 54.4 | 25 | 18 | 20 |
| CdTe NC/PVA 3# | 1.97 | 3.4 × 10−4 | 233 | 70.2 | 42 | 20 | 21 |
| CdTe NC/PVA 4# | 1.89 | 3.4 × 10−4 | 146 | 62.3 | 54 | 21 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Sui, J.; Chai, Z.; Wu, W. Temperature-Dependent Excitonic Photoluminescence and Nonlinear Absorption of CdTe Nanocrystal/Polyvinyl Alcohol Films. Nanomaterials 2021, 11, 1761. https://doi.org/10.3390/nano11071761
Chang Q, Sui J, Chai Z, Wu W. Temperature-Dependent Excitonic Photoluminescence and Nonlinear Absorption of CdTe Nanocrystal/Polyvinyl Alcohol Films. Nanomaterials. 2021; 11(7):1761. https://doi.org/10.3390/nano11071761
Chicago/Turabian StyleChang, Qing, Jingrong Sui, Zhijun Chai, and Wenzhi Wu. 2021. "Temperature-Dependent Excitonic Photoluminescence and Nonlinear Absorption of CdTe Nanocrystal/Polyvinyl Alcohol Films" Nanomaterials 11, no. 7: 1761. https://doi.org/10.3390/nano11071761
APA StyleChang, Q., Sui, J., Chai, Z., & Wu, W. (2021). Temperature-Dependent Excitonic Photoluminescence and Nonlinear Absorption of CdTe Nanocrystal/Polyvinyl Alcohol Films. Nanomaterials, 11(7), 1761. https://doi.org/10.3390/nano11071761






